Оптимальная последовательность применения ингибиторов рецептора эпидермального фактора роста у пациентов с распространенным немелкоклеточным раком легкого, имеющих в опухоли активирующие мутации гена EGFR
Автор: Коломейцева Алина Андреевна, Феденко Александр Александрович
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Обзоры
Статья в выпуске: 6 т.19, 2020 года.
Бесплатный доступ
Актуальность. Успехи лечения больных EGFR-позитивным немелкоклеточным раком легкого (НМРЛ) напрямую связаны с применением ингибиторов рецептора эпидермального фактора роста (EGFR). В настоящее время для лечения этой группы пациентов применяются три поколения ингибиторов EGFR. Актуальным представляется вопрос о том, какой препарат или какая последовательность их применения будет оптимальным вариантом лечения для конкретного пациента. Цель исследования -проанализировать современные данные о применении ингибиторов EGFR в терапии больных распространенным EGFR-позитивным НМРЛ, а также оценить возможные механизмы резистентности к ним и определить оптимальную терапевтическую последовательность ингибиторов EGFR различных поколений. Материал и методы. В обзор включены данные рандомизированных клинических исследований, а также результаты исследования, проведенного в условиях реальной клинической практики, изучающих эффективность ингибиторов EGFR и варианты последующей терапии в случае развития лекарственной резистентности к ним. Результаты. Выбор оптимального варианта терапии первой линии больных EGFR-позитивным НМРЛ зависит от множества факторов но, на наш взгляд, терапия афатинибом с последующим переходом на осимертиниб при появлении мутации Т790М в опухоли позволяет максимально продлить малотоксичную таргетную терапию и отсрочить применение цитостатических препаратов. Заключение. Учитывая доминирующий механизм развития резистентности - появление мутации Т790М гена EGFR, именно последовательное применение ингибиторов EGFR второго и третьего поколений, на наш взгляд, представляется оптимальным вариантом лечения больных EGFR-позитивным НМРЛ.
Нмрл, ингибиторы egfr, мутация t790m, гефитиниб, эрлотиниб, афатиниб, осимертиниб, резистентность
Короткий адрес: https://sciup.org/140254388
IDR: 140254388 | УДК: 616.24-006.6:575.224 | DOI: 10.21294/1814-4861-2020-19-6-119-125
Текст обзорной статьи Оптимальная последовательность применения ингибиторов рецептора эпидермального фактора роста у пациентов с распространенным немелкоклеточным раком легкого, имеющих в опухоли активирующие мутации гена EGFR
Применение ингибиторов рецептора эпидермального фактора роста (EGFR) произвело настоящий прорыв в лечении пациентов с распространенным немелкоклеточным раком легкого (НМРЛ), имеющих в опухоли активирующие мутации гена EGFR . В настоящее время ингибиторы тирозинкиназы (ИТК) EGFR вытеснили классические цитостатические препараты и являются препаратами выбора для первой линии лечения этой группы больных.
Во многих странах мира, в том числе и в Российской Федерации, в арсенал онколога входит три поколения ИТК EGFR. К первому поколению относят обратимые ингибиторы EGFR – гефитиниб и эрлотиниб; второе поколение включает необратимый блокатор семейства рецепторов ErbB – афатиниб; к третьему поколению этой группы препаратов относится необратимый ингибитор осимертиниб, специфично блокирующий мутированный EGFR , в том числе при наличии мутации резистентности к ИТК EGFR Т790М. В рандомизированных клинических исследованиях применение ИТК EGFR всех трех поколений статистически значимо улучшало медиану выживаемости без прогрессирования (мВБП) в сравнении со стандартной химиотерапией больных НМРЛ, чья опухоль несла частые мутации EGFR (Del19 или L858R) [1–8]. Помимо впечатляющей эффективности, ингибиторы EGFR обладают более совершенным профилем переносимости, чем традиционная платиносодержащая химиотерапия. Нежелательные явления предсказуемы, контролируемы и редко приводят к полной отмене препарата. Доступность трех поколений ИТК EGFR для лечения НМРЛ с мутациями EGFR поднимает закономерный вопрос о том, какой препарат или какая последовательность применения ИТК EGFR будет оптимальным вариантом лечения для конкретного пациента.
