Определение глутатиона и его окислительно-восстановительного статуса в изолированных вакуолях красных свекольных клеток
Автор: Прадедова Е.В., Нимаева О.Д., Путилина Т.Е., Семенова Н.В., Собенин А.М., Саляев Р.К.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.12, 2016 года.
Бесплатный доступ
Глутатион вакуолей красной свеклы (Beta vulgaris L.) измеряли с использованием трех хорошо известных методов: спектрофлуориметрический метод с ортофталевым альдегидом (OPT); спектрофотометрический метод с 5,5'-дитиобис-2-нитробензойной кислотой (DTNB); высокоэффективная жидкостная хроматография (ВЭЖХ). Содержание восстановленного (GSH) и окисленного глутатиона (GSSG) различалось в зависимости от метода исследования. При ОПТ концентрация глутатиона составляла: GSH - 0,05 мкмоль / мг белка; GSSG - 0,019 мкмоль / мг белка и общего глутатиона (GSHtotal) - 0,097 мкмоль / мг белка. В случае определения с DTNB концентрация глутатиона составляла: GSH - 0,091 мкмоль / мг белка; GSSG - 0,031 мкмоль / мг белка; GSHtotal - 0,153 мкмоль / мг белка. Концентрация глутатиона, определяемая по ВЭЖХ, была ниже: GSH - 0,039 мкмоль / мг белка; GSSG - 0,007 мкмоль / мг белка; GSHtotal - 0,053 мкмоль / мг белка. Коэффициент редокс GSH / GSSG также зависит от метода определения: с ОПТ - 3.11; с DTNB - 2,96 и ВЭЖХ - 5,57. Редокс-отношение глутатиона в вакуолях было намного ниже, чем тканевые экстракты красной свеклы, которые в зависимости от метода определения были: 7.23, 7.16 и 9.22. Результаты показали, что вакуоли клеток паренхимы красной свеклы содержат глутатион. Несмотря на низкое значение окислительно-восстановительного отношения GSH / GSSG, в вакуолях пул уменьшенного глутатиона преобладал над пулом окисленного глутатиона.
Короткий адрес: https://sciup.org/14323988
IDR: 14323988
Текст научной статьи Определение глутатиона и его окислительно-восстановительного статуса в изолированных вакуолях красных свекольных клеток
Редокс-система глутатиона вызывает большой интерес и всесторонне изучается не только у человека и животных, но и растений. Изменение активности этой системы служит критерием отклонения от физиологической нормы при патологических состояниях (Arrigo,1999; Forman et al., 2008). Количество глутатиона и его редокс-состояние являются показателями устойчивости организма к действию срессирующих факторов биотической и абиотической природы (Agrawal et al., 1992; Dixon et al., 1998). Однако органы и ткани многоклеточных организмов различаются содержанием глутатиона. Согласно данным литературы, в растениях основной пул глутатиона сосредоточен в фотосинтезирующих и запасающих органах (Noctor et al., 2011). В самих клетках глутатион тоже распределен неравномерно по внутриклеточным структурам. В клетках растений относительно большие концентрации глутатиона выявлены в цитозоле, хлоропластах и митохондриях. Известно, что в растительной клетке запасающую функцию выполняет центральная вакуоль. Можно было бы ожидать накопление глутатиона в этом компартменте, но его присутствие в вакуоли до сих пор вызывает сомнение и, по мнению некоторых исследователей, является тканеспецифичным или видоспецифичным (Noctor et al., 2011). Однако отмечено, что на компартментацию глутатиона в клетках растений заметное влияние оказывают факторы среды. В некоторых случаях в стрессовых условиях глутатион накапливался в вакуолях, что приводило к многократному повышению его концентрации (Queval et al., 2011). На сегодняшний день установлен избирательный транспорт глутатиона в вакуоль посредством ABC-транспортеров тонопласта, которые преимущественно транспортируют окисленный глутатон (GSSG). Этот факт позволил предположить, что центральная вакуоль вносит существенный вклад в поддержание высокого редокс-соотношения глутатиона (GSH/GSSG) в цитозоле (Coleman et al., 1997; Dixon et al., 1998 Zechmann, Müller, 2010; Noctor et al., 2011).
Несмотря на многочисленные исследования функций глутатиона, его компартментация в растительных клетках изучена недостаточно. Мало информации о пуле вакуолярного глутатиона. Более полное представление о содержании и редокс-статусе глутатиона в вакуолях можно получить при изучении разных тканей у растений, находящихся в различных условиях существования. Целью настоящего исследования явилось определение глутатиона в вакуолях клеток покоящихся корнеплодов столовой свеклы (Beta vulgaris L.). Основная ткань корнеплода – запасающая паренхима, в клетках которой вакуоль занимает 90% клеточного объема. В период физиологического покоя корнеплод подвержен действию низких температур и других стрессирующих факторов. Особенности исследуемого объекта, а также литературные данные, указывающие на аккумуляцию глутатиона в запасающих тканях и компартментацию в вакуолях при стрессе, послужили основанием для предположения, что в вакуолях корнеплода может содержаться глутатион в относительно высоких концентрациях. С помощью трех различных методов (спектрофлуориметрического, спектрофотометрического и хроматографического) была предпринята попытка определить во фракциях изолированных вакуолей содержание глутатиона и соотношение его восстановленного (GSH) и окисленного (GSSG) пулов.
MATERIALS AND METHODS
Выделение вакуолей
Корнеплоды столовой свеклы ( Beta vulgaris L.) хранили при 4°С. Из тканей корнеплодов выделяли вакуоли с помощью модифицированного макрообъемного метода (Salyaev et al ., 1981). Фракцию изолированных вакуолей дополнительно очищали от примесей органелл (пластид, ядер) и клеточных стенок в ступенчатом градиенте плотности сахарозы-KCl (1.050–1.080–1.145–1.180 г/см3). Растворы разной удельной плотности для градиента готовили, смешивая два матричных раствора: 1 М КCl и 1.8 М сахароза (20 мМ трис-HCl, рН 7.4) (Salyaev et al ., 1981). Градиент центрифугировали в течение 20 мин при 125 g. Фракцию вакуолей отделяли и наблюдали с помощью светового микроскопа NU-2Е («Car Zeiss», Германия). Чистоту вакуолярных фракций контролировали биохимическими методами: определяли ДНК (маркер ядер, пластид и митохондрий) и активность маркерных ферментов (NADP- и NAD-малатдегидрогеназы и др.) (Edwards et al. , 1985; Levites, 1986).
Стандартная операция экстракции глутатиона из анализируемого образца
Проводили «кислотную экстракцию». Для этого фракции изолированных вакуолей шокировали в охлажденном растворе для экстракции (5% метафосфорная кислота, 1 мМ EDTA, 0.1% муравьиная кислота), в соотношении 1 : 2 (объем органелл : объем раствора).
В тканях корнеплодов также определяли глутатион. Ткань гомогенизировали в этом же растворе в соотношении 1 : 5 (масса : объем) (Rellan-Alvarez et al., 2006). Экстракты центрифугировали при 13500 g в течение 15 мин при 4°С.
Нестандартная операция экстракции глутатиона из анализируемого образца
Фракции изолированных вакуолей в соотношении 1 : 1 (объем органелл : объем раствора) шокировали в охлажденном реакционном растворе: 100 мМ Na-фосфатный буфера (рН 8.3), 5 мМ EDTA, 0.3 мМ NADPH, 0.02 МЕ глутатионредуктазы пекарских дрожжей (GR). Ткань корнеплода свеклы гомогенизировали в этом же реакционном растворе в соотношении 1 : 1 (масса ткани : объем раствора). В присутствии NADPH и GR в вакуолях и гомогенате восстанавливался окисленный глутатион (GSSG) до восстановленной формы (GSH). Для эффективного восстановления образцы инкубировали 15 мин. Затем проводили «кислотную экстракцию», как описано выше.
