Overall review the current tend and difficulties of antimicrobial compounds in composite food packaging applications
Автор: Melesse Emiru, Filinskaya Y. A., Alkhair Ali, Bannikova O. A., Eyeberdiyeva Marjen
Журнал: Вестник Воронежского государственного университета инженерных технологий @vestnik-vsuet
Рубрика: Фундаментальная и прикладная химия, химическая технология
Статья в выпуске: 3 (93), 2022 года.
Бесплатный доступ
Food waste/spoilage caused by microbial cell has recently emerged as a major food insecurity and environmental concern. Additionally, food spoilage contributes to the economic crisis and healthy problems. As a result, an active packaging system is still required to keep the food safe and to protect its quality from foreign contaminants. The purpose of this review was to summarize the current solutions and difficulties of antimicrobial compounds in composite food packaging applications. Specifically, the extrusion and antimicrobial coating methods for incorporating antimicrobial compounds into packaging systems and their optimum processing parameters for common polymer composites were revealed. The common inorganic and organic antimicrobial substances/compounds with their quantities adding to the packaging system and their antimicrobial activity (reduction, partially deactivation and completely deactivation) were presented. The difficulties in creating a package with antimicrobial properties concerning issues of migration of antimicrobial additives from the package to the food product, accumulation of antimicrobial additives in the food product, as well as their processing temperature were elaborated. Therefore, this review work contributes to open up the entire scientific knowledge on antimicrobial compounds used in polymer composite materials for food packaging application and helps to develop important results for large scale operations
Antimicrobial compounds, microorganisms, composites, food spoilage, food packaging, challenges
Короткий адрес: https://sciup.org/140297637
IDR: 140297637 | УДК: 640 | DOI: 10.20914/2310-1202-2022-3-204-213
Текст научной статьи Overall review the current tend and difficulties of antimicrobial compounds in composite food packaging applications
According to an FAO report, food contamination by microorganisms is the world's bottleneck of produced food. The primary problem in food factories is food waste due to microbial contamination. Based on the FAO report, more than 1.3 million metric tons of edible human foods are wasted because of traditional ways of harvesting, storage, and transportation practices, as well as market and consumer waste globally [1,2].The migration of harmful materials and permeability of the foreign materials to the food containers are the current issue related with food contamination have grown globally. The physical, chemical, and biological methods of system can make food decay [3,4]. The fresh foods can be out of date, and drop shelf-life due to microbial spoilage and putrefaction. Hence, around 45; 35; 30 and 20% of fruit and vegetables, fish, cereals, dairy and meat products lost yearly respectively. These mostly wasted by living organisms (microorganisms). Additionally, plastic waste released worldwide exceeds 400 million tons per year and their non-sustainability and non-recyclability are the current issues. Hence, predicts the production rate of plastic waste is expected to enlarge fourfold in 2050 [5, 6]. Next to food contamination by microorganisms and negative impact of convectional food packaging materials, the consumers pertain. Excellent shelf-life and excellent properties of packaging materials are the main goal and concern of the moment food factories [7].
The combination of nano particles and polymer is known as nano-composite, and it is a promising material for food packaging. The food packaging system is categorized into three parts: primary (which coats and communicates with the food), secondary (which covers the primary packaging system), and tertiary (which is the outer covering used for bulk handling, distribution, and further storage) [8]. The best packaging system characterized by good thermal, surface, mechanical, low barrier, green, suitable optical and excellent antimicrobial properties [9,10] represented as Figure 1. In this paper only the antimicrobial materials in food application system are discussed.
The goal of this review was to reveal information about antimicrobial materials, techniques for using in packaging materials, percentile reduction of microorganism activity, and difficulties in creating antimicrobial packaging material properties.