Различия в клинической эффективности ингибиторов EGFR в зависимости от поколения
Имея различные фармакологические характеристики и механизм действия, по-разному воздействуя на рецептор, ингибиторы EGFR проявляют и неодинаковую противоопухолевую активность. ИТК EGFR первого поколения, гефитиниб и эрлотиниб, нековалентно и обратимо связываются с EGFR («дикого» типа и мутантными формами) [9, 10]. Исследования III фазы продемонстрировали, что медиана ВБП при применении ИТК первого поколения в первой линии в сравнении со стандартными платиносодержащими режимами химиотерапии составила 9,2–13,1 мес против 4,6–6,3 мес соответственно. При этом медиана продолжительности жизни в группах значимо не различалась [1–5].
Механизм действия ИТК EGFR второго поколения афатиниба обусловлен ковалентным и необратимым связыванием со всеми возможными гомо- и гетеродимерами ErbB и их необратимым блокированием [11–13]. Преимущество афатиниба над стандартными платиносодержащими режимами химиотерапии первой линии по показателю ВБП у больных НМРЛ с активирующими мутациями EGFR было продемонстрировано в двух исследованиях III фазы, LUX-Lung 3 и LUX-Lung 6–13,6 мес против 6,9 мес и 11 мес против 5,6 мес соответственно [6, 7]. По совокупным данным двух исследований, в отличие от ИТК EGFR первого поколения, результаты планового анализа пациентов с мутацией EGFR с делецией в экзоне 19 (Del19) показали, что применение афатиниба приводило к статистически значимому улучшению общей выживаемости по сравнению с химиотерапией [14]. Кроме того, у пациентов с метастатическим поражением центральной нервной системы в комбинированном анализе исследований LUX-Lung 3 и LUX-Lung 6 афатиниб также продемонстрировал преимущество по ВБП по сравнению со стандартной платиносодержащей ПХТ (8,2 мес против 5,4 мес; отношение рисков (ОР) 0,50; p=0,0297) [15]. В исследовании LUX-Lung 7 афатиниб продемонстрировал статистически значимое улучшение мВБП (ОР: 0,73, 95 % ДИ: 0,57–0,95, p=0,017) и времени до прекращения лечения (ВПЛ; ОР: 0,73, 95 % ДИ: 0,58–0,92, p=0,0073) по сравнению с гефитинибом у пациентов с частыми мутациями EGFR (Del19/L858R) [16].
Кроме того, афатиниб оказался эффективным у пациентов, имеющих в опухоли легкого редкие мутации EGFR , такие как G719X, L861Q и S7681, инсерции в экзоне 20 и другие. Его активность была показана в объединенном анализе исследований LUX-Lung 2, 3 и 6, который включил 38 пациентов с редкими мутациями EGFR . Частота объективного ответа на афатиниб составила 71 %, медиана ВБП – 11 мес [17].
Ингибитор EGFR третьего поколения является специфическим ингибитором мутантного EGFR, вызывая необратимое ковалентное связывание с ним, при этом не действуя на «дикий» тип гена EGFR . Осимертиниб проявляет специфичность в отношении мутации EGFR T790M, появление которой обусловливает резистентность к ИТК EGFR [18]. В исследовании AURA3 осимертиниб продемонстрировал впечатляющую активность по сравнению с режимом химиотерапии пеметрек-сед + производные платины при применении в качестве второй линии лечения после прогрессирования на ингибиторах EGFR первого и второго поколений, обусловленного появлением мутации EGFR T790M, с частотой объективного ответа (71 % и 31 %, p<0,001) и медианой ВБП (10,1 мес и 4,4 мес, ОР 0,30; 95 % ДИ 0,23–0,41; р<0,001) соответственно. Следует отметить, что преимущество осимертиниба по показателю мВБП наблюдалось также и у пациентов с метастатическим поражением центральной нервной системы (8,5 мес и 4,2 мес; ОР 0,32; 95 % ДИ 0,21–0,49) [8, 19].
Применение осимертиниба в первой линии лечения у пациентов с активирующими мутациями EGFR (Del19 или L858R) в исследовании FLAURA показало существенное, статистически значимое улучшение ВБП по сравнению с ингибиторами EGFR первого поколения эрлотинибом и гефити-нибом – 17,7 мес против 9,7 мес (ОР: 0,46, 95 % ДИ: 0,37–0,57, p<0,001), а также значимое улучшение показателя медианы общей выживаемости – 38,6 мес против 31,8 мес (ОР: 0,799, 95 % ДИ: (0,641–0,997, p=0,0462) [20,21].