Спектрофлуориметрический метод определения глутатиона
Метод основан на взаимодействии о-фталевого альдегида (OPT) с SH-группой GSH, в результате формируется флуоресцентный конъюгат (Hissin et al., 1976; Agrawal et al., 1992). Определенное количество экстракта, помещали в реакционный раствор: 100 мМ Na-фосфатный буфер (рН 8.0), 5 мМ EDTA, 4 мкг/мл OPT. После 15-мин инкубации при комнатной температуре проводили измерения на спектрофлуориметре «RF-5301 PC» («Shimadzu», Япония) при длине волны экстинкции 350 нм и эмиссии 420 нм. Сульфгидрильные группы GSH экранировали с помощью тиолового реагента N-этилмалеимида (NEM), который добавляли к экстракту в конечной концентрации 2 мМ (Hissin et al., 1976).
Для определения общего или суммарного глутатиона (GSH + GSSG) к образцам добавляли аликвоту раствора 100-мМ Na-фосфатного буфера (рН 8.0) с 2 мM дитиотрейтола (DTT) или 2-меркаптоэтанола (ME). Эта процедура позволяла восстановить дисульфидные группы GSSG (Agrawal et al ., 1992). Затем проводили «кислотную экстракцию», как описано выше, и последующее измерение GSH с OPT.
Учитывая разведение, рассчитывали концентрацию глутатиона по калибровочной кривой, построенной для стандартных концентраций химически чистого GSH.
Исследование взаимодействия аминокислот с о-фталевым альдегидом
Готовили образцы на основе 100-мМ Na-фосфатного буфера (рН 8.0). Они содержали 100 мкМ химически чистых аминокислот: цистин глутаминовая кислота; глицин и гистидин. В эти образцы добавляли 2 мМ DTT, ME или NEM, инкубировали 15 мин, затем вносили 4 мкг/мл OPT и проводили измерение, как описано выше. Полученные результаты выражали в условных единицах, которые представляли отношение величин оптической плотности к молярной концентрации исследуемой аминокислоты.
Определение аминокислот
В образцах вакуолей и тканевого экстракта определяли содержание аминокислот. Измерение проводили согласно протоколу на автоматическом анализаторе аминокислот ААА 339 (Чехия).
Спектрофотометрический метод определения глутатиона
Метод основан на взаимодействии 5.5'-дитиобис-2-нитробензойной кислоты (DTNB) с SH-группой GSH (Tietze, 1969). В результате реакции образуется хромофор 5-тио-2-нитробензойная кислота (TNB) с максимумом оптической плотности при 412 нм (Anderson, 1985). Для определения содержания суммарного глутатиона (GSHсум) использовали реакционную среду, содержащую 100 мМ Na-фосфатный буфер (рН 8.3 или 9.2), 5 мМ EDTA, 6 мМ DTNB, 0.3 мМ NADPH, в которую вносили образец. Реакцию запускали, добавляя 0.02 МЕ GR дрожжей, и после 3-мин инкубации регистрировали образование TNB на спектрофотометре «SPECORD S100» («Analytik Jena», Германия) при длине волны 412 нм или планшетном фотометре «ImmunoChem-2100» («High Technology», США) при длине волны 405 нм (Smith et al., 1984). Для предотвращения специфичного взаимодействия DTNB с SH- группами GSH в образцы добавляли тиоловые реагенты NEM (2 мМ) или 2-винилпиридин (2%). В том случае, когда определяли GSSG, в образцы добавляли 2-винилпиридин (2%), затем проводили такое же измерение, как для GSHсум (Smith et al., 1984). Концентрацию глутатиона рассчитывали с помощью калибровочной кривой, построенной для стандартных концентраций химически чистого GSH или с помощью коэффициента экстинкции для GSH (ε412=13.6 мМ-1см-1) (Ellman, 1959).
Метод высокоэффективной жидкостной хроматографии
После «кислотной экстракции» проводили пробоподготовку методом твердофазной экстракции на картриджах Sep-Pak С18 (Rellán-Álvarez et al., 2006). Предварительно картриджи промывали концентрированным метанолом (5 мл), затем 0.5% муравьиной кислотой (5 мл). После подготовки на картриджи наносили образцы. Затем картриджи промывали деионизованной водой, подкисленной муравьиной кислотой, и проводили элюирование раствором, содержащим 5-% метанол и 0.5-% муравьиную кислоту. Элюат выпаривали на роторном испарителе «IKA RV 10 basic» («WENK LabTec GmbH», Германия), полученный экстракт растворяли в заданном объеме 50% метанола. Измерения проводили на жидкостном микроколоночном хроматографе «Милихром А-02» (Россия): колонка 2х75 мм сорбент ProntoSil 120-5-С18 AQ-0838 (обращено фазный); элюент А – 0,2 М LiClO4 и 0,005 М HClO4 в H2O; элюент Б – CH3CN; T=40ºС; τ=0,34; λ=210 нм, υ=100 мкл/мин; градиентный режим хроматографирования – градиент 10 мин от 5 до 100% CH3CN. Учитывая разведение, рассчитывали концентрацию глутатиона по калибровочной кривой, построенной для стандартных концентраций химически чистых GSH и GSSG.
Условные единицы, в которых выражали концентрацию глутатиона
Содержание глутатиона в образцах выражали в мкмолях или нмолях GSH на мг белка. Последний определяли по методу Bredford (1976) или методу Shafner с соавт. (1973).
Используемые реактивы
Хлористый калий, сахароза, натрий фосфорнокислый двузамещенный, метанол, этанол, соляная и муравьиная кислоты были отечественного производства, квалификации х.ч. Остальные перечисленные выше реактивы также соответствовали квалификации х.ч. и были произведены фирмой «Sigma» (США).
Статистический анализ
Все эксперименты выполняли в 4-5 биологических повторностях, каждая из которых была представлена тремя аналитическими. В таблицах и графиках приведены средние арифметические значения и их стандартные отклонения. Достоверность различий оценивали по критерию Стьюдента.
RESULTS
В вакуолях, изолированных из клеток корнеплодов столовой свеклы, определяли глутатион с помощью трех широко используемых методов. Содержание и редокс-состояние вакуолярного глутатиона сравнивали с содержанием и редокс-состоянием глутатиона тканевого экстракта корнеплода.
Определение с помощью спектрофлуориметрического метода с о-фталевым альдегидом
На растворах химически чистого глутатиона проводили апробирование метода с OPT. Для этого готовили 1-мМ растворы GSH и GSSG (Рис.1). Специфичность взаимодействия OPT с SH-группой проверяли с помощью тиолового реагента – NEM (Pastore et al. , 2003; Forman et al ., 2008; Ranawat, Bansal, 2009). NEM, образующий устойчивую связь с SH-группой, препятствует взаимодействию OPT с GSH (Рис. 1).
Для восстановления SH-групп, а в нашем случае сульфгидрильных групп дисульфидной формы GSSG, при анализе с OPT некоторые авторы рекомендуют применять ME или DTT (Agrawal et al ., 1992). В результате восстановления этими соединениями 1 мМ GSSG следует ожидать 2 мМ GSH, так как из одной молекулы GSSG образуется две молекулы GSH. ME или DTT в конечной концентрации 2 мМ добавляли к образцам 1-мМ GSSG. Результаты показали, что оба соединения восстанавливали GSSG, но более эффективным был DTT. В его присутствии в растворах 1-мМ GSSG в среднем определяли 1.8 мМ GSH, что несколько отличалось от ожидаемой концентрации – 2 мМ (Рис. 1).