This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License
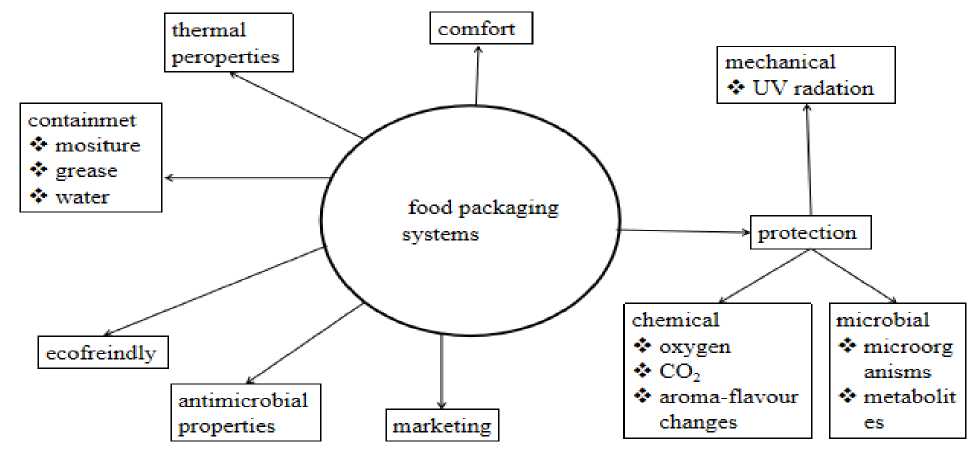
Figure 1. Over all purpose and properties of food packaging systems
-
2. Common Antimicrobial which is used for preparation of packaging materials
The use of antimicrobial compounds in food packaging systems is gaining popularity due to health concerns and government regulations. The common antimicrobial substance/compounds can be sorted into inorganic and organic matters according the Figure 2. The literature reported a wide range of antimicrobial agents such as nano particles of metallic elements [11], metallic oxides [12], clay [13,14], essential oils such as lemon oil, rosemary oil, sun flower oil, lemongrass, carvacrol oil, bergamot oil, metha pipertia L.oil, Mentha villosa Huds oil, eucalyptus globulus oil, cinnamon oil, and Acid compounds such as acetic acid, ascorbic acid, citric acid, lactic acid, glacial acetic acid [15], natural agents [16–18], biopolymers [19, 20], enzymes [21–23], synthetic antimicrobial agents [24]. Antimicrobial substances/ compounds have been studied for their ability to inhibit microbial growth in foods, including organic/natural (essential oils, CO 2 , organic acids, antibiotics, and so on) and inorganic (particularly Silver, Zinc metal/oxide nano particles) but their commercial availability remains limited [25, 26]. The silver (Ag) metal/oxide antimicrobial is used commercially as an antimicrobial agent in food packaging applications in the United States and Japan [27]. The use of silver (Ag) metal/oxide as an antimicrobial agent in food packaging solutions is expected to increase in the European Union (E.U.) used as additives in food surface.
Antimicrobial substances/compounds extracted from natural resources are drawn to the meat, bread, and pastry industries. The meat industry has a strong preference for natural extract antimicrobial agents derived from plants (cloves, ginger, rosemary, thyme, garlic, cinnamon, and so on) [28, 29] as well as natural antimicrobial compounds derived from fungal cells (nisin, pediocin and various bacteri-ocins). Furthermore, technological advancements in the meat industry have been used to improve organoleptic properties and packaging performance by blocking/completely inhibiting microbial activities in the food packaging system [30, 31]. The use of synthetic antimicrobial compounds in the packaging of pastry and bread [32, 33] as well as vegetables and fruits [34–36] has been reported in the literature.
Natural/inorganic antimicrobial compounds in the literature have elucidated the antimicrobial activities of common food spoiling microorganisms. The antimicrobial packaging systems have been tested for Salmonella enterica, E. coli, S. aureus , and other pathogens [37].As a result, the yeast and molds fungal strains have also been developed [38].
