Ограниченный протеолиз как способ снижения аллергенности запасных глобулинов семян
Автор: Кердиварэ А.М.
Журнал: Сельскохозяйственная биология @agrobiology
Рубрика: Обзоры, проблемы
Статья в выпуске: 3 т.53, 2018 года.
Бесплатный доступ
По данным SDAP (структурная база данных аллергенных белков), запасные 11S и 7S глобулины семян арахиса, сои и некоторых других растений являются аллергенами. Структурной основой доменов двудоменных субъединиц 11S и 7S глобулинов служит β-баррель, соединенный с группой α-спиралей. В процессе эволюции в аминокислотных последовательностях запасных глобулинов за пределами структурного модуля β-баррель-α-спирали появились протяженные неупорядоченные вставки с повышенной чувствительностью к протеолитической атаке. Эти вставки определяют закономерности ограниченного протеолиза запасных глобулинов в прорастающих семенах и in vitro. В настоящем обзоре анализируются экспериментальные данные, полученные при исследовании ограниченного протеолиза запасных глобулинов семян арахиса (Arachis hypogaea L.), сои (Glycine max L.) и некоторых других растений. Установлено, что ограниченный протеолиз 11S глобулина арахиса (A. Cherdivară с соавт., 2017) приводит к разрушению С-концевой области a-цепей, включая участок, образующий группу a-спиралей...
Запасные глобулины семян, протеолиз, аллергенность, ige эпитопы, арахис, соя
Короткий адрес: https://sciup.org/142216550
IDR: 142216550 | УДК: 631.576.33:577.15.004.14:577.152.3 | DOI: 10.15389/agrobiology.2018.3.475rus
Текст обзорной статьи Ограниченный протеолиз как способ снижения аллергенности запасных глобулинов семян
Основную часть пищевых растительных белков представляют собой запасные белки семян. Подавляющее число запасных белков семян входит в состав двух консервативных семейств — 7S (вицилины) и 11S глобулинов (легумины) (1). Их аминокислотные последовательности унаследованы от вицилино- и легуминоподобных белков споровых растений (2). В свою очередь, последние произошли от бактериальных оксалатдекарбоксилаз (3). Все эти белки принадлежат обширному суперсемейству ку-пинов, объединяющему десятки функционально разнообразных семейств белков, в структуре которых присутствует β -баррель из антипараллельных
β -стрендов (4).
Третичная структура субъединиц олигомерных молекул оксалатдекарбоксилаз и запасных глобулинов дополнена группой α -спиралей (2). В субъединицах этих белков присутствуют два домена, каждый из которых образован структурным модулем β -баррель- α -спирали. Двудоменная структура оксалатдекарбоксилаз сформировалась на раннем эволюционном этапе в результате дупликации этого модуля, присутствующего в их однодоменном бактериальном предшественнике (3).
В процессе эволюции в аминокислотных последовательностях запасных глобулинов семян, в целом консервативных, появились протяженные вариабельные гидрофильные вставки, вынесенные на поверхность их олигомерных структур (2, 3). Эти вставки определяют чувствительность нативных молекул запасных глобулинов к быстрому ограниченному протеолизу, с которого и начинается их деградация в прорастающих семенах и in vitro (2). Последующий массированный протеолиз запасных глобулинов происходит по поочередному механизму (5), приводящему к полному разрушению их молекул (3).
В семенах арахиса (6-8), сои (9-11) и многих других культурных растений, используемых в пищевом рационе, — орехов (12-14), миндаля (15) и горчицы (16), запасные 7S и 11S глобулины являются аллергенами. Эта информация, а также ряд дополнительных сведений внесены в базу данных SDAP (Structural database of allergenic proteins) (17). В некоторых из запасных глобулинов — 7S глобулинах арахиса (18) и чечевицы (19), 11S глобулинах арахиса (20), сои (21, 22) и гречихи (23) — идентифицированы антигенные детерминанты (IgE эпитопы), ответственные за IgE связывание. Многие из IgE эпитопов принадлежат областям последовательностей запасных глобулинов с повышенной чувствительностью к протеолитической атаке, что показывает принципиальную возможность снижения аллергенности запасных глобулинов семян посредством их ограниченного протеолиза.
В настоящем обзоре для подтверждения этой гипотезы проанализированы экспериментальные данные, полученные при исследовании ограниченного протеолиза запасных глобулинов семян арахиса ( Arachis hypogaea L.), сои ( Glycine max L.) и некоторых других растений.