Убедившись в специфичности метода с OPT и верности расчетов, сделанных на основе калибровочной кривой, построенной для химически чистого GSH, мы провели исследование на изолированных вакуолях и экстрактах ткани. В вакуолях был выявлен GSH. Его концентрация незначительно отличалась от концентрации глутатиона тканевого экстракта (Рис. 2А). Если пробы прединкубировали с NEM, то вопреки ожиданию, протекала слабо выраженная реакция, которую не наблюдали в случае с растворами химически чистого GSH. По всей видимости, OPT неспецифично взаимодействовал с другими соединениями экстракта. С учетом этого эффекта в исследуемых образцах были определены концентрации GSH, которые представлены в таблице 1.
Когда образцы прединкубировали с DTT или ME, согласно процедуре, предложенной Agrawal с соавт. (1992), количество GSH возрастало на два порядка. Все указывало на неспецифичную реакцию используемых восстановителей и OPT с неустановленными соединениями экстрактов (Табл.1). Подобный эффект ранее описывали и другие исследователи (Scaduto, 1988).
Известно, что с помощью OPT определяют некоторые аминокислоты: глицин; триптофан и гистидин. Однако для визуализации реакций аминокислот с OPT требуются определенные условия, отличные от условий наших экспериментов (Dawson et al ., 1986). Тем не менее, мы исследовали аминокислотный состав в вакуолях клеток корнеплодов столовой свеклы и выявили разнообразные свободные аминокислоты, в том числе входящие в состав глутатиона глицин, глутамат и цистеин (находился в окисленном состоянии в виде цистина), а также гистидин
(Табл. 2). Предстояло установить, могут ли перечисленные свободные аминокислоты взаимодействовать с OPT в условиях нашего эксперимента. Для этого готовили 100-мкМ растворы цистина, глицина, глутамата и гистидина. В эти растворы вносили OPT и испытуемые соединения. В отсутствие восстанавливающих агентов в растворах свободных аминокислот наблюдали слабую флуоресценцию (Табл. 3). Однако с DTT и особенно с ME флуоресценция резко увеличивалась, что говорило о выраженных неспецифичных взаимодействиях между испытуемыми соединениями. Возможно, интенсивная флуоресценция, наблюдаемая в присутствии DTT и ME в образцах вакуолей и тканевого экстракта, была вызвана взаимодействием ряда аминокислот с тиоловыми реагентами и OPT (Табл. 1). Несмотря на способность ME и DTT восстанавливать химически чистый GSSG в стандартных образцах, для определения количества GSSG в растительных образцах, как показал наш опыт, эти соединения применять не следует.
Другой распространенный подход для определения GSSG основан на его восстановлении до двух молекул GSH с помощью фермента глутатионредуктазы (GR) (Noctor et al., 2011). Согласно методическому подходу Agrawal с соавт. (1992) в одни образцы сначала добавляли аликвоту раствора, содержащего фосфатный буфер, GR пекарских дрожжей и NADPH, затем после непродолжительной инкубации при комнатной температуре проводили «кислотную экстракцию» глутатиона. В другие образцы добавляли аликвоту охлажденного раствора фосфатного буфера без GR и NADPH, и сразу подвергали «кислотной экстракции». Концентрацию глутатиона рассчитывали, учитывая разведение. Как показали результаты, количество GSH в образцах с GR заметно возрастало (Рис. 3). Оно представляло суммарное содержание глутатиона:
GSH сум = GSH + 2GSH, (1)
GSSG = (GSH сум – GSH)/2. (2)
где: GSH сум – общее содержание GSH и GSSG; GSH – восстановленный глутатион; 2GSH – две молекулы восстановленного глутатиона, образованные при восстановлении GR одной молекулы GSSG.
Концентрацию окисленного глутатиона рассчитывали по формуле 2. В образцах с тиоловым реагентом NEM так же, как и в предыдущих экспериментах отмечали неспецифичную реакцию, которую учитывали при расчетах.
После определения концентраций GSH, GSSG и GSH сум , можно было установить редокс-соотношение восстановленного и окисленного пулов глутатиона (GSH/GSSG) и восстановленного и суммарного пулов глутатиона (GSH/GSH сум ) (Agrawal et al ., 1992; Noctor et al ., 2011). В результате у вакуолей соотношения GSH/GSSG и GSH/GSHсум, характеризующие редокс-статус глутатиона, оказались заметно ниже, чем у тканевых экстрактов. Это было обусловлено высоким содержанием GSSG в вакуолях (Табл. 1).
Определение глутатиона с помощью спектрофотометрического метода с реактивом Эллмана
На сегодняшний день метод с реактивом Эллмана является одним из наиболее используемых подходов для выявления глутатиона, несмотря на то, что он так же, как и метод с OPT, дает приблизительную оценку содержания глутатиона (Bielavski, Joy, 1986; Forman et al. , 2008). Этот метод основан на реакции GSH с DTNB, которую называют реактивом Эллмана. Следует отметить, что DTNB специфично связывается с SH-группами всех соединений, поэтому ее можно использовать для измерения как небелковых, так и белковых тиолов (Owens, Belcher, 1965).
Согласно методическому протоколу в «кислых экстрактах», из которых удалены белки, с помощью DTNB определяют суммарные небелковые тиолы. В ходе реакции DTNB взаимодействует с GSH, образуется конъюгат и анион 5-тио-2-нитробензойной кислоты (TNB). Предполагают следующую последовательность реакций (Pastore et al ., 2003):
GSH + ArSSAr → GSSAr + ArSH (1)
ArSSG + GSH → GSSG + ArSH (2)
где: GSH – восстановленный глутатион; GSSG – окисленный глутатион; ArSSAr – DTNB; GSSAR – конъюгат GSH и красителя; ArSH – TNB.
Таким образом, модификация одной сульфгидрильной группы сопровождается освобождением одного аниона TNB (Owens, Belcher, 1965; Pastore et al ., 2003).
Результаты экспериментов, проводимых с химически чистым GSH, подтвердили высокую специфичность метода с DTNB, а также корректность наших расчетов (Рис. 4). Был отмечен стабилизирующий эффект на химически чистый GSH раствора MPA. Концентрация GSH в растворе MPA была несколько выше, чем в буферном растворе, что соотносилось с ранее полученными данными, согласно которым глутатион более стабилен в кислых условиях (Pastore et al., 2003). Для экранирования SH-групп GSH использовали NEM. С NEM реакция практически не развивалась. Однако так же, как и в случае с OPT, происходило неспецифичное взаимодействие, которое в среднем составляло 5% от общей реакции без тиолового реагента (Рис. 4).
Для расчета концентрации GSH в образцах можно использовать как калибровочные кривые, так и коэффициент экстинкции для TNB (Ellman, 1959). Мы применили оба подхода при определении глутатиона в вакуолях и экстрактах ткани. Величины концентраций глутатиона в исследуемых образцах, рассчитываемые двумя способами, практически не различались (Рис. 5). В присутствии тиоловых реагентов NEM и 2-винилпиридина развивалась слабая неспецифичная реакция. С NEM она была выше, чем с 2-винилпиридином. В дальнейшем для экранирования SH-групп применяли только 2-винилпиридин, в отличие от NEM, это соединение давало низкую неспецифичную реакцию и не подавляло активность GR.