Table 1 lists the selective antimicrobial compounds used in food packaging systems. Among them, nisin coated with ally isothiocyanate can completely inhibit Salmonella microbial activity, while dihydroxylated coumarins coated with methanolic extract can completely inhibit T. mentagrophytes and R. solani microbial activities, respectively. Chitosan coated with Lauric acid in starch film and Ethylene in co-polymer films, respectively, reduce the microbial activity of E. coli and L.monocytogenes .
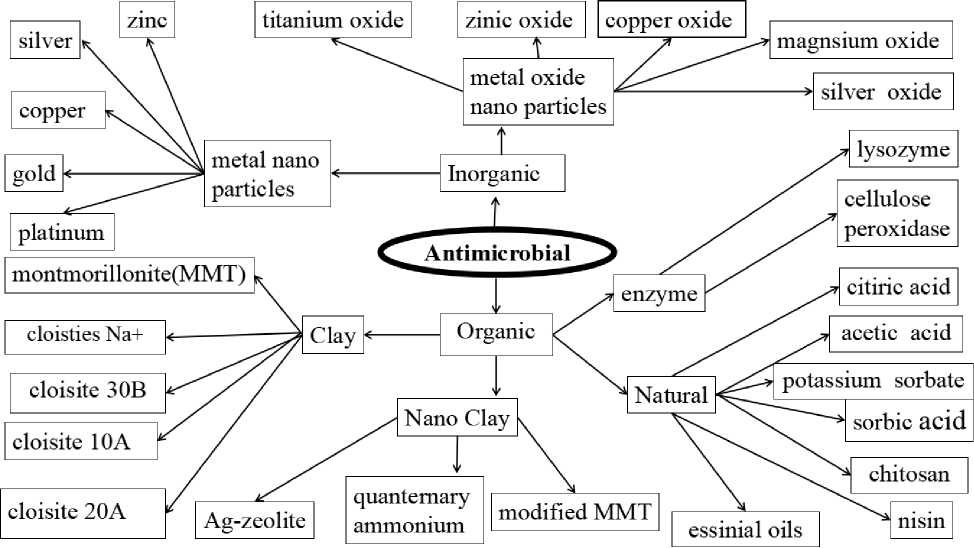
Figure 2. Type of antimicrobial substances/compounds used in food packaging system [39, 40]
Table 1.
Natural antimicrobial compounds and antimicrobial activity
|
Antimicrobial agent |
Coated with |
Antimicrobial activity |
Type of microorganism |
Reference |
|
Chitosan |
Lauric acid in starch film |
reduction |
Subtilis E. coli |
[41] |
|
Ethylene in copolymer films |
2–5 log reduction |
E. coli, L.monocytogenes |
[42] |
|
|
Reduction |
Negative and positive bacteria |
[42] |
||
|
Nisin |
Ally isothiocyanate |
Completely inactive |
Salmonella |
[43] |
|
Poly lactic acid |
Completely inactive |
Listeria monocytogene |
[44] |
|
|
Polyethylene films |
3 to 7 log Reduction |
Listeria monocytogene |
[45] |
|
|
essential oils of mustard and cinnamon |
inhibit spoilage |
Aspergillus flavus, Endomyces fibuliger, |
[46] |
|
|
methanolic extract of pomogranate peel |
inhibition of 10–25 mm |
S. aureus |
[47] |
|
|
grape seed extract |
inhibition |
E. coli |
[48] |
|
|
methanolic extracts dihydroxylated coumarins |
100% inhibition |
T. mentagrophytes and R. solani |
[49] |
|
|
olive leaf extract |
Inhibition |
Candida albicans |
[50] |
-
3. Methods for incorporate antimicrobial compounds and difficulties in packaging systems
-
3.1 Antimicrobial coating methods
-
3.2 Extrusion technologies
Antimicrobial packaging materials are critical for food preservation and safety because they prevent spoilage caused by fungal and bacterial microorganisms [51, 52]. This packaging material has the advantage of increasing shelf-life, dimension microbe growth phases, and protecting foods and preserving their original quality, taste, and protection for an extended period of time [53–55]. This promising packaging method is used in meat, fruits, dairy products, and vegetables [56–58].