Эффективность осимертиниба у пациентов, имеющих в опухоли редкие мутации EGFR , также активно изучается в настоящее время. В исследовании II фазы, проведенном в Корее, 37 пациентов с малораспространенными мутациями EGFR (G719X, L861Q, S768I и др.) получали терапию осимертинибом. ЧОО составила 50 % (95 % ДИ:
33–67 %), медиана длительности ответа – 11,2 мес (95 % ДИ: 7,7–14,7 мес) [22].
Резистентность к ингибиторам EGFR
Независимо от того, какой ингибитор EGFR будет выбран в качестве первой линии лечения и как долго будет длиться ответ на него, неизбежно развивается резистентность и, как следствие, прогрессирование болезни. Вероятно, это обусловлено тем, что опухоли с мутантным геном могут быть исходно крайне неоднородными. Понимание характера развития опухоли на клеточном уровне имеет решающее значение для рационального терапевтического подхода, направленного на задержку развития резистентности, и выбора оптимального лечения после появления резистентности у каждого отдельно взятого пациента.
В настоящее время определение механизма устойчивости к ингибиторам EGFR возможно с помощью проведения повторных биопсий опухоли или определения циркулирующей опухолевой ДНК в плазме крови [23, 24]. К наиболее частым механизмам развития резистентности к ИТК EGFR первого и второго поколений, который наблюдается примерно в 70 % случаев, относится появление клона опухолевых клеток, несущих мутацию T790M в экзоне 20 EGFR [25–28]. По данным исследований AURA2 и AURA extension, процент выявляемости T790M зависит от того, какая драйверная мутация определялась исходно. При делеции в 19 экзоне EGFR частота выявления мутации резистентности достигает 80 %, тогда как при мутации L858R не превышает 60 % [29, 30]. Еще в 20–40 % случаев резистентность обусловлена активацией дополнительных сигнальных путей в опухолевой клетке, таких как PI3KCA, амплификация MET и HER2 . У части больных резистентность к ИТК EGFR обусловлена морфологической трансформацией аденокарциномы в мелкоклеточный рак, механизм подобной трансформации остается до конца не изученным.
В отличие от ИТК первого и второго поколений механизмы развития резистентности к осимер-тинибу в настоящее время не вполне изучены и представляются неоднородными. В когорте из 19 пациентов, получающих осимертиниб в первой линии лечения, предполагаемые механизмы резистентности были идентифицированы в 9 случаях и включали мутации генов MEK1 , KRAS , PI3KCA , HER2 или JAK2 либо амплификацию генов KRAS , MET или EGFR . Двое пациентов приобрели третичную мутацию резистентности гена EGFR , C797S [31, 32]. Вследствие этого возможные варианты таргетной терапии после первой линии лечения осимертинибом по-прежнему неясны.
О резистентности к осимертинибу, применяемому во второй и последующих линиях лечения, известно чуть больше. Как правило, механизм развития резистентности зависит от того, сохра- няет ли опухоль или утратила мутацию T790M. Мутация EGFR C797S встречается приблизительно в 20–40 % случаев, кроме того, наблюдаются трансформация в мелкоклеточную гистологию, амплификация HER2, MET или FGFR и мутации в гене BRAF. EGFR-опосредованная резистентность (C797S, L792X и L718Q) чаще возникает в случаях, когда мутация T790M сохранена [33–35].
Выбор последующей терапии после развития резистентности к ингибиторам EGFR
С учетом неизбежного развития резистентности на терапии ингибиторами EGFR последующий выбор варианта терапии приобретает первостепенное значение [36, 37]. Для пациентов, прогрессирующих на ИТК EGFR первого и второго поколений с положительной мутацией T790M, препаратом выбора является осимертиниб [8]. Если прогрессирование болезни произошло на осимертинибе, назначенном в первой линии лечения, и нет возможности включить пациента в клиническое исследование, единственным вариантом эффективного лечения остаются платиносодержащие режимы химиотерапии или их комбинация с атезолизумабом и бевацизумабом, которая в исследовании IMpower 150 показала свою активность, независимо от наличия активирующих мутаций EGFR [38].
В связи с этим, на наш взгляд, именно терапевтическая последовательность, предполагающая, что за ингибиторами EGFR первого или второго поколений следует осимертиниб, представляется оптимальным вариантом, позволяя увеличить продолжительность лечения препаратами направленного действия, отложить необходимость применения токсичных схем химиотерапии и достичь длительной выживаемости больных с драйверными мутациями EGFR.