Учитывая неспецифичное взаимодействие, получили величины концентраций, которые представлены в таблице 4. Результаты показали довольно высокое содержание GSH в вакуолях, которое всего лишь в 2 раза было ниже содержания глутатиона в тканевом экстракте. Количество GSSG также было относительно высоким (Табл. 4, Метод I). В целом концентрация глутатиона, определяемая с DTNB, оказалось выше, чем определяемая с OPT. Ранее при сравнении этих методов отмечали завышенные величины концентраций с OPT и объясняли этот эффект более низкой специфичностью альдегида (Scaduto, 1988). В нашем исследовании были получены противоположные результаты.
Существенный недостаток методов с DTNB и OPT, состоит в том, что они не позволяют без дополнительных процедур выявлять GSSG. Согласно распространенному подходу после «кислотной экстракции» с помощью рециркулирующего анализа следует:
-
I. Определить суммарный восстановленный глутатион
GSH + GSSG + NADPH 2 → 3GSH + NADP (1) 3GSH + 3ArSSAr → 3GSSAR + 3ArSH, (2) 3ArSSG + 3GSH → 3GSSG + 3ArSH, (3)
-
II. Определить окисленный глутатион, после экранирования SH-групп GSH 2-винилпиридином GSSG + NADPH 2 → 2GSH + NADP, (4) GSH + ArSSAr → GSSAR + ArSH, (5) ArSSG + GSH → GSSG + ArSH, (6)
где: GSH – восстановленный глутатион; GSSG – окисленный глутатион; ArSSAr – DTNB; ArSH – TNB; ArSSG – конъюгат GSH и TNB.
Весь метод включает следующие этапы. После «кислотной экстракции» с MPA, которая позволяет избавиться от белков, а кислые условия препятствуют спонтанному окислению GSH во время пробоподготовки, проводится определение GSHсум с помощью рециклирующего анализа с GR и NADPH (формулы 1-3) (Pastore et al., 2003). Далее выполняется еще одна процедура, позволяющая определить GSSG, в ходе которой с помощью тиолового реагента (NEM или 2-винилпиридина) устраняется GSH (Forman et al., 2008). Затем осуществляется каталитическое восстановление GSSG до 2GSH с помощью GR и NADPH (формулы 4-6). Таким образом, в образцах определяются GSHсум и GSSG, а содержание тиольной формы GSH можно установить, если вычесть концентрацию GSSG из концентрации GSHсум. Следует отметить, что рециклирующий метод не отличается высокой точностью из-за возможности цикличных реакций, если в среде избыточное количество GR и NADPH находятся длительное время (Pastore et al., 2003):
GSSG + NADPH 2 → 2GSH + NADP, (4) GSH + ArSSAr → ArSSG + ArSH, (5) ArSSG + GSH → GSSG + ArSH, (6) GSSG + NADPH 2 → 2 GSH + NADP, (7) 2 GSH + 2ArSSG → 2GSH + 2ArSH. (8)
где: GSH – восстановленный глутатион; GSSG – окисленный глутатион; ArSSAr – DTNB; ArSH – TNB; ArSSG – конъюгат GSH и TNB.
Формулы 7 и 8 отражают повторное восстановление GSSG и взаимодействие с DTNB образовавшихся молекул GSH, которые уже вступали в реакцию с молекулами красителя. При наличии в среде избыточного количества NADPH и DTNB происходит циклическое восстановление GSSG и образование большого количества TNB, которое не соответствует реальному содержанию GSH. Поэтому, согласно методическому протоколу, строго лимитируется время инкубации, оно не должно превышает 5 мин (Pastore et al., 2003). В связи с этим считают, что анализ с DTNB дает довольно приблизительную оценку содержания GSSG (Bielavski, Joy, 1986; Forman et al., 2008). Действительно, концентрация GSSG, выявляемая этим методом, оказалась довольно высокой, поэтому, как и в случае с OPT-методом, при определении с DTNB применили другой подход. Вышеописанный способ обозначили как «Метод I», а другой подход, о котором речь пойдет ниже, обозначили как «Метод II». Если по «Методу I» инкубацию образцов с GR и NADPH проводили после «кислотной экстракции», то по «Методу II» – до «кислотной экстракции», так же как это было описано для метода с OPT. Сначала одну часть исследуемого образца инкубировали в среде с GR и NADPH (Wang, 1995), а затем с помощью MPA удаляли белок и вносимую GR. Далее в полученном экстракте определяли GSHсум. Одновременно другую часть анализируемого образца сразу подвергали «кислотной экстракции». На стандартных образцах химически чистого GSH была показана высокая стабильность сульфгидрильной формы глутатиона в кислых растворах MPA (Рис. 4). В полученных экстрактах определяли содержание GSH. Концентрацию GSSG в этом случае рассчитывали, вычитая количество GSH из количества GSHсум (Табл. 4, Метод II). В результате содержание GSSG оказалось заметно ниже, чем при определении
«Методом I». «Метод II» позволил избежать этап дериватизации GSH с 2-винилпиридином, а также циклическое восстановление GSSG ферментом GR, наблюдаемое при рециклирующем анализе «Метода I». Снижение величин GSSG привело к снижению показателей редокс-состояния глутатиона. Тем не менее, при сопоставлении данных, полученных разными способами, была отмечена одна и та же закономерность. В тканевом экстракте концентрации GSH сум и GSH, а также показатели редокс-состояния глутатиона, были выше, чем во фракциях изолированных вакуолей.
Определение глутатиона с помощью хроматографического метода
Высокоэффективная жидкостная хроматография (HPLC) считается более чувствительным и специфичным способом определения веществ. Обратно-фазовая HPLC с УФ-детекцией позволяет определять глутатион без предварительной дериватизации и не требует восстановления GSSG (Vignaud et al ., 2004) (Рис. 6).