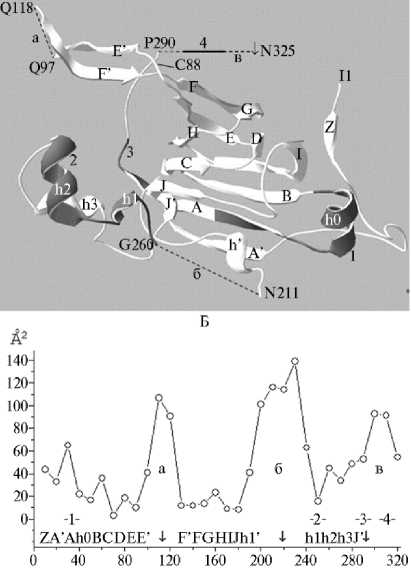
Ограниченный протеолиз 11S глобулинов и IgE эпитопы, идентифицированные в их последовательностях. Структура α -цепей субъединицы Ara h3 (pdb|3c3v) 11S глобулина арахиса (24), типичная для 11S глобулинов семян сои (25, 26) и ряда других растений (27, 28), образована β -баррелем из антипараллельных β -стрендов BCDEFGHI, соединенных с группой α -спиралей h1, h2, h3, и дополнена β -стрендами Z, A ′ -A, E ′ -F ′ и J-J ′ , а также α -спиралями h0 и h1 ′ . Три гидрофильные неупорядоченные области в α -цепях 11S глобулинов потенциально чувствительны к ограниченному протеолизу (2): петля между β -стрендами E и F (E ′ -F ′ ), петля между β -баррелем и α -спиралями и С-концевой участок (рис. 1, табл. 1, соответственно области а, б и в).
Для 11S глобулинов характерна следующая последовательность реакций ограниченного протеолиза (2). Процесс начинается с отщепления чувствительной к протеолизу гидрофильной С-концевой области (см. рис. 1, табл. 1, область в). Дальнейшее действие протеиназ приводит к расщеплению петли между β -баррелем и α -спиралями (см. рис. 1, табл. 1, область б). В зависимости от индивидуальных особенностей структуры 11S глобулина и специфичности протеиназы петля между β -стрендами E и F может либо оставаться интактной, либо расщепляться (см. рис. 1, табл. 1, 476
область а). Положение точек расщепления α -цепей 11S глобулинов опре-
Номера аминокислотных остатков
Рис. 1. Третичная структура α -цепи 11S глобулина арахиса ( Arachis hypogaea ) Ara h3 (pdb|3c3v) .
А — ленточная диаграмма. Неупорядоченные области (а, б, в) в α -це-пях 11S глобулинов потенциально чувствительны к ограниченному протеолизу (2). Стрелкой отмечена пептидная связь N325-G326, расщепляющаяся при созревании молекулы Ara h3. Остаток Cys88 участвует в формировании дисульфидной связи между α - и β -цепями. Темные участки диаграммы соответствуют последовательностям IgE эпитопов 1-4.
Б — доступная растворителю площадь аминокислотных остатков ASA (accessibility surface area) в последовательности α -цепи. Приведенные значения ASA (31), выраженные в Å 2 , соответствуют средним величинам, рассчитанным для групп из 10 остатков в аминокислотной последовательности α -цепи. График соответствует модельной четвертичной структуре гомогексамера Ara h3, построенной при использовании в качестве шаблона мономера pdb|3c3v (31). Стрелками обозначено положение точек расщепления α -цепи при ограниченном гидролизе трипсином (32). Цифрами показано положение IgE эпитопов 1-4 в аминокислотной последовательности Ara h3.
1. Точки расщепления α -цепей ( ↓ ) при ограниченном протеолизе запасных 11S глобулинов семян сои Glycine max Gly m G1-G5, арахиса Arachis hypogaea Ara h3 и abl14270 (ARAhy), подсолнечника Helianthus an-nuus aaa33374 (HELan), овса Avena sativa aaa32720 (AVEsa), кедра Pinus sibirica caa77569 (PINsi) и тыквы Cucurbita maxima pdb|2e9q (CUCma)
|
Вторичная структура |
Субъединица |
Протеиназа |
||
|
A′A - BCD --- E --- а |
FGHIJ h1 h2h3J′ |
|||
|
б |
в |
|||
|
↓ |
↓ |
Gly m G1-G5 |
Папаин (30) |
|
|
** |
** ↓ |
* ↓ * |
Gly m G2 |
Трипсин (34) |
|
** ↓ |
** ↓ |
* ↓ * |
Gly m G2 |
Трипсин (32) |
|
↓ |
↓ |
Gly m G5 |
Папаин (35) |
|
|
* ↓ |
↓ |
* ↓ ** |
Ara h3 |
Папаин (31) |
|
↓ |
↓ |
↓ |
ARAhy |
Трипсин (32) |
|
↓ |
↓ |
↓ |
HELan |
Папаин (36) |
|
↓ |
↓ |
↓ |
AVEsa |
Папаин (37) |
|
↓ |
↓ |
PINsi |
Папаин (38) |
|
|
↓ |
↓ |
CUCma |
Папаин (39) |
|
П р и м е ч а н и е. Разрушающийся при ограниченном протеолизе участок h1h2h3J’ выделен подчеркиванием. Звездочками отмечено положение IgE эпитопов, идентифицированных в субъединицах 11S глобулинов сои Gly m G1 (21) и Gly m G2 (22) и арахиса Ara h3 (20).