На сегодняшний день имеются немногочисленные данные, оценивающие совокупный эффект последовательной терапии ингибиторами EGFR у пациентов с НМРЛ и положительной мутацией EGFR . Так, впечатляющие результаты получены при изучении последовательности назначения афатиниба в первой линии лечения и осимерти-ниба – после прогрессирования, обусловленного появлением мутации резистентности T790M, как в рамках анализов подгрупп в клинических исследованиях, так и в условиях реальной клинической практики. В ретроспективном объединенном анализе трех рандомизированных исследований LUX-Lung 3, 6, 7, включившем 37 пациентов, получавших последовательное лечение афатинибом и осимертинибом, медиана продолжительности терапии обоими препаратами составила 20,2 мес. Медиана продолжительности жизни на момент анализа не была достигнута [39].
В наблюдательном, глобальном, многоцентровом исследовании GioTag была проведена оценка общей продолжительности последовательного лечения афатинибом и осимертинибом у больных НМРЛ с положительной мутацией EGFR с приобретенной резистентностью T790M, которые получали лечение в условиях реальной клинической практики [40]. В исследование было включено 204 пациента из 10 стран. Из характеристик следует отметить, что у ряда включенных в анализ больных были метастазы в головной мозг (10,3 %), а также были пациенты с соматическим статуcом ECOG 2–3 (15,3 %). У подавляющего большинства пациентов (73,5 %) на момент начала терапии афатинибом в опухоли присутствовала мутация EGFR Del19. Перед началом терапии осимертинибом у всех пациентов было документально подтверждено наличие мутации T790M. Медиана продолжительности терапии для всей выборки последовательного лечения афатинибом и осимертинибом составила 28,1 мес (90 % ДИ: 25,9–31,3). Хотя клиническая эффективность последовательного лечения афа-тинибом и осимертинибом наблюдалась во всех подгруппах пациентов, максимальную выгоду от данной терапевтической последовательности получили пациенты с ECOG 0–1 по сравнению с ≥2 (31,3 мес в сравнении с 22,2 мес) и с сочетанием удовлетворительного соматического статуса ECOG 0–1 и наличием мутации EGFR Del19 (36,4 мес (29,2–46,7)). При медиане наблюдения 30,3 мес медиана общей выживаемости во всей популяции больных составила 41,3 мес (90 % ДИ: 36,8–46,3) и 45,7 мес (90 % ДИ: 45,3–51,5) у пациентов с делецией в 19 экзоне EGFR [40, 41].
Таким образом, полученные в наблюдательном исследовании GioTag результаты свидетельствуют о том, что последовательная терапия афатинибом и осимертинибом является высокоэффективным вариантом лечения, особенно для пациентов с делецией в 19 экзоне EGFR .
Недавно опубликованные данные доклинических исследований показали, что частота появления мутации T790M в клеточных линиях, обработанных афатинибом, была выше, чем при обработке эрлотинибом. Возможно, это связано с тем, что афатиниб подавляет большинство сопутствующих аберраций в клетках с мутацией Del19, при этом обладая минимальным воздействием на T790M [42].
В настоящее время нет данных об эффективности последовательной терапии ингибиторами EGFR первого поколения и осимертиниба. Продолжается исследование по применению осимер-тиниба после прогрессирования на гефитинибе у больных с приобретенной мутацией T790M в сравнении с осимертинибом в первой линии лечения [43].
Возможные доступные опции лекарственной терапии больных EGFR-позитивным НМРЛ представлены на рис. 1.
Учитывая доминирующий механизм развития резистентности – появление мутации T790M гена
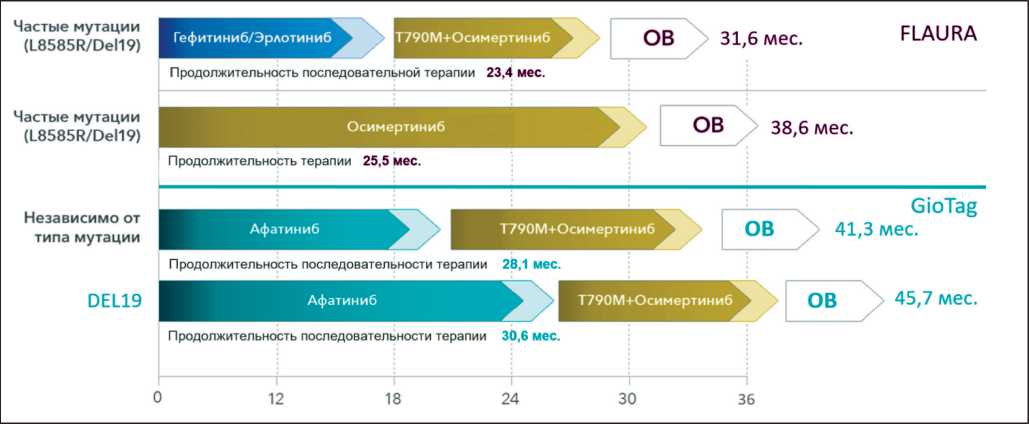
Рис. 1. Варианты последовательной терапии EGFR-позитивного НМРЛ Fig. 1. Sequential treatment options for EGFR-positive NSCLC
EGFR , именно последовательное применение ингибиторов EGFR второго и третьего поколений, на наш взгляд, представляется оптимальным вариантом лечения этой когорты пациентов. Такой подход позволяет максимально отсрочить применение цитостатических препаратов у больных EGFR-позитивным НМРЛ.