Antimicrobial compounds can be mixed into two parts in food packaging systems. The first stage involves direct contact with the food surface (such as foils in this application), and the second involves antimicrobial agents blended into packaging systems (here there is no direct contact the antimicrobial compounds with food) [59–61]. Essential oils, nano metal oxides, chitosan, and nisin, among other antimicrobial agents, can be blended/coated with films or carpeted the surface of food, when the film being ediblE. The movement of the agents to the food in this case is classified as partial or complete migration to the food [62, 63].Casting and extrusion
Мелессе Е. и др. Вестник ВГУИТ, 2022, Т. 84, №. 3, С. 204-213 techniques are the most common routes for antimicrobial substance incorporation among the various types of antimicrobial packaging methods.
Organic and inorganic compounds can be mixed in the coating route for the synthesis of active packaging in food sectors. Among the inorganic nano metal oxides that can be coated to the surface of the food or material covalent or hydrogen bonding interaction are titanium oxide, copper oxide, zinc oxide, silver oxide, magnesium oxides, and nano encapsulation. These nano particles (NPs) depend such as material type [64,65], particle size, shape [66]functionality, hydrophilic-hydrophobic properties and usage concentration [67]. According to the table 2, the application of coating technique has been reported for varies investigation. The PPE/PEE coated at temperature of 75℃with rosemary oil, garlic oil, allylisothiocyanate, and trans-cinnamal-dehyde showed antimicrobial activity against of E. coli, SaL. typhimurium, E. sakazaki, B. cereus [68]. Carboxymethyl cellulose (CMC), agar, carrageenan, coated with ZnO NPs revealed its E. coli and L. monocytogenes activities [69] and agar film coated natamycin reported antimicrobial assay against aspergillosis niger and Saccharomyces cerevisiae in strawberries [70]However, the coating technology faces the following challenges. The main limitations are the change in surface structure of antimicrobial substances, particle aggregation, volatile substances losing their antimicrobial properties during drying, and chemical compatibility with solvents and polymers. The antimicrobial compounds distribution in the package system, indirect contact with the food, and non-aggressive thermal treatment, and it can be good for the synthesis of active packaging systems. Temperature labile antimicrobial substances can be blended through coating routes with little loss of activity in active packaging materials. Other methods of mixing inorganic nano metals in packaging systems, such as chemical/physical deposition, are more priceless and require smart processing equipment. Metal oxides such as Ag 2 O, ZnO, TiO 2 , MgO, and CuO are the most effective surface modification, fiction-alization, and deposition agents [71].
Extrusion is the most common method for incorporating natural extracts or inorganic nano particles into the film surface for packaging applications [72]. Furthermore, this route blends the natural extracts (bio-active compounds) before entering the stage of the processed polymer's melting temperature (inside the extrusion) to ensure uniform distribution in the film. Nonetheless, it faces three challenges: homogeneous dispersion of inorganic metal nano particles in the polymer matrix,
According to table 2, a substantial amount of literature has been published on the incorporation of antimicrobial agents via extrusion. At 160–190 °C, the LLDPE material was extruded with grapefruit seed extract. However, the antimicrobial activity was rendered ineffective [73]. The surface coating is preferably for the natural agents (bio-active compounds), despite of its low temperatures and simple technique, poor adhesion is the main limitation to develop the active packaging systems using the coating techniques [74]. Furthermore, films have been investigated reported from chitosan/essential oil-coated PP [75], cinnamaldehyde, garlic oil and rosemary oil-coated PP/LDPE [68], oregano essential oil and citral-coated PP/EVOH [76], chitosan-coated plastic [77], thyme and oregano-coated LDPE. Interestingly, the active packaging materials synthesized through extrusion route shows more effective against E. coli, Salmonella typhimurium , and L. monocytogenes .