делено по результатам N-концевого секвенирования фрагментов (29, 30) или по совокупности косвенных данных (специфичность протеиназы, последовательность формирования фрагментов и их молекулярные массы). О потенциальной чувствительности к ограниченному протеолизу неупорядоченных областей (см. рис. 1, а, б, в) в аминокислотных последовательностях 11S глобулинов свидетельствует их относительно высокая доступность рас- творителю ASA, показанная на примере Ara h3 (см. рис. 1, Б).
Отщепляемый С-концевой участок, охватывающий область α-спиралей h1h2h3 и β-стренда J’ (см. табл. 1), разрушается до коротких пептидов, что характерно не только для Ara h3 (31), но и для других 11S глобулинов (2). Следует отметить, что N-концевая область α-цепи Ara h3, как и всех исследованных 11S глобулинов (2), нечувствительна к протеолитической атаке. Три из четырех IgE эпитопов, идентифицированных в субъединице 11S глобулина арахиса Ara h3 (20) (см. рис. 1, табл. 1), принадлежат к С-концевой области α-цепи, разрушающейся при гидролизе папаином (31). То есть ограниченный протеолиз субъединицы Ara h3 может привести к существенному снижению ее аллергенности.
В состав гетерогексамерной молекулы 11S глобулина арахиса входят девять субъединиц, весьма консервативных в С-концевой области α -цепей, где присутствуют три из четырех IgE эпитопов Ara h3 (см. рис. 1, 2, табл. 1). О потенциальной способности к связыванию IgE каждой из последовательностей других субъединиц 11S глобулина арахиса, гомологичных второму и третьему IgE эпитопам Ara h3, можно судить по результатам определения PD-индексов (property-based peptide similarity index for two sequences) (40). Метод основан на сопоставлении физико-химических свойств каждой из аминокислот последовательности IgE эпитопа, идентифицированного в белковом аллергене, с каждой из аминокислот близкого по первичной структуре участка последовательности исследуемого белка. По мере возрастания различий между сравниваемыми участками этих последовательностей величина PD индексов увеличивается от 0 (последовательности идентичны) до предельной величины, равной 10, выше которой присутствие соответствующего IgE эпитопа маловероятно (40).
Присутствие второго и третьего IgE эпитопов Ara h3 (см. рис. 1, Б) в гомологичных участках последовательностей шести из восьми субъединиц 11S глобулина арахиса, неидентичных Ara h3 (31), весьма вероятно (величина соответствующих PD-индексов не превышает 2,9). Наконец, последовательности четвертого IgE эпитопа Ara h3 (см. рис. 1, Б) и соответствующих предполагаемых IgE эпитопов в двух других субъединицах 11S глобулина арахиса (aag01363 и abf93402) идентичны. То есть ограниченный протеолиз не только субъединицы Ara h3, но и всей гетерогексамерной молекулы 11S глобулина арахиса может сопровождаться существенным снижением его аллергенности.
Рис. 2. Аминокислотные последовательности С-концевой области α -цепей 11S глобулинов арахиса Arachis hypogaea (A1 — pdb|3c3v, A2 — abl14270) и сои Glycine max (G1 — pdb|1fxz; G2 — baa00154) . Цифры слева соответствуют нумерации аминокислотных остатков в полных последовательностях субъединиц. В нижних строчках показаны идентичные аминокислотные остатки (*) и их консервативные (:) и полуконсерва-тивные (.) замещения. Стрелки соответствуют пептидным связям, расщепляемым трипсином ( ↓ ) (29, 32, 34) и папаином ( ↑ ) (30, 32). Жирным шри-
hl'
Al 185 EFLRYQQQSRQSRRRSLPYSPYSPQSQPR^QEEREFSPRGQHSR R A2 185 EFLRYQQQSRQSRRRSLPLSPYSPQ--PG QEDREFSPQGQHGR>Lr G1 172 EFLKYQQ-EQGGHQSQ------------- К
G2 170 EFLKYQQQQQGGSQSQ hl h2h3
Al 229 E R AGQEEEHEGGNIFSGFTPEFLAQAFQVDDRQIVQNLRGENESE A2 227 E R AGQEQENEGGNIFSGFTSEFLAQAFQVDDRQIVQNLRGENESE G1 188 gTk hqqeeeneggsilsgftleflehafsvd-kqiaknlqgenege G2 187 g?k-L-qqeeenegsnilsgfapeflkeafgvn-mqivrnlqgeneee
J'
Al 274 eqgaivtvr>Lgglrilspdrk rgadeeeeydedeyeydeedrrrgr A2 272 EQGAIVTVK GGLRILSPDRK SP-DEEEEYDEDEYAEEERQQDRRR G1 232 DKGAIVTVK GGLSVIKPPTD EQQQRPQEEEEEEEDEKPQCKGKDK G2 230 DSGAIVTVK GGLRVTAPAMR>LkPQ---QEEDDDDEEEQPQCVETDK фтом выделены последовательности IgE эпитопов, подчеркиванием — аминокислотные остатки Arg и Lys повышенной доступности растворителю (ASA >100 Е) в модельных олигомерных структурах А1 и G1.