Заключение
Наличие нескольких препаратов для таргетной анти-EGFR терапии коренным образом изменило лечение больных НМРЛ с мутациями EGFR. Однако перед клиницистом стоит непростая задача оптимального выбора имеющихся препаратов и последовательного их применения для того, чтобы извлечь максимальную клиническую пользу для пациента. Решение этой задачи зависит от множества факторов, таких как эффективность и переносимость препаратов, применяемых в первой линии лечения, их влияние на качество жизни, возможность выполнить жидкостную биопсию или повторно получить материал из ткани опухоли для
Список литературы Оптимальная последовательность применения ингибиторов рецептора эпидермального фактора роста у пациентов с распространенным немелкоклеточным раком легкого, имеющих в опухоли активирующие мутации гена EGFR
- Zhou C., Wu Y.L., Chen G., Feng J., Liu X.Q., Wang C., Zhang S., Wang J., Zhou S., Ren S., Lu S., Zhang L., Hu C., Hu C., Luo Y., Chen L., Ye M., Huang J., Zhi X., Zhang Y., Xiu Q., Ma J., Zhang L., You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011 Aug; 12(8): 735-42. doi: 10.1016/S1470-2045(11)70184-X.
- RosellR., Carcereny E., GervaisR., Vergnenegre A., MassutiB., Fe-lip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M., Porta R., Cobo M., Garrido P., Longo F., Moran T., Insa A., De Marinis F., Corre R., Bover I., IllianoA., Dansin E., de Castro J., MilellaM., ReguartN., Altavil-la G., Jimenez U., Provencio M., Moreno M.A., Terrasa J., Muñoz-Langa J., Valdivia J., Isla D., Domine M., Molinier O., Mazieres J., Baize N., Garcia-Campelo R., Robinet G., Rodriguez-Abreu D., Lopez-Vivanco G., Gebbia V., Ferrera-Delgado L., Bombaron P., Bernabe R., Bearz A., Artal A., Cortesi E., Rolfo C., Sanchez-Ronco M., Drozdowskyj A., Queralt C., de Aguirre I., Ramirez J.L., Sanchez J.J., Molina M.A., Taron M., Paz-Ares L. ; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012 Mar; 13(3): 239-46. doi: 10.1016/S1470-2045(11)70393-X.
- Wu Y.L., Zhou C., Liam C.K., Wu G., Liu X., Zhong Z., Lu S., Cheng Y., Han B., Chen L., Huang C., Qin S., Zhu Y., Pan H., Liang H., Li E., Jiang G., How S.H., Fernando M.C.L., Zhang Y., Xia F., Zuo Y. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015; 26(9): 1883-89. doi: 10.1093/annonc/mdv270.
- Mok T.S., Wu Y.L., ThongprasertS., Yang C.H., Chu D.T., SaijoN., Sunpaweravong P., Han B., Margono B., Ichinose Y., Nishiwaki Y., Ohe Y., Yang J.J., ChewaskulyongB., JiangH., DuffieldE.L., Watkins C.L., Armour A.A., Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009 Sep 3; 361(10): 947-57. doi: 10.1056/NEJMoa0810699.
- Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I., Fujita Y., Okinaga S., Hirano H., Yoshimori K., Harada T., Ogura T., Ando M., Miyazawa H., Tanaka T., Saijo Y., Hagiwara K., Morita S., Nukiwa T.; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010 Jun 24; 362(25): 2380-8. doi: 10.1056/NEJMoa0909530.