Анализ проводили согласно методу, детали которого описали Rellán-Álvarez и соавт. (2006). Содержание GSH и GSSG рассчитывали по калибровочным кривым, построенным для химически чистого глутатиона. Концентрацию GSH сум определяли, суммируя концентрации GSH и 2GSSG. Полученные с помощью этого метода величины концентраций GSH и GSH сум в вакуолях и тканевом экстракте оказались заметно ниже, чем величины концентраций, установленные методами с OPT и DTNB (Табл. 1, 4 и 5). Однако содержание
GSSG в вакуолях также было относительно высоким, что повлияло на показатели редокс-состояния глутатиона, которые были относительно
GSH/GSSG заметно варьировало, однако для вакуолей оно во всех случаях было намного ниже (3.11; 2.96; 6.94; 5.57), чем для экстрактов ткани низкими (Табл. 5)
(7.23; 7.16; 13.88; 9.22). Соотношение GSH/GSH сум
Таким образом, с помощью методов спектрофлуориметрии, спектрофотометрии и HPLC в вакуолях выявили глутатион. Концентрация вакуолярного глутатиона была лишь в 1.5-2.5 раза ниже концентрации GSH тканевого экстракта. При у вакуолей также было ниже, чем у тканевого экстракта, и составляло: для вакуолей – 0.61; 0.59 0.78; 0.74; для экстрактов ткани – 0.78; 0.78; 0.87 0.82 (Табл. 1, 4, 5). Несмотря на то, что в вакуолях пул окисленного глутатиона был относительно анализе разными способами соотношение высоким, пул восстановленного глутатиона
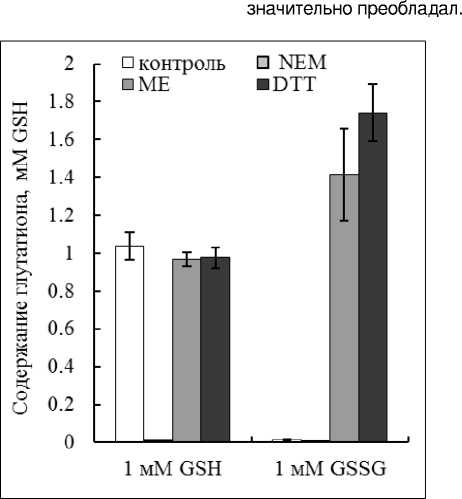
Figure 1. Исследование эффективности метода с о -фталевым альдегидом (OPT), проводимое с химически чистым глутатионом (Sigma). Готовили 1-мМ растворы GSH и GSSG. Для
|
Образец |
Содержание глутатиона, мкмоль/мг белка |
||||||
|
GSH |
GSSG |
GSH cум |
GSH/GSSG |
GSH/GSH cум |
GSH с ME |
GSH с DTT |
|
|
Вакуоли |
0.059 ±0.01 |
0.019± 0.003 |
0.097± 0.02 |
3.11 |
0.61 |
9.86±1.28 |
5.46±0.89 |
|
Экстракт ткани |
0.094 ±0.01 |
0.013± 0.002 |
0.120± 0.01 |
7.23 |
0.78 |
4.91±0.69 |
4.66±0.54 |
Где: GSH сум – суммарное содержание глутатиона, равное GSH + 2GSSG ; GSH с ME – проба с 2 мМ 2-меркаптоэтанола; GSH с DTT – проба с 2 мМ дитиотрейтола. Уровень значимости p < 0.05
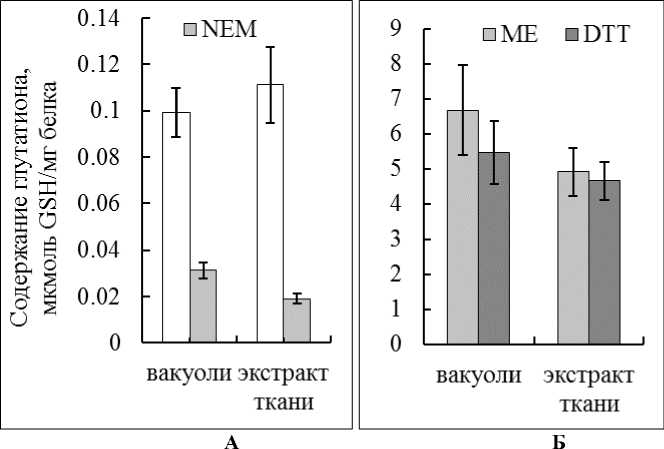
Figure 2. Содержание GSH в образцах изолированных вакуолей и тканевого экстракта, определяемое с OPT: А – без восстановителей SH-групп; Б – с 2 мМ 2-меркаптоэтанола (ME) или 2 мМ дитиотрейтола (DTT). Специфичность реакции определяли с тиоловым реагентом N-этилмалеимидом (NEM) (2 мМ).
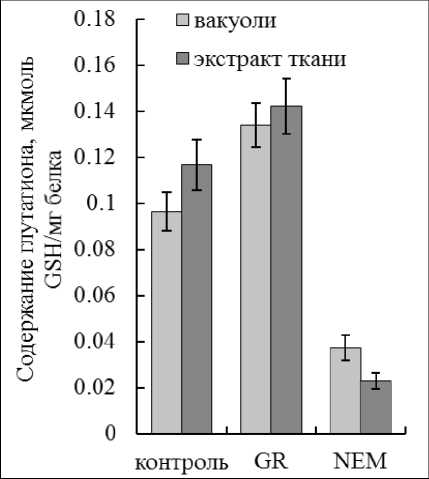
Figure 3. Определение суммарного глутатион с OPT. Контроль – пробы не инкубировали с глутатионредуктазой дрожжей; GR – пробы прединкубировали с глутатионредуктазой дрожжей в присутствии NADPH; NEM – пробы прединкубировали с NEM.
Table 2. Содержание аминокислот в анализируемых образцах
|
образец |
цистин, мкМ |
глицин, мкМ |
глутаминовая кислота, мкМ |
гистидин, мкМ |
|
Вакуоли |
0.768 |
0.819 |
4.155 |
0.387 |
|
экстракт ткани |
0.504 |
0.213 |
3.639 |
0.381 |
Table 3. Взаимодействие аминокислот с OPT в условиях проводимого эксперимента
|
Образец с вносимым реагентом |
цистин, у.е. |
глицин, у.е. |
глутаминовая кислота, у.е. |
Гистидин, у.е. |
|
контроль |
0.755 |
0.381 |
0.846 |
0.373 |
|
NEM |
0.224 |
0.228 |
0.253 |
0.297 |
|
ME |
9.135 |
13.434 |
29.807 |
12.862 |
|
ME + NEM |
8.948 |
3.082 |
19.199 |
2.471 |
|
DTT |
4.726 |
3.027 |
9.025 |
3.844 |
|
DTT + NEM |
0.422 |
0.887 |
1.092 |
1.946 |
Для проведения эксперимента брали: аминокислоты – 100 мкМ; NEM, ME, DTT – 2 мМ. Взаимодействие аминокислоты с о-фталевым альдегидом выражали в удельных единицах (у.е.), представляющих отношение оптической плотности и молярной концентрации исследуемой аминокислоты. Уровень значимости p < 0.001.
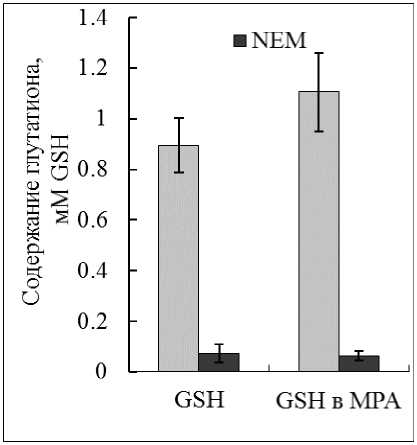
Figure 4. Оценка специфичности метода c DTNB. Готовили 1-мМ раствор химически чистого глутатиона в натрий-фосфатном буфере (GSH) или 5-% метафосфорной кислоте
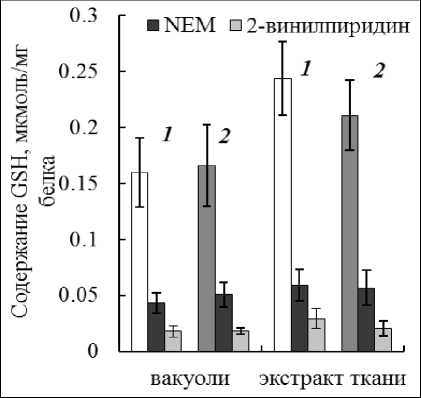
Figure 5. Содержание GSH сум в вакуолях и экстракте ткани: (1) – рассчитанное по калибровочной кривой; (2) – с учетом коэффициента экстинкции для TNB. Реагенты, связывающие SH-группы NEM и 2-винилпиридин.