Additionally, the LDPE incorporated with garlic oil extruded with a temperature above 100℃ and the antiviral and antimicrobial activities have been decreased [78]. At this point, the authored reported garlic oil and trans-cinnamaldehyde have excellent antimicrobial activity than allyl isothiocyanate [68]. According to current research, rosemary oleoresin is the least effective naturally derived antimicrobial compound against L. innocua and E. coli in an agar medium when coated onto LDPE/polyamide films compared to trans-cinnamaldehyde, thymol, and carvocrol [79].
Zinc oxide nanoparticles' antimicrobial activities in food packaging applications have been reported in the literaturE. It contained agar, carrageenan, and CMC polymers. It had less of an effect when combined with CMC than when combined with agar and carrageenan. The Gram-positive bacteria has been inhibited by the metal nano particles (example zinc oxide), but it can't surprise for the negative bacteria. This activity of ZnO NPs was also observed in CA films [80]. Gram-positive Staphylococcus aureus bacteria were more inhib-ited/had lower activity than Gram-negative bacteria (E. coli, Citrobacter freundii, and Klebsiella pneumonia). The ZnO mixed with sage starch was also more effective against Gram positive (S. aureus) bacteria than Gram negative bacteria (E. coli) [81]. This happened due to the different cell walls of them. Gram-positive bacteria have a single thick cell wall (referred to as multi-layers of glycopeptide), whereas Gram-negative bacteria have a complex cell wall (thin glycopeptide layer) that is protected by an outer membrane [74,82]. Furthermore, the pores found on the cell wall of Gram-positive bacteria allow zinc oxide to penetrate, causing leakage into the intracellular part and cell death [83]. However, the Gram-negative bacteria's outer cell membrane protects the zinc oxide from penetration and attachment [80]. When compared to other inorganic compounds (especially metal oxides), zinc oxide nano particles have a higher antimicrobial activity (against E. coli, Bacillus atrophaeus, and Salmonella aureus) [84]. When compared to silver oxide, zinc oxide is less expensive and less toxic to humans and animals, making it appealing for food packaging applications (AgNPs) [40].The percentage of agents (antimicrobial materials) determined the affinity of the selective microorganisms to be deactivated, partially inhibited, or completely inhibited. In the strawberry packaging application, a 5wt percent sliver nano particle (Nano-Ag) blend by solvent evaporation performed welL. TiO2 (0-20 wt.%) and - CD-thymol (0-5 wt.%) incorporated with PLA via solvent casting, hot-pres processing, and injection process demonstrated that the produced active nanocomposite packaging films have applications in E. coli and Alternaria alternata, respectively.
Table 2.