Перспективы снижения аллергенности 11S глобулина сои посредством ограниченного протеолиза не столь однозначны. С одной стороны, ограниченный протеолиз субъединицы Gly m G1 приводит к удалению 478
участка α -спирали h1h2h3- β -стренд J ′ , где присутствуют оба идентифицированных IgE эпитопа (см. табл. 1, рис. 2). Наличие двух соответствующих IgE эпитопов в гомологичных последовательностях субъединиц Gly m G3, Gly m G4 и Gly m G5 не исключено (PD-индексы от 0 до 5,9). С другой стороны, только один из IgE эпитопов, идентифицированных в 11S глобулине сои Gly m G2 (см. табл. 1), может быть удален при его ограниченном протеолизе папаином (см. рис. 2). При этом присутствие соответствующего IgE эпитопа в гомологичных участках последовательностей других субъединиц 11S глобулина сои маловероятно (величина PD-индексов близка к 10).
Ограниченный протеолиз 7S глобулинов и IgE эпитопы, идентифицированные в последовательности 7S глобулина арахиса Ara h1. N- и С-концевые домены субъединиц 7S глобулинов струк- турно эквивалентны α- и β-цепям 11S глобулинов, но отличаются от по- следних по нескольким признакам (41): область междоменного линкера в 7S глобулинах не расщеплена, в их структурах отсутствует междоменная дисульфидная связь. В 7S глобулинах известной третичной структуры из семян канавалии (42), фасоли (43), сои (44), вигны (45, 46) и сосны (47) присутствует ряд неупорядоченных областей, потенциально чувствительных к ограниченному протеолизу (2) (табл. 2, рис. 3).
В субъединице 7S глобулина арахиса Ara h1, проявляющей наибольшую аллергенность среди запасных глобулинов семян (59), установлено присутствие 21 IgE эпитопа (18). IgE эпитопы 1-3 принадлежат N-концевой последовательности, удаляемой посттрасляционно (60). IgE эпитопы 4-9 (см. рис. 3, А), локализованные в чувствительной области N-концевого удлинения (см. рис. 3, Б, область а), которое изобилует остатками, соответствующими субстратной специфичности папаина (61), удаляются при начальной атаке этим ферментом (50). Последующее действие папаина приводит к разрушению чувствительной области между α-спиралью h3 и β-стрендом J′ (см. табл. 2, область б), специфично удлиненной в последовательности Ara h1, где локализован IgE эпитоп 13 (см. рис. 3, A).
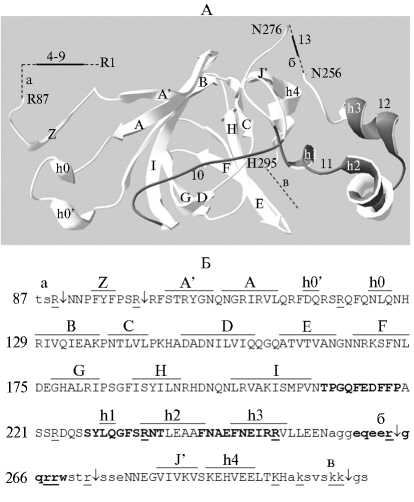
Рис. 3. Структура N-концевого домена 7S глобулина арахиса Ara h1 (pdb|3smh).
А — ленточная диаграмма третичной структуры. Неупорядоченные области а, б и в показаны штриховыми линиями. Темные участки диаграммы соответствуют последовательностям IgE эпитопов 4-13 в зрелой молекуле Ara h1 (18).
Б — первичная структура. Неупорядоченные области: а — N-концевое удлинение, б — между α -спиралью h3 и β -стрендом J ′ , в — С-концевая область. Стрелки соответствуют пептидным связям, расщепляемым папаином (50). Подчеркиванием выделены аминокислотные остатки с повышенной доступностью для растворителя (ASA > 100 Е), соответствующие субстратной специфичности папаина (61). Эти остатки присутствуют в кристаллической структуре олигомера pdb|3smh, а также его модели (pdb|3s7e в качестве шаблона) (50) в областях б и в. Жирным шрифтом показаны последовательности IgE эпитопов 10-13.
Следует отметить относительно высокую доступность для растворителя у участка α -спиралей h1-h3, — в 3,5 раза выше, чем ASA у остальной части N-концевого домена Ara h1. Поэтому заманчиво попытаться подобрать условия ограниченного протеолиза, обеспечивающие разрушение этого потенциально чувствительного участка α -спиралей, где присутствуют IgE эпитопы 11 и 12 (см. рис. 3, А).