Table 4 . Содержание глутатиона в образцах, установленное с помощью метода с DTNB
|
образец |
Содержание глутатиона, мкмоль/мг белка |
|||||||||
|
Метод I инкубация с GR после «кислотной экстракции» |
Метод II прединкубация с GR до «кислотной экстракции» |
|||||||||
|
GSH |
GSSG |
GSH cум |
GSH/ GSSG |
GSH/ GSH сум |
GSH |
GSSG |
GSH cум |
GSH/ GSSG |
GSH/ GSH сум |
|
|
Вакуоли |
0.091 ±0.013 |
0.031 ±0.007 |
0.153 ±0.027 |
2.94 |
0.59 |
0.118 ±0.023 |
0.017 ±0.003 |
0.152 ±0.028 |
6.94 |
0.78 |
|
Экстракт ткани |
0.179 ±0.012 |
0.025 ±0.009 |
0.229 ±0.031 |
7.16 |
0.78 |
0.222 ±0.028 |
0.016 ±0.003 |
0.254 ±0.034 |
13.88 |
0.87 |
Где: GSH cум – суммарное содержание глутатиона, равное GSH+2GSSG. Уровень значимости p < 0.05
Table 5 . Содержание глутатиона в образцах, установленное с помощью метода HPLC
|
Образец |
Концентрация глутатиона, мкмоль /мг белка |
||||
|
GSH |
GSSG |
GSHcум |
GSH/GSSG |
GSH/ GSH cум |
|
|
Вакуоли |
0.039 ±0.009 |
0.007 ±0.001 |
0.053 ±0.011 |
5.57 |
0.74 |
|
Экстракт ткани |
0.083 ±0.003 |
0.009 ±0.002 |
0.101 ±0.007 |
9.22 |
0.82 |
Статистически достоверны при p < 0.001
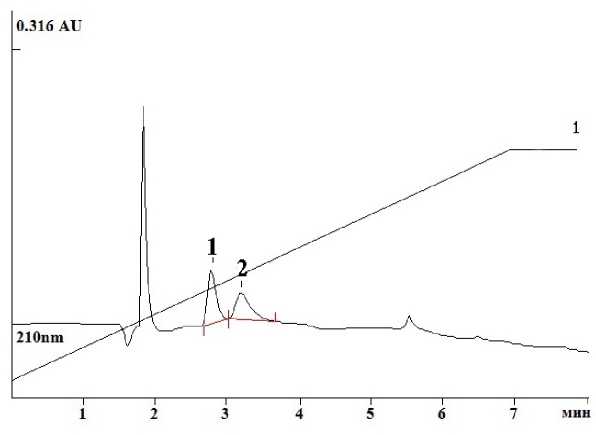
Figure 6. Определение глутатиона методом HPLC с УФ-детекцией. Стандартные растворы GSH (1) и GSSG (2) .
DISCUSSION
Содержание глутатиона в растении в значительной степени зависит от вида растения, типа исследуемой ткани, стадии развития, условий произрастания и отчасти от метода анализа (Noctor et al., 2011). На сегодня разработаны разные методы для определения глутатиона, в их числе количественные методы для измерения в условиях in vitro на основе спектрофлуориметрического, спектрофотометрического анализа и HPLC, а также методы для измерения в условиях in situ на основе лазерной (конфокальной или двухфотонной) сканирующей микроскопии. Однако ни один из этих методов не предоставляет исчерпывающей информации (Noctor et al., 2011). Дополнительные трудности при сравнении и обобщении результатов создают единицы, в которых выражаются относительные концентрации глутатиона. В связи с этим значительное варьирование полученных данных для одного и того же объекта обусловлено не только ограничениями используемого метода, но и условными единицами, когда концентрацию глутатиона выражают на грамм сырого веса или миллграмм белка, или на отдельную клетку либо целое растение. Поэтому у разных видов или разных органов одного вида растений были определены как десятки наномолей GSH на грамм сырого веса или миллиграмм белка, так и миллимоли на отдельную клетку (Zaharieva, Abadia, 2003).
Согласно данным литературы, в корнеплодах Beta vulgaris (сахарной свеклы) содержание глутатиона, определяемое спектрофотометрическим методом с DTNB, составило: для GSH – 28.7-46.1 нмоль/г сырого веса; для GSSG – 8.8-9.2 нмоль/г сырого веса (Zaharieva, Abadia, 2003). А при определении глутатиона в том же объекте методом HPLC/масс-спектрометрии концентрация глутатиона оказалась заметно выше и составила: для GSH – 92.1 нмоль/г сырого веса; для GSSG – 46.1 нмоль/г сырого веса (Rellán-Álvarez et al., 2006б). Для более объективного сравнения наших данных с данными этих исследований концентрацию глутатиона в экстрактах ткани корнеплодов столовой свеклы пересчитали в нмоль/г сырого веса. Результаты, полученные с помощью метода с DTNB, показали, что в корнеплодах столовой свеклы концентрация GSH соответствовала 135.3 нмоль/г сырого веса, а GSSG – 6.2-10.9 нмоль/г сырого веса. А в случае определения методом HPLC с УФ-детекцией она была: для GSH – 61.5 нмоль/г сырого веса; для GSSG – 6.8 нмоль/г сырого веса. При определении с DTNB концентрация глутатиона оказалась выше, тогда как при определении HPLC несколько ниже концентраций GSH в корнеплодах сахарной свеклы. Наблюдаемые различия могут быть обусловлены сортовыми качествами, особенностями используемых методов, а также разными стадиями развития и условиями произрастания растения. В нашем исследовании корнеплоды находились в фазе покоя, тогда как корнеплоды сахарной свеклы – в фазе активного роста, при этом условия их выращивания в представленных работах заметно различались (Zaharieva, Abadia, 2003; Rellán-Álvarez et al., 2006).
Довольно часто отмечали выраженную динамику в содержании глутатиона в зависимости от стадии развития растения. Наибольшим содержанием GSH характеризовались активно фотосинтезирующие листья (Bielavski, Joy, 1986 Matamoros et al., 1999; Dixit et al., 2001; Rellán-Álvarez et al., 2006; Requejo, Tena, 2012). К концу вегетации у стареющих листьев количество глутатиона снижалось, тогда как у запасающих органов, наоборот, повышалось. Например, в корнях и корневищах Picrorhiza kurroa концу вегетации содержание глутатиона увеличивалось практически в два раза (Gangola et al., 2013). В период покоя повышение GSH (от 2 до 8 раз) происходило у хвойных и листопадных деревьев, что можно объяснить снижением метаболизма при воздействии низких температур (Siller-Cepeda et al., 1991).
Следует отметить, что у покоящихся корнеплодов столовой свеклы содержание GSH оказалось высоким в вакуолях. В то же время, в вакуолях не всех типов тканей удавалось определить GSH. Так, иммуногистохимическим методом в вакуолях листьев (мезофилл и трихомы) и корней Arabidopsis thaliana, Nicotiana tabacum и Cucurbita pepo либо не выявляли, либо определяли незначительное количество GSH (Müller et al., 2004; Zechmann et al., 2006; Kolb et al., 2010; Zechmann, Müller, 2010). Его отсутствие в вакуолях объясняли возможной быстрой деградацией после транспортировки через тонопласт (Müller et al., 2004; Kolb et al., 2010). Однако те же исследователи показали GSH в вакуолях клеток-спутниц флоэмы и паренхимных клеток сосудистых пучков (Zechmann, Müller, 2010). Интересно, что на этих же объектах другие исследователи выявляли GSH в вакуолях. При безводном фракционировании листьев A. thaliana методом HPLC после дериватизации монохлоробиманом (MCB) концентрация GSH, определяемая в вакуолях, находилась в пределах 613-733 мкМ, тогда как в цитозоле – 3.0-3.5 мМ (Krueger et al., 2009). Содержание вакуолярного глутатиона, установленное в диапазоне микромолярных концентраций, согласуется с более ранними исследованиями (Rennenberg, 1982). В другом случае, также у A. thaliana, в вакуолях клеток листьев концентрация GSH составила 17.6 нмоль на грамм сырого веса или 30 мкМ при окраске MCB. Вакуолярная концентрация GSH при пересчете на объем органеллы была в среднем в 100 раз меньшей, чем в других компартментах, и соответствовала 5.4% от суммарного содержания глутатиона в клетке (Queval et al., 2011). Вычисленная с учетом занимаемого объема концентрация глутатиона в вакуолях клеток мезофилла N. tabacum составила примерно 20 мкМ и соответствовала 17% от общего содержания (Rennenberg, 1982). Изучались и другие растения. Например, в протопластах, изолированных из мезофилла листьев Hordeum vulgare, содержание GSH и GSSG в вакуолях составляло от общего содержания 0.9% и 12%, соответственно (Dietz et al., 1992). Доля вакуолярного глутатиона, достигающая 30% от содержания глутатиона целой клетки, была определена при помощи безводного фракционирования листьев Pisum sativum (Klapheck et al., 1987).