Development techniques, antimicrobial compounds and applications
|
Polymer |
Antimicrobial agent |
Percentage (%) |
method |
Temperature |
application |
references |
|
LDPE |
garlic oil |
2,4,6,8w/w |
extrusion |
170 |
[78] |
|
|
PLA |
Waste Orange Peels Extracts |
0.25-2.00% wt |
hot pressing/extrusion |
175, 100 bar |
yellowish color increasing with addition level and anunacceptable browning at the 2% dosage |
[85] |
|
PET and HDPE |
TiO 2 NPs |
extrusion |
260 |
milk |
[86] |
|
|
PLA |
green tea extract (GTE) |
extrusion |
antioxidant activity |
[87] |
||
|
polypropylene polyethylene |
rosemary oil, allyl isothiocyanate, trans-cinnamaldehyde, garlic oil |
0.6-1.2%, v/v |
coat |
75 |
E. coli, SaL. typhimurium, E. sakazaki, B. cereus |
[68] |
|
PLA |
a-tocopherol |
extrusion |
193 |
inhibitor of lipid oxidation of whole milk powder |
[88] |
|
|
HDPE LDPE |
catechin, quercetin and tea extract |
extrusion |
improved their thermal resistance |
[72] |
||
|
CMCagar, carrageenan |
ZnO NPs |
solution casting |
80 |
E. coli and L. monocytogenes |
[69] |
|
|
agar film |
natamycin |
0%, 0.33%, 0.66%, 0.99%, 1.33%, w/w |
solvent casting |
100 |
Aspergillus niger and Saccharomyces cerevisiae |
[70] |
|
PLA |
Nano-Ag |
5wt% |
Solvent evaporation |
Strawberry |
[89] |
|
|
TiO 2 |
0-20 wt.% |
solvent casting and hot-press processing |
E. coli |
|||
|
P-CD-thymol |
0-5 wt.% |
injection process |
Alternaria alternata |
|||
|
cellulose |
chitosan |
solution casting |
||||
|
PHB |
bacterial cellulose nanofibers |
melt compounding technique |
Gram-positive bacteria |
|||
|
cellulosic paper |
chitosan |
dip-coating |
Advantages and challenges of antimicrobial compounds incorporated in composite food packaging systems
TiO2, monolaurin, clove leaf oil, enterocin, and pomegranate peel extract, when combined with chitosan/PVA, cellulose/chitosan, starch, agar, and zein, demonstrated promising results in cheese product packaging applications. Natural extracts of mentha pipetia and bunium percicum garllic acid incorporated with PLA/NC have been used in Protected food (ground beef) applications. According to table 3, natural extract antimicrobial materials are more popular than inorganic (metal and metal oxide nano particles) antimicrobial materials in food packaging applications due to their safety, low cost, non-thermal processing requirements, and edibility.
The advantage of the bio-based nano-composite packaging materials in synthesis of food packaging materials has been reported in the Figure 3.
Table 3.
Type of food, packaging material, and antimicrobial compounds [90]
|
Protected food type |
Polymer |
Antimicrobial material |
|
Cheese |
Chitosan/PVA |
TiO 2 |
|
Cellulose/Chitosan |
Monolaurin |
|
|
Starch |
Clove leaf oil |
|
|
Agar |
Enterocin |
|
|
Zein |
Pomegranate peel extract |
|
|
Peanuts(roasted) |
Banana flour |
Garlic essential oil |
|
Salami |
Whey protein |
Cinnamoum cassia, Rosmarinus officinalis oils |
|
Ham |
Chitosan/starch |
Gallic acid |
|
Rainbow trout fillet |
Chitosan |
Grape seed extract |
|
Cucumber |
Limonene |
|
|
Tomato |
TiO 2 nano particles |
|
|
Poultry |
Ginger oil |
|
|
Strew berries |
Chitosan/CMC, PLA, Gelatin |
Chitosan/citric acid, AgNPs, mentha spicata oil, butylated hydroxyanisole |
|
Shrimps |
Chitosan |
Carvacrol |
|
Gelatin |
ZnO/clove oil |
|
|
Chicken |
Thyme oil |
|
|
Chitosan |
Acerola residue extract |
|
|
Pullulan |
Nisin |
|
|
Crap fillets |
Alginate/CMC |
ZNO/Ziziphora clinopodioides oil |
|
Ground beef |
PLA/NC |
Mentha pipetia, Bunium percicum garllic acid |
|
Salmon |
PLA |
Glycerol monolaurate |
|
Fish |
Thymol |
|
|
Iceberg lettuce |
Cellulose |