Субъединицы 7S глобулина арахиса в составе его гетеротримерной молекулы чрезвычайно консервативны. Весьма вероятно, что IgE эпитопы, идентифицированные в Ara h1, присутствуют и в других субъединицах этого белка: соответствующие PD-индексы не превышают 2,5. Ограниченный протеолиз папаином приводит к существенному снижению аллергенности всей гетероолигомерной молекулы 7S глобулина арахиса в связи с удалением более чем третьей части IgE эпитопов.
Таким образом, приведенные в настоящем обзоре результаты исследований ограниченного протеолиза 11S и 7S глобулинов арахиса, сои и некоторых других растений свидетельствуют о перспективности использования этого метода для существенного снижения аллергенности запасных глобулинов семян.
Список литературы Ограниченный протеолиз как способ снижения аллергенности запасных глобулинов семян
- Dunwell J.M. Structure, function, and evolution of vicilin and legumin seed storage proteins. In: Biotechnology of biopolymers -from synthesis to patents/A. Steinbüchel, Y. Doi (eds.). Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2005: 967-997.
- Shutov A.D., Wilson K.A. Seed storage globulins: their descent from bacterial ancestors and mechanisms of degradation. In: Globulins: biochemistry, production and role in immunity/S.D. Milford (ed.). Nova Science Publishers, NY, 2014: 71-104.
- Rudakova A.S., Cherdivară A.M., Wilson K.A., Shutov A.D. Seed storage globulins: origin and evolution of primary and higher order structures. Biochemistry (Moscow), 2015, 80: 1354-1361 ( ) DOI: 10.1134/S000629791510017X
- Dunwell J.M., Culham A., Carter C.E., Sosa-Aguirre C.R., Goodenpugh P.W. Evolution of functional diversity in the cupin superfamily. Trends Biochem. Sci., 2001, 26: 740-746 ( ) DOI: 10.1016/S0968-0004(01)01981-8
- Rupley J.A. Susceptibility to attack by proteolytic enzymes. Method. Enzymol., 1967, 11: 905-917.
- Hourihane J.O. Peanut allergy -current status and future challenges. Clin. Exp. Allergy, 1997, 27: 1240-1246 ( ) DOI: 10.1111/j.1365-2222.1997.tb01167.x
- Kleber-Janke T., Crameri R., Appenzeller U., Schlaak M., Becker W.M. Selective cloning of peanut allergens, including profilin and 2S albumins, by phage display technology. Int. Arch. Allergy Imm., 1999, 119: 265-274 ( ) DOI: 10.1159/000024203
- Al-Muhsen S., Clarke A.E., Kagan R.S. Peanut allergy: an overview. Can. Med. Assoc. J. (CMAJ), 2003, 168: 1279-1285.
- Helm R.M., Cockrell G., Connaughton C., Sampson H.A., Bannon G.A., Beilinson V., Livingstone D., Nielsen N.C., Burks A.W. A soybean G2 glycinin allergen. 1. Identification and characterization. Int. Arch. Allergy Imm., 2000, 123: 205-212 ( ) DOI: 10.1159/000024445
- Krishnan H.B., Kim W.S., Jang S., Kerley M.S. All three subunits of soybean beta-conglycinin are potential food allergens. J. Agr. Food Chem., 2009, 57: 938-943 ( ) DOI: 10.1021/jf802451g
- Wang T., Qin G.X., Sun Z.W., Zhao Y. Advances of research on glycinin and β-conglycinin: a review of two major soybean allergenic proteins. Crit. Rev. Food Sci., 2014, 54: 850-862 ( ) DOI: 10.1080/10408398.2011.613534
- Beyer K., Grishina G., Bardina L., Grishin A., Sampson H.A. Identification of an 11S globulin as a major hazelnut food allergen in hazelnut-induced systemic reactions. J. Allergy Clin. Immunol., 2002, 110: 517-523 ( ) DOI: 10.1067/mai.2002.127434
- Wang F., Robotham J.M., Teuber S.S., Sathe S.K., Roux K.H. Ana o 2, a major cashew (Anacardium occidentale L.) nut allergen of the legumin family. Int. Arch. Allergy Imm., 2003, 132: 27-39 ( ) DOI: 10.1159/000073262
- Sharma G.M., Irsigler A., Dhanarajan P., Ayuso R., Bardina L., Sampson H.A., Roux K.H., Sathe S.K. Cloning and characterization of an 11S legumin, Car i 4, a major allergen in pecan. J. Agr. Food Chem., 2011, 59: 9542-9552 ( ) DOI: 10.1021/jf2017447
- Willison L.N., Tripathi P., Sharma G., Teuber S.S., Sathe S.K., Roux K.H. Cloning, expression and patient IgE reactivity of recombinant Pru du 6, an 11S globulin from almond. Int. Arch. Allergy Imm., 2011, 156: 267-281 ( ) DOI: 10.1159/000323887