При исследовании субклеточного перераспределения глутатиона у мутантов A. thaliana, дефицитных по каталазе (cat2), которые испытывали окислительный стресс, отмечали резкое повышение концентрации GSH в вакуолях: с 5.4%, от общего клеточного содержания в контрольных растениях до 26% в опытных. Тогда как в других компартментах, таких как цитозоль, митохондрии, пероксисомы и ядра, содержание глутатиона резко снижалось (Queval et al., 2011).
Благодаря тому, что редокс-пара глутатиона является одной из наиболее избыточных в клетке, соотношение GSH и GSSG (GSH/GSSG) считают важным критерием в оценке метаболической активности и глубины стрессового состояния как целого растения, так и компартментов клетки. Довольно часто не абсолютную величину пула глутатиона, а GSH/GSSG рассматривают, как один из самых важных факторов, контролирующих генную экспрессию и белковые функции, а также как важный индикатор редокс-окружения клетки (Arrigo 1999; Schafer и Buettner 2001). Согласно сложившемуся мнению, при определении и сравнении устойчивости растений к стрессу, соотношение GSH/GSSG может быть более важным показателем, чем количество GSH (Wang, 1995). Это соотношение в вакуолях клеток корнеплодов в нашем исследовании варьировало в зависимости от метода определения GSH, однако оно всегда было меньше, чем у экстрактов ткани, что соотносится с представленными ранее результатами, свидетельствующими в пользу того, что у растений соотношение GSH/GSSG в вакуолях поддерживается на невысоком уровне (Noctor et al., 2011). Что же касается тканей покоящихся корнеплодов, то GSH/GSSG у них оказалось довольно высоким (7.2; 13.9; 9.2). Следует отметить, что величины GSH/GSSG, определяемые для клеток, тканей и органов разных растительных объектов, довольно широко варьируют. Например, у протопластов Hordeum murianum они изменялись в пределах от 0.62 до 3.81 (Andrzejewska, 2012). У низших растений, в частности у Chlorogonium elongatum, соотношение GSH/GSSG варьировало от 1.9 до 2.6 (Agrawal et al., 1992). Зерна разных зерновых культур отличались содержанием глутатиона и его редокс-состоянием, например, GSH/GSSG составило для: Triticum aestivum – 6.87.5; Hordeum vulgare – 4.6-5.5; Secale cereale – 4.8; Avena sativa – 1.9; Fagopyrum esculentum – 11.1 (Zieliñski et al., 1999). Причину такой широкой вариации величин GSH/GSSG видят как в физиологических особенностях и онтогенетических изменениях, так и в адаптации к стрессовым воздействиям. В некоторых исследованиях показана корреляция между высоким содержанием глутатиона, высоким GSH/GSSG соотношением и устойчивостью растений к неблагоприятным факторам среды. В листьях растений Cucumis sativus, чувствительных к холоду, при пониженных температурах увеличивалось количество GSSG и не изменялось содержание GSHсум, что приводило к снижению GSH/GSSG соотношения, тогда как в листьях холодоустойчивых растений P. sativum увеличивалось содержание не только GSSG, но и GSHсум (Wise and Naylor, 1987). В целом при изменении температурных условий у C. sativus величины соотношения GSH/GSSG могли изменяться от 7.5 до 1.5. Уровень GSH/GSSG напрямую зависел от низкотемпературного закаливания или генетически детерминированной физиологической устойчивости. У растений, закаленных или устойчивых к пониженным температурам, величины GSH/GSSG при низкотемпературной экспозиции были значительно выше (Wise and Naylor, 1987).
Довольно часто, наряду с GSH/GSSG, для оценки редокс-состояния глутатиона используют другой показатель, который является отношением восстановленного глутатиона к суммарному глутатиону (GSH/GSH сум ). Это соотношение также служит объективной мерой редокс-состояния глутатиона. При оптимальных условиях оно обычно варьирует в пределах 0.9-0.95 (характерно для листьев растений) (Noctor et al ., 2011). Хотя в некоторых работах приводят и более низкие значения. Например, у проростков Glycine max , в зависимости от условий, происходило варьирование этого показателя в пределах от 0.08 до 0.2 (Chen et al ., 2009).
Величины редокс-состояния (GSH/GSSG и GSH/GSH сум ) позволяют сопоставить результаты, определяемые разными методами на различных растительных объектах, и получить представление об интенсивности редокс-процессов, которая может изменяться в онтогенезе растения и при воздействии факторов среды.
Применяя разные методы, мы получили различные концентрации глутатиона. Тем не менее, результаты позволили сделать вывод о том, что в вакуолях клеток запасающей паренхимы покоящихся корнеплодов столовой свеклы присутствует глутатион в относительно высокой концентрации. В вакуолях так же, как и в других компартментах клетки, пул восстановленного глутатиона преобладает над окисленным. Низкие показатели редокс-состаяния глутатиона в вакуолях свидетельствуют в пользу накопления или интенсивного образования окисленного глутатиона. ACKNOWLEDGMENT
Работа выполнена при поддержке Российского фонда фундаментальных исследований и Правительства Иркутской области, проект № 14-4404059 р_сибирь_а.