- Sequist L.V., Yang J.C., Yamamoto N., O'Byrne K., Hirsh V., Mok T., Geater S.L., OrlovS., Tsai C.M., BoyerM., Su W.C., Bennouna J., Kato T., Gorbunova V., Lee K.H., Shah R., Massey D., Zazulina V., Shahidi M., Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013 Sep 20; 31(27): 3327-34. doi: 10.1200/jc0.2012.44.2806.
- Wu Y.L., Zhou C, Hu C.P., Feng J., Lu S., Huang Y, Li W, Hou M, Shi J.H., Lee K.Y., Xu C.R., Massey D, Kim M, Shi Y, Geater S.L. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014 Feb; 15(2): 213-22. doi: 10.1016/S1470-2045(13)70604-1.
- PirkerR, PirkerR, BuderA, FilipitsM. Osimertinib in advanced EGFR T790M-positive non-small-cell lung cancer: the clinical impact of AURA3. Translational Cancer Res. 2017; 6: 265-9. doi: 10.21037/ tcr.2017.03.12.
- Sharma S,V,, Bell D,W,, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007 Mar; 7(3): 169-81. doi: 10.1038/nrc2088.
- Spicer J,F, Rudman SM. EGFR inhibitors in non-small cell lung cancer (NSCLC): the emerging role of the dual irreversible EGFR/HER2 inhibitor BIBW 2992. Target Oncol. 2010 Dec; 5(4): 245-55. doi: 10.1007/ s11523-010-0140-y.
- Solca F,, Dahl G, Zoephel A, Bader G, Sanderson M,, Klein C, Kraemer O, Himmelsbach F,, Haaksma E, Adolf G,R. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012 Nov; 343(2): 342-50. doi: 10.1124/jpet.112.197756.
- Modjtahedi H, Cho B,C,, MichelM,C,, Solca F, A comprehensive review of the preclinical efficacy profile of the ErbB family blocker afatinib in cancer. Naunyn Schmiedebergs Arch Pharmacol. 2014 Jun; 387(6): 505-21. doi: 10.1007/s00210-014-0967-3.
- Li D, Ambrogio L, Shimamura T,,, Kubo S, Takahashi M, Chirieac L,R,, Padera R,F,, Shapiro G,I, Baum A, Himmelsbach F,, Rettig WJ, Meyerson M, Solca F, Greulich H, Wong K,K. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008 Aug 7; 27(34): 4702-11. doi: 10.1038/ onc.2008.109.
- Yang J,C,, Wu Y,L,, SchulerM,, Sebastian M, Popat S,, Yamamoto N, Zhou C, Hu C,P,, O'Byrne K, Feng J, Lu S, Huang Y,, Geater S,L,, LeeK,Y,, Tsai CM,, Gorbunova V',, Hirsh V',, Bennouna J, OrlovS,, MokT, Boyer M, Su W,C,, Lee K,H,, Kato T, Massey D, Shahidi M,, Zazulina V, Sequist L,V Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015; 16(2): 141-51. doi: 10.1016/S1470-2045(14)71173-8.
- SchulerM, Wu Y,L,, Hirsh V, O'ByrneK, Yamamoto N, Mok T, Popat S,, Sequist L,V,, Massey D, Zazulina V,',, Yang J,C, First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol. 2016 Mar; 11(3): 380-90. doi: 10.1016/j. jtho.2015.11.014.
- Park K,, Tan E,H, O'Byrne K,, Zhang L, Boyer M, Mok T,, Hirsh K, Yang J,C, Lee K,H, Lu S, Shi Y, Kim S,W, Laskin J,, Kim D,W,, Arvis C,D,, Kölbeck K, Laurie SA,, Tsai CM,, Shahidi M,, Kim M,, Massey D,, Zazulina V,, Paz-Ares L, Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016; 17(5): 577-89. doi: 10.1016/S1470-2045(16)30033-X.
- YangJ,C,, Sequist L,V, Geater S,L,, Tsai CM, Mok T,S, SchulerM,, Yamamoto N,, Yu CJ,, Ou S,H,, Zhou C,, Massey D,, Zazulina V,, Wu Y,L, Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 2015 Jul; 16(7): 830-8. doi: 10.1016/S1470-2045(15)00026-1.
- Hirano T,, Yasuda H,, Tani T,, Hamamoto J,, Oashi A,, Ishioka K,, Arai D,, Nukaga S,, Miyawaki M,, Kawada I,, Naoki K,, Costa D,B,, Kobayashi S,S,, Betsuyaku T,, Soejima K, In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget. 2015 Nov 17; 6(36): 38789-803. doi: 10.18632/oncotarget.5887.