- Palomares O., Cuesta-Herranz J., Vereda A., Sirvent S., Villalba M., Rodrigues R. Isolation and identification of an 11S globulin as a new major allergen in mustard seeds. Annals of Allergy, Asthma and Immunology, 2005, 94: 586-592 ( ) DOI: 10.1016/S1081-1206(10)61138-6
- Ivanciuk O., Schein C.H., Braun W. SDAP: database and computational tools for allergenic proteins. Nucleic Acids Res., 2003, 31: 359-362 ( ) DOI: 10.1093/nar/gkg010
- Shin D.S., Compadre C.M., Maleki S.J., Kopper R.A., Sampson H., Huang S.K., Burks A.W., Bannon G.A. Biochemical and structural analysis of the IgE binding sites on Ara h 1, an abundant and highly allergenic peanut protein. J. Biol. Chem., 1998, 273: 13753-13759 ( ) DOI: 10.1074/jbc.273.22.13753
- Vereda A., Andreae D.A., Lin J., Shreffler W.G., Ibanez M.D., Cuesta-Herranz J., Bardina L., Sampson H.A. Identification of IgE sequential epitopes of lentil (Len c 1) by means of peptide microarray immunoassay. J. Allergy Clin. Immunol., 2010, 126: 596-601 ( ) DOI: 10.1016/j.jaci.2010.06.023
- Rabjohn P., Helm E.M., Stanley J.S., West C.M., Sampson H.A., Burks A.W., Bannon G.A. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J. Clin. Invest., 1999, 103: 535-542 ( ) DOI: 10.1172/JCI5349
- Beardslee T.A., Zeece M.G., Sarath G., Markwell J.P. Soybean glycinin G1 acidic chain shares IgE epitopes with peanut allergen Ara h 3. Int. Arch. Allergy Imm., 2000, 123: 299-307 ( ) DOI: 10.1159/000053642
- Helm R.M., Cockrell G., Connaughton C., Sampson H.A., Bannon G.A., BeilinsonV., Nielsen N.C., Burks A.W. A soybean G2 glycinin allergen. 2. Epitope mapping and three-dimensional modeling. Int. Arch. Allergy Imm., 2000, 123: 213-219 ( ) DOI: 10.1159/000024446
- Yoshioka H., Ohmoto T., Urisu A., Mine Y., Adachi T. Expression and epitope analysis of the major allergenic protein Fag e 1 from buckwheat. J. Plant Physiol., 2004, 161: 761-767 ( ) DOI: 10.1016/j.jplph.2004.01.010
- Jin T., Guo F., Chen Y.W., Howard A., Zhang Y.Z. Crystal structure of Ara h 3, a major allergen in peanut. Mol. Immunol., 2009, 46: 1796-1804 ( ) DOI: 10.1016/j.molimm.2009.01.023
- Adachi M., Takenaka Y., Gidamis A.B., Mikami B., Utsumi S. Crystal structure of soybean proglycinin A1aB1b homotrimer. J. Mol. Biol., 2001, 305: 291-305 ( ) DOI: 10.1006/jmbi.2000.4310
- Adachi M., Kanamori J., Masuda T., Yagasaki K., Kitamura K., Mikami B., Ursumi S. Crystal structure of soybean 11S globulin: glycinin A3B4 homohexamer. PNAS USA, 2003, 100: 7395-7400 ( ) DOI: 10.1073/pnas.0832158100
- Tandang-Silvas M.R., Carrazco-Pena L., Barba de la Rosa A.P., Osuna-Castro J.A., Utsumi S., Mikami B., Maruyama N. Expression, purification and preliminary crystallization of amaranth 11S proglobulin seed storage protein from Amaranthus hypochondriacus L. Acta Crystallogr. F, 2010, 66: 919-922 ( ) DOI: 10.1107/S1744309110021032
- Tandang-Silvas M.R.G., Fukuda T., Fukuda C., Prak K., Cabanos C., Kimura A., Itoh T., Mikami B., Utsumi S., Maruyama N. Conservation and divergence on plant seed 11S globulins based on crystal structures. Biochim. Biophys. Acta, 2010, 1804: 1432-1442 ( ) DOI: 10.1016/j.bbapap.2010.02.016
- Shutov A.D., Kakhovskaya I.A., Bastrygina A.S., Bulmaga V.P., Horstmann C., Müntz K. Limited proteolysis of β-conglycinin and glycinin, the 7S and 11S storage globulins from soybean (Glycine max (L.) Merr.): structural and evolutionary implications. Eur. J. Biochem., 1996, 241: 221-228 ( ) DOI: 10.1111/j.1432-1033.1996.0221t.x
- Shutov A., Rudakova A., Rudakov S., Kakhovskaya I., Schallau A., Maruyama N., Wilson K. Limited proteolysis regulates massive degradation of glycinin, storage 11S globulin from soybean seeds: an in vitro model. J. Plant Physiol., 2012, 169: 1227-1233 ( ) DOI: 10.1016/j.jplph.2012.06.004
- Cherdivară A., Rudakova A., Rudakov S., Şutov A. Alergenul Ara h3, globulina de rezervă din seminţele de arahide. 1. Proteoliza limitată cu papaină . Studia Universitatis Moldaviae, seria Ştiinţe reale şi ale naturii, 2017, 1(101): 37-40 (in Romanian).