Clove and oregano oils |
|
Ostrich meat |
Kefiran/polyurethane |
Zataria multiflora oil |
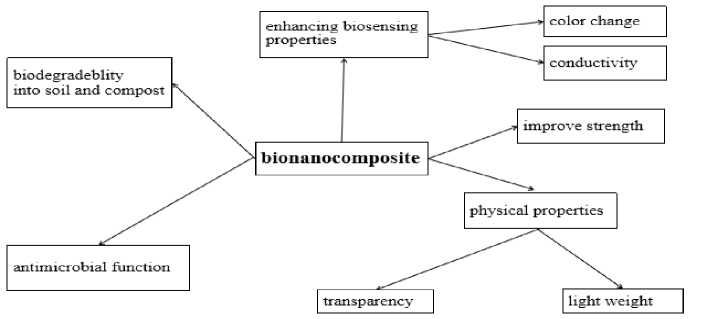
Figure 3. Bio-based nano-composites have an advantage in the synthesis of food packaging systems
The combination of nano particles and polymer is known as nano-composite, and it is a promising material for food packaging [91, 92]. According to some published research, nano particles may migrate from packaging materials (bio nanocomposites) to foods [40].If the nano particle concentration is high, these migration particles may cause rancidity (oxidation of food). Despite the fact that Zn, Ag+ ions, and clay have been transferred from chitosan, polypropylene(pp), and potato starch-based nanobased composites [39, 92], their migration to food is negligible and classified below the quantification limit. However, due to the high concentration transfer of nano particles from food packaging composites, the critical concern at this stage is that these nano particles can migrate into the main parts of the human body (brain, liver, fetus, and spleen), production cost, nano particles migrate to foods and have an environmental concern. The nano particles have a high surface area, which causes a high reaction with heavy metals, implying contamination of the soil and water bodies [40]. Therefore, it is a major challenge to prevent the migration of nano particles from bio nanocomposite materials to food mass when considering smart food packaging.
The most promising antimicrobial agents have eco-friendliness, safety, and lower scented human risk to consumers by reducing food contamination by microorganisms. When compared to conventional composites, the extraction process of bio-active compounds used in food packaging as antimicrobial agents from natural resources is expensive and limited in raw material availability.
Conclusion
Antimicrobial compounds combined with food packaging systems have the potential to keep fresh food for a long timE. The current art of questioning in the food sectors and research areas is to design active packaging system for the purpose of shelf-life and safety of food. Currently, the cost of natural antimicrobial compounds and safety of metallic/oxide nano particles, as well as regulatory concerns, are limiting the production and synthesis of antimicrobial food packaging systems. Because of the low energy intensive, non-thermal requirement, and the agents do not migrate into the food, the coating technique of incorporating antimicrobial compounds into the food packaging composite has received more attention than the extrusion method, and it can be good for developing active packaging materials. Incorporating natural extracts (especially essential oils) with antimicrobial properties into food packaging systems holds more promise than inorganic antimicrobial (such as metal/metal oxide nano particles). When compared to silver oxide, the zinc oxide nano particle is less expensive and less toxic to humans and animals, making it appealing for food packaging services. Researchers and academics can improve coating and extrusion techniques to incorporate natural antimicrobial compounds in polymer film to create an active composite food packaging system and further investigate the health effects of metal nano particles.
Acknowledgment
The authors appreciate for Moscow State University of Food Production.
Список литературы Overall review the current tend and difficulties of antimicrobial compounds in composite food packaging applications
- World Health Organization. WHO model list of essential medicines - 22nd list, 2021. Technical Document 2021.