- Mok T,S, Wu Y,-L,, Ahn M,-J, Garassino M,C, Kim H,R, Rama-lingam S,S,, Shepherd FA,, He Y,, Akamatsu H,, Theelen W,S,, Lee C,K,, Sebastian M,, Templeton A,, Mann H,, Marotti M,, Ghiorghiu S,, Pa-padimitrakopoulou VA,; AURA3 Investigators. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017 Feb 16; 376(7): 629-640. doi: 10.1056/NEJMoa1612674.
- Soria J,C,, Ohe Y,, Vansteenkiste J,, Reungwetwattana T,, ChewaskulyongB,, LeeK,H,, DechaphunkulA,, ImamuraF,, NogamiN,, Kurata T,, Okamoto I,, Zhou C,, Cho B,C,, Cheng Y,, Cho E,K,, Voon P,J,, Planchard D, Su W,C,, Gray J,E, Lee SM, Hodge R,, Marotti M, Ru-kazenkov Y,, Ramalingam S,S,; FLAURA Investigators. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018 Jan 11; 378(2): 113-125. doi: 10.1056/NEJMoa1713137.
- Ohe Y,, Imamura F,, Nogami N,, Okamoto I,, Kurata T,, Kato T,, Sug-awara S, Ramalingam S,S,, UchidaH,, HodgeR,, Vowler S,L,, WaldingA,, Nakagawa K. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019 Jan 1; 49(1): 29-36. doi: 10.1093/jjco/hyy179.
- Cho J.H, Lim S.H., An H.J, Kim K.H, Park K.U., Kang E.J., Choi Y.H, Ahn M.S., Lee M.H, Sun J.M, Lee S.H, Ahn J.S., Park K, Ahn MJ, Osimertinib for Patients With Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J Clin Oncol. 2020 Feb 10; 38(5): 488-495. doi: 10.1200/JCO.19.00931.
- Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald N,Q, Butler A, JonesD, RaineK, Latimer C, Santos C,R, NohadaniM, EklundA,C, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012 Mar 8; 366(10): 883-892. doi: 10.1056/NEJMoa1113205.
- Murtaza M, Dawson SJ, Tsui D,W, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong A,S, Marass F, Humphray S, Hadfield J, Bentley D, Chin TM, Brenton J,D, Caldas C, Rosenfeld N, Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013; 497(7447): 108-12. doi: 10.1038/nature12065.
- ArcilaM,E, Oxnard G,R, NafaK, Riely GJ, Solomon S,B, Za-kowskiM,F, KrisM,G, Pao W, Miller VA, LadanyiM. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011 Mar 1; 17(5): 1169-80. doi: 10.1158/1078-0432. CCR-10-2277.
- Sequist L,V, Waltman B,A, Dias-Santagata D, Digumarthy S, TurkeA,B, FidiasP, BergethonK, ShawA,T, Gettinger S, CosperA,K, AkhavanfardS, HeistR,S, Temel J, Christensen J,G, Wain J,C, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman J,A, Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011 Mar 23; 3(75): 75ra26. doi: 10.1126/scitranslmed.3002003.
- Yu HA, Arcila M,E, Rekhtman N, Sima C,S, Zakowski M,F, Pao W, KrisM,G, Miller VA, LadanyiM, Riely GJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013 Apr 15; 19(8): 2240-7. doi: 10.1158/1078-0432.CCR-12-2246.
- Yang J,C,, Ahn M,J,, Kim D,W,, Ramalingam S,S,, Sequist L,V,, Su W,C,, Kim S,W,, Kim J,H,, Planchard D,, Felip E,, Blackhall F,, Haggstrom D,, Yoh K,, Novello S,, Gold K,, Hirashima T,, Lin C,C,, Mann H, Cantarini M, Ghiorghiu S, Jänne P,A. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol. 2017 Apr 20; 35(12): 1288-1296. doi: 10.1200/JCO.2016.70.3223.
- Ahn MJ, Tsai CM, Shepherd FA, Bazhenova L, Sequist L,V, Hida T, Yang J,C,H, Ramalingam S,S, Mitsudomi T, Jänne PA, Mann H, Cantarini M,, Goss G. Osimertinib in patients with T790M mutationpositive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer. 2019 Mar 15; 125(6): 892-901. doi: 10.1002/cncr.31891.