- Cherdivarăă A., Rudakova A., Rudakov S., Şutov A. Alergenul Ara h3, globulina de rezervă din seminţele de arahide. 2. Proteoliza limitată cu tripsină . Studia Universitatis Moldaviae, seria Ştiinţe reale şi ale naturii, 2017, 1(101): 41-45 (in Romanian).
- Cherdivarăă A., Rudakova A., Şutov A. Globulinele de rezervă 7S din seminţe ca alergeni. Imunoreactivitatea încrucişată între globulinele de rezervă 7S şi 11S . Studia Universitatis Moldaviae, seria Ştiinţe reale şi ale naturii, 2016, 1(91): 56-60 (in Romanian).
- Shutov A.D., Senyuk V.I., Kakhovskaya I.A., Pineda J. High molecular mass products of hydrolysis of soybean glycinin by trypsin. Biochemistry (Moscow), 1993, 58: 174-182.
- Rudakova A., Rudakov S., Kakhovskaya I., Wilson K., Yagasaki K., Utsumi S., Shutov A. Limited proteolysis controls massive degradation of glycinin, storage 11S globulin from soybean seeds. Materialele simpozionului naţional «Agrobiodiversitatea vegetalǎ în Republica Moldova: evaluatea, conservarea şi utilizarea» . Chişinǎu, 2008: 396-402.
- Макаева Э., Лаптева Н., Рудакова А., Рудаков С., Каховская И., Шутов А. 11S глобулин подсолнечника: кинетика ограниченного и кооперативного протеолиза папаином. . Studia Universitatis Moldaviae, seria Ştiinţe reale şi ale naturii, 2009, 1(21): 24-28 (in Russ).
- Shutov A.D., Rudakova A.S., Klimova N.V., Lapteva N.A., Makaeva E.F., Wilson K. Limited proteolysis of oat 11S globulin by papain. Studia Universitatis Moldaviae, seria Ştiinţe reale şi ale naturii, 2014, 1(71): 85-90.
- Лаптева Н., Макаева Э., Рудаков С., Рудакова А., Каховская И., Шутов А. Смешанный тип протеолиза 11S глобулина кедра папаином. . Studia Universitatis Moldaviae, Ştiinţe reale şi ale naturii, 2010, 6(36): 9-13 (in Russ).
- Rudakova A.S., Rudakov S.V., Kakhovskaya I.A., Shutov A.D. 11S storage globulin from pumpkin seeds: regularities of proteolysis by papain. Biochemistry (Moscow), 2014, 79: 820-825 ( ) DOI: 10.1134/S0006297914080100
- Ivanciuk O., Midoro-Horiuti T., Schein C.H., Xie L., Hillman G.R., Goldblum R.M., Braun W. The property distance index PD predicts peptides that cross-react with IgE antibodies. Mol. Immunol., 2009, 46: 873-883 ( ) DOI: 10.1016/j.molimm.2008.09.004
- Lawrence M.C. Structural relationships of 7S and 11S globulins. In: Seed proteins/P.R. Shewry, R. Casey (eds.). Kluwer Academic Publishers, Dordrecht, 1999: 517-541.