Список литературы Определение глутатиона и его окислительно-восстановительного статуса в изолированных вакуолях красных свекольных клеток
- Agrawal S.B., Agrawal M., Lee E.H., Kramert G.F. and Pillai P. (1992) Changes in polyamine and glutathione contents of a green alga, Chlorogonium elongatum (dang) france exposed to mercury. Environ. Exp. Botany, 32, 145-151
- Andrzejewska R. (2012) Induction of glutathione s-tansferase and glutathione content by herbicide and safener treatment in barley Hordeum murianum L. cells culture. Practical Aplications of Environmental Research, 12, 201-210
- Anderson M.E. (1985) Glutathione and Glutathione Disulfide in Biological Samples. Methods Enzymol., 113, 548-555
- Arrigo A.P. (1999). Gene expression and the thiol redox state. Free Radic. Biol. and Med., 27, 936-944
- Bielavski W. and Joy K.W. (1986) Reduced and oxidised glutathione and glutathione-reductase activity in tissues of Pisum sativum. Planta, 169, 267-272
- Bradford M. (1976) A rapid and sensitive method for the quantitation of protein utilising the principal of protein-dye binding. Anal. Biochem., 72, 248-254
- Chen L., Liu S.C., Gai1 J.Y., Zhu1 Y.L., Yang L.F. and Wei G.P. (2009) Effects of nitrogen forms on the growth, ascorbate-glutathione cycle and lipid peroxidation in developing seeds of vegetable soybean. Province African Journal of Agricultural Research, 4, 1178-1188
- Coleman J.О.D., Blake-Kall M.M.A. and Davies T.G.E. (1997) Detoxification of xenobiotics by plants: chemical modification and vascular compartmemation. Trends. Plant Sci., 2, 144-151
- Dawson R.M.C., Elliot D.C., Elliott W.H. and Jones K.M. (1986) Data for biochemical research. Oxford: Oxford Science Publications, OUP, 580 p
- Dietz, K.-J., Brune A. and Pfanz H. (1992) Trans-Tonoplast Transport of the Sulfur Containing Compounds Sulfate, Methionine, Cysteine and Glutathione. Phyton., 32, 37-40
- Dixit V., Pandey V. and Shiam R. (2001) Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J. Exp. Botany, 52, 1101-1109
- Dixon D.P., Cummins L., Cole D.J. and Edwards G.E. (1998) Glutathione-mediated detoxification system in plants. Curr. Opin. Plant Biol., 1, 258-266
- Edwards G.E., Nakamoto H., Bunell J.N. and Hatch M.D. (1985) Pyruvate, Pi dikinase and NADPmalate dehydrogenase in C4 photosynthesis: properties and mechanism of light/dark regulation. Annu. Rev. Plant Physiol., 36, 255-286
- Ellman G. (1959) Tissue sulfhydryl groups. Arc. Biochem. Biophys., 82, 70-77
- Forman H.J., Zhang H. and Rinna A. (2008) Glutathione: Overview of its protective roles, measurement, and biosynthesis. Molecular Aspects of Medicine DOI: 10.1016/j.mam.2008.08.006
- Gangola M.P., Parkash J., Ahuja P.S. and Dutt S. (2013) Components of antioxidant system of Picrorhiza kurrooa exhibit different spatio-temporal behavior. Mol. Biol. Rep DOI: 10.1007/s11033-013-2772-3
- Hissin P.J. and Hilf R. (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues // Anal. Biochem., 74, 214-226
- Klapheck S., Latus C. and Bergmann L. (1987) Localisation of glutathione synthetase and of glutathione in leaf cells of Pisum sativum L. J. Plant Physiol., 101,131-123
- Kolb D., Muller M., Zellnig G. and Zechmann B. (2010) Cadmium induced changes in subcellular glutathione contents within glandular trichomes of Cucurbita pepo L. Protoplasma, 243, 87-94
- Krueger S., Niehl A., Lopez Martin M.C., Steinhauser D., Donath A., Hildebrandt T., Romero L.C., Hoefgen R., Gotor C. and Hesse H. (2009) Analysis of cytosolic and plastidic serine acetyltransferase mutants and subcellular metabolite distributions suggests interplay of the cellular compartments for cysteine biosynthesis in Arabidopsis. Plant Cell Environ., 32, 349-367
- Levites E.V. (1986) Genetics of plant isozymes. Nauka: Sibir. Dep., 144 p
- Matamoros M.A., Moran J.F., Iturbe-Ormaetxe I., Rubio M.C. and Becana M. (1999) Glutathione and homoglutathione synthesis in legume root nodules. Plant Physiol., 121, 879-888
- Muller M., Zechmann B. and Zellnig G. (2004) Ultrastructural localization of glutathione in Cucurbita pepo plants. Protoplasma, 223, 213-219
- Noctor G., Queval G., Mhamdi A., Chaouch S. and Foyer C.H. (2011) Glutathione. The Arabidopsis Book. Published By: The American Society of Plant Biologists URL: http://www.bioone.org/doi/full/10.1199/tab.0142
- Owens C.W.I. and Belcher R.V. (1965) A Colorimetric Micro-Method for the Determination of Glutathione. Biochem. J., 94, 705-711
- Pastore A., Federici G., Bertini E. and Piemonte F. (2003) Analysis of glutathione: implication in redox and detoxification. Clinica Chimica Acta, 333, 19-39
- Queval G., Jaillard D., Zechmann B. and Noctor G. (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ., 34, 21-32
- Ranawat P. and Bansal M.P. (2009) Delineating the Molecular Mechanism behind Regulation of Spermatogenesis by Selenium: Involvement of Mitogen Activated Protein Kinase; JNK. Am. J. Biomed. Sci., 1, 226-241
- Rellan-Alvarez R. Hernandez L.E., Abadia J. and Alvarez-Fernández A. (2006б) Direct and simultaneous determination of reduced and oxidized glutathione and homoglutathione by liquid chromatography-electrospray/mass spectrometry in plant tissue extracts. Anal. Biochem., 356, 254-264
- Rellan-Alvarez R., Ortega-Villasante C., Alvarez-Fernandez A., del Campo F. F. and Hernandez L.E. (2006) Stress Responses of Zea mays to Cadmium and Mercury. Plant and Soil., 279, 41-50
- Rennenberg H. (1982) Glutathione metabolism and possible biological roles in higher plants. Phytochemistry, 21, 2771-2781
- Requejo R. and Tena M. (2012) Influence of glutathione chemical effectors in the response of maize to arsenic exposure. J. Plant Physiol., 169, 649-659
- Salyaev R.K., Kuzevanov V.Y., Khaptogaev S.B. and Kopytchuk V.N. (1981) Isolation and purification of vacuoles and vacuolar membranes from plant cells. Russ. J. Plant Physiol., 25, 1295-1305
- Scaduto R.I. (1988) Dithiothreitol and amino acids interfere with the fluorometric determination of glutathione with orthophthaldehyde. Anal Biochem., 174, 265-70
- Schafer F.Q. and Buettner G.R. (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med., 30, 1191-1212
- Schaffner W. and Weissmann C. (1973) A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem., 56, 502-514
- Siller-Cepeda J.H., Chen T.H.H. and Fuchigami L.H. (1991) High Performance Liquid Chromatography Analysis of Reduced and Oxidized Glutathione in Woody Plant Tissues. Plant Cell Physiol., 32, 1179-1185
- Smith I.K., Kendall A.C., Keys A.J., Turner J.C. and Lea P.J. (1984) Increased levels of glutathione in a catalase-deficient mutant of barley (Hordeum vulgare L.). Plant Sci. Lett., 37, 29-33
- Tietze F. (1969) Enzymic Method for Quantitative Determination of Nanogram Amounts of Total and Oxidized Glutathione: Applications to Mammalian Blood and Other Tissues. Anal. Biochem., 27, 502-522
- Wang C.Y. (1995) Temperature Preconditioning Affects Glutathione Content and Glutathione Reductase Activity in Chilled Zucchini Squash. J. Plant Physiol., 145, 148-152
- Wise R.R. and Naylor A.W. (1987) Chilling-enhanced photooxidation. Evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol., 83, 278-282
- Vignaud C., Rakotozafy L., Falguieres A., Potus J. and Nicolas J. (2004) Separation and identification by gel filtration and high-performance liquid chromatography with UV or electrochemical detection of the disulphides produced from cysteine and glutathione oxidation. J. Chromatogr. A., 1031,125-133
- Zaharieva T.B. and Abadia J. (2003) Iron deficiency enhances the levels of ascorbate, glutathione, and related enzymes in sugar beet roots. Protoplasma, 221, 269-275
- Zechmann B. and Müller M. (2010) Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma, 246, 15-24
- Zechmann B., Zellnig G. and Muller M. (2006) Immunocytochemical localization of glutathione precursors in plant cells. J. Electron Microscopy, 55, 173-181
- Zieliñski H., Honke J., Troszyñska A. and Kozlowska H. (1999) Reduced-Oxidized Glutathione Status as a Potential Index of Oxidative Stress in Mature Cereal Grain. American Association of Cereal Chemists, 76, 944-948