- LeX, Puri S, NegraoM,V, NilssonM,B, Robichaux J, Boyle T, Hicks J,K, Lovinger K,L, Roarty E, Rinsurongkawong W, Tang M, Sun H, Elamin Y Lacerda L,C, Lewis J, Roth JA, Swisher S,G, Lee JJ, William W,NJr, Glisson B,S, Zhang J, Papadimitrakopoulou VA,, GrayJ,E, Heymach J,VLandscape of EGFR-Dependent and Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC. Clin Cancer Res. 2018 Dec 15; 24(24): 6195-6203. doi: 10.1158/1078-0432.CCR-18-1542.
- Piotrowska Z, Thress K, Mooradian MJ, Heist R,S, Azzoli C,G, Temel J,S, Evans T, MET amplification (amp) as a resistance mechanism to osimertinib. Clin. Oncol. 2017; 35: 9020.
- TanC,S, KumarakulasingheN,B, HuangY,Q,,Ang Y,L,E, ChooJ,R, Goh B,C,, Soo R,A. Third generation EGFR TKIs: current data and future directions. Mol Cancer. 2018 Feb 19; 17(1): 29. doi: 10.1186/s12943-018-0778-0.
- Thress K,S, Paweletz C,P, Felip E, Cho B,C, Stetson D, Dougherty B, Lai Z, Markovets A, Vivancos A, Kuang Y, Ercan D, Matthews S,E, Cantarini M, Barrett J,C, Jänne P,A, Oxnard G,R. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015 Jun; 21(6): 560-2. doi: 10.1038/nm.3854.
- Oxnard G,R, Hu Y, Mileham K,F, HusainH, Costa D,B, Tracy P, Feeney N, Sholl LM, Dahlberg S,E, Redig AJ, Kwiatkowski D,J, Rabin M,S, Paweletz C,P, Thress K,S, Jänne PA. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. 2018 Nov 1; 4(11): 1527-1534. doi: 10.1001/jamaon-col.2018.2969.
- Yang Z., Yang N., Ou Q, Xiang Y., Jiang T., Wu X., Bao H., Tong X., WangX., Shao Y.W., Liu Y., Wang Y., Zhou C. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res. 2018 Jul 1; 24(13): 3097-3107. doi: 10.1158/1078-0432.CCR-17-2310.
- Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: which tyrosine kinase inhibitor and when? Future Oncol. 2018; 14(11): 1117-32. doi: 10.2217/fon-2017-0636.
- Hirsh V. Turning EGFR mutation-positive non-small-cell lung cancer into a chronic disease: optimal sequential therapy with EGFR tyrosine kinase inhibitors. Ther Adv Med Oncol. 2018 Jan 22; 10: 1758834017753338. doi: 10.1177/1758834017753338.
- ReckM., Mok T.S.K., NishioM., JotteR.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., Rodriguez-Abreu D., Moro-Sibilot D., Thomas C.A., Barlesi F., Finley G., Lee A., Coleman S., Deng Y., Kow-anetzM., Shankar G., Lin W., Socinski M.A.; IMpower150 Study Group. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019 May; 7(5): 387-401. doi: 10.1016/ S2213-2600(19)30084-0.
- SequistL.V., Wu Y.L., Schuler M. Subsequent therapies post-afatinib among patients with EGFRmutation-positive NSCLC in LUX-Lung (LL) 3, 6 and 7. Ann Oncol. 2017; 28(suppl 2): doi: 10.1093/annonc/ mdx380.051.
- HochmairM.J., MorabitoA., HaoD., YangC.T., SooR.A., Yang J.C., Gucalp R., Haimos B., Wang L., Golembesky A., Märten A., Cufer T. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol. 2018 Nov; 14(27): 2861-2874. doi: 10.2217/fon-2018-0711.
- HochmairM.J., MorabitoA., HaoD., YangC.T., SooR.A., Yang J.C., Gucalp R., Haimos B., Wang L., Märten A., Cufer T. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol. 2019 Sep; 15(25): 2905-2914. doi: 10.2217/fon-2019-0346.
- Kohsaka S., PetronczkiM., SolcaF., MaemondoM. Tumor clonal-ity and resistance mechanisms in EGFR mutation-positive non-small-cell lung cancer: implications for therapeutic sequencing. Future Oncol. 2019 Feb; 15(6): 637-52. doi: 10.2217/fon-2018-0736.
- Osimertinib Treatment on EGFR T790M Plasma Positive NSCLC Patients (APPLE) [Internet]. URL: https://clinicaltrials.gov/ct2/show/ NCT02856893 (cited 14.11.2019).