- Ko T.P., Ng J.D., McPherson A. The three-dimensional structure of canavalin from jack bean (Canavalia ensiformis). Plant Physiol., 1993, 101: 729-744 ( ) DOI: 10.1104/pp.101.3.729
- Lawrence M.C., Izard T., Beuchat M., Blagrove R.J., Colman P.M. Structure of phaseolin in 2.2 Å resolution: implications for a common vicilin/legumin structure and the genetic engineering of seed storage proteins. J. Mol. Biol., 1994, 238: 748-776 ( ) DOI: 10.1006/jmbi.1994.1333
- Maruyama N., Adachi M., Takahashi K., Yagasaki A., Kohno M., Takenaka Y., Okuda E., Nakagawa S., Mikami B., Utsumi S. Crystal structures of recombinant and native soybean β-conglycinin β-homotrimers. Eur. J. Biochem., 2001, 268: 3595-3604 ( ) DOI: 10.1046/j.1432-1327.2001.02268.x
- Itoh T., Garcia R.N., Adachi M., Maruyama Y., Tecson-Mendoza E.M., Mikami B., Utsumi S. Structure of 8S alpha globulin, the major seed storage protein of mung bean. Acta Crystallogr. D, 2006, 62: 824-832 ( ) DOI: 10.1107/S090744490601804x
- Fukuda T., Maruyama N., Salleh M.R., Mikami B., Utsumi S. Characterization and crystallography of recombinant 7S globulins of Adzuki bean and structure-function relationships with 7S globulins of various crops. J. Agr. Food Chem., 2008, 56: 4145-4153 ( ) DOI: 10.1021/jf072667b
- Jin T., Wang Y., Chen Y.W., Fu T.J., Kothary M.H., McHugh T.H., Zhang Y. Crystal structure of Korean pine (Pinus koraiensis) 7S seed storage protein with copper ligands. J. Agr. Food Chem., 2014, 62: 222-228 ( ) DOI: 10.1021/jf4039887
- Chruszcz M., Maleki S.J., Majorek K.A., Demas M., Bublin M., Solberg R., Hurlburt B.K., Ruan S., Mattisohn C.P., Breiteneder H., Minor W. Structural and immunologic characterization of Ara h 1, a major peanut allergen. J. Biol. Chem., 2011, 286: 39318-39327 ( ) DOI: 10.1074/jbc.M111.270132
- Cabanos C., Urabe H., Tandang-Silvas M.R., Utsumi S., Mikami B., Maruyama N. Crystal structure of the major peanut allergen Ara h 1. Mol. Immunol., 2011, 49: 115-123 ( ) DOI: 10.1016/j.molimm.2011.08.004
- Cherdivarăă A., Rudakova A., Rudakov S., Shutov A. Proteoliza limitată a alergenului Ara h1, globulina de rezervă 7S din seminţele de arahide . Studia Universitatis Moldaviae, seria Ştiinţe reale şi ale naturii, 2016, 6(96): 10-15 (in Romanian).
- Kawai M., Susuki S., Asano M., Miwa T., Shibai H. Characterization of 30-kDa fragments derived from beta-conglycinin degradation process during germination and seedling growth of soybean. Bioscience, Biothechnology and Biochemistry, 1997, 61: 794-799 ( ) DOI: 10.1271/bbb.61.794
- Seo S., Tan-Wilson A., Wilson K.A. Protease C2, a cysteine endopeptidase involved in the continuing mobilization of soybean β-conglycinin seed proteins. Biochim. Biophys. Acta, 2001, 1545: 192-206 ( ) DOI: 10.1016/S0167-4838(00)00277-6
- Zakharov A., Carchilan M., Stepurina T., Rotari V., Wilson K., Vaintraub I. A comparative study of the role of the major proteinases of germinated common bean (Phaseolus vulgaris L.) and soybean seeds in the degradation of their storage proteins. J. Exp. Bot., 2004, 55: 2241-2249 ( ) DOI: 10.1093/jxb/erh247
- Shutov A.D., Rudakova A.S., Rudakov S.V., Kakhovskaya I.A., Schallau A.A., Wilson K.A., Maruyama N. Degradation of β-conglycinin β-homotrimer by papain: independent occurrence of limited and extensive proteolyses. Bioscience, Biothechnology and Biochemistry, 2013, 77: 2082-2086 ( ) DOI: 10.1271/bbb.130440
- Senyuk V., Rotari V., Becker C., Zakharov A., Horstmann C., Müntz K., Vaintraub I. Does an asparaginyl-specific cysteine endopeptidase trigger phaseolin degradation in cotyledons of kidney bean? Eur. J. Biochem., 1998, 258: 546-558 ( ) DOI: 10.1046/j.1432-1327.1998.2580546.x
- Rotari V.I., Senyuk V.I., Jivotovskaya A.V., Horstmann C., Vaintraub I.A. Proteinase A-like enzyme from germinated kidney bean seeds. Its action on phaseolin and vicilin. Physiol. Plantarum, 1997, 100: 171-177 ( ) DOI: 10.1111/j.1399-3054.1997.tb03469.x
- Rudakova A., Rudakov S., Lapteva N., Morari D., Stepurina T., Rotari V., Kakhovskaya I., Wilson K., Fukuda T., Utsumi S., Shutov A. In vivo and in vitro limited proteolysis of phaseolin: facts, suggestions and problems. Studia Universitatis Moldaviae, seria Ştiinţe reale şi ale naturii, 2007, 1(01): 101-111.
- Jivotovskaya A.V. Senyuk V.I., Rotari V.I., Horstmann C., Vaintraub I.A. Proteolysis of phaseolin in relation to its structure. J. Agr. Food Chem., 1996, 44: 3768-3772 ( ) DOI: 10.1021/jf960129l
- Pele M. Peanut allergens. Romanian Biothechnological Letters, 2010, 15: 5204-5212.
- Wichers H.J., De Beyer T., Savelkoul F.J., van Amerongen A. The major peanut allergen Ara h1 and its cleaved-off N-terminal peptide; possible implications for peanut allergen detection. J. Agr. Food Chem., 2004, 52: 4903-4907 ( ) DOI: 10.1021/jf049697o
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem. Bioph. Res. Co., 1967, 27(2): 157-162 ( ) DOI: 10.1016/S0006-291X(67)80055-X