- Morris M.A., Padmanabhan S.C., Cruz-Romero M.C., Cummins E. et al. Development of active, nanoparticle, antimicrobial technologies for muscle-based packaging applications. Meat Sci. 2017. vol. 132. pp. 163-78. https://doi.org/10.1016/j.meatsci.2017.04.234
- Saravanan A., Kumar P.S., Hemavathy R.V., Jeevanantham S. et al. Methods of detection of food-borne pathogens: a review. Environmental Chemistry Letters. 2021. vol. 19. no. 1. pp. 189-207. https://doi.org/10.1007/s10311-020-01072z
- Nile S. H. et al. Nanotechnologies in food science: applications, recent trends, and future perspectives. Nano-micro letters. 2020. vol. 12. no. 1. pp. 1-34. https://doi.org/10.1007/s40820-020-0383-9
- Alabi O.A., Ologbonjaye K.I., Awosolu O., Alalade O.E. Public and environmental health effects of plastic wastes disposal: a review. J Toxicol Risk Assess. 2019. vol. 5. no. 021. pp. 1-13. https://doi.org/10.23937/2572-4061.1510021
- Hong L.G., Yuhana N.Y., Zawawi E.Z.E. Review of bioplastics as food packaging materials. AIMS Mater Sci. 2021. vol. 8. pp. 166-184. https://doi.org/10.3934/matersci.2021012
- Gutiérrez T.J. Polymers for food applications: News. Polymers for food applications. Springer, Cham, 2018. pp. 1-4. https://doi.org/10.1007/978-3-319-94625-2
- Grönman K., Soukka R., Järvi-Kääriäinen T., Katajajuuri J.M. et al. Framework for sustainable food packaging design. Packaging Technology and Science. 2013. vol. 26. https://doi.org/10.1002/pts.1971
- Jariyasakoolroj P., Leelaphiwat P., Harnkarnsujarit N. Advances in research and development of bioplastic for food packaging. Journal of the Science of Food and Agriculture. 2020. vol. 100. no. 14. pp. 5032-5045. https://doi.org/10.1002/jsfa.9497.
- Debeaufort F. Active biopackaging produced from by‐products and waste from food and marine industries. FEBS Open bio. 2021. vol. 11. no. 4. pp. 984-998. https://doi.org/10.1002/2211-5463.13121
- Arfat Y.A., Ejaz M., Jacob H., Ahmed J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohydrate Polymers. 2017. vol. 157. pp. 65-71. https://doi.org/10.1016/j.carbpol.2016.09.069.
- Siripatrawan U, Kaewklin P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018. vol. 84. https://doi.org/10.1016/j.foodhyd.2018.04.049.
- Nouri A., Yaraki M.T., Ghorbanpour M., Agarwal S. et al. Enhanced Antibacterial effect of chitosan film using Montmorillonite/CuO nanocomposite. International Journal of Biological Macromolecules. 2018. vol. 109. pp. 1219-1231. https://doi.org/10.1016/j.ijbiomac.2017.11.119.
- Salmas C., Giannakas A., Katapodis P., Leontiou A. et al. Development of ZnO/Na-montmorillonite hybrid nanostructures used for PVOH/ZnO/Na-montmorillonite active packaging films preparation via a melt-extrusion process. Nanomaterials. 2020. vol. 10. no. 6. pp. 1079. https://doi.org/10.3390/nano10061079
- Xing Y., Xu Q., Li X., Chen C. et al. Chitosan-based coating with antimicrobial agents: Preparation, property, mechanism, and application effectiveness on fruits and vegetables. Int J Polym Sci. 2016. vol. 2016. https://doi.org/10.1155/2016/4851730.
- Yu H.H., Kim Y.J., Park Y.J., Shin D.M. et al. Application of mixed natural preservatives to improve the quality of vacuum skin packaged beef during refrigerated storage. Meat Sci. 2020. vol. 169. https://doi.org/10.1016/j.meatsci.2020.108219
- Huang T., Qian Y., Wei J., Zhou C. Polymeric Antimicrobial food packaging and its applications. Polymers (Basel). 2019. vol. 11. https://doi.org/10.3390/polym11030560
- Liang S., Wang L. A natural antibacterial-antioxidant film from soy protein isolate incorporated with cortex Phellodendron extract. Polymers (Basel). 2018. vol. 10. https://doi.org/10.3390/polym10010071
- Nguyen T.T., Dao U.T.T., Bui Q.P.T., Bach G.L.et al. Enhanced antimicrobial activities and physiochemical properties of edible film based on chitosan incorporated with Sonneratia caseolaris (L.) Engl. leaf extract. Progress in Organic Coatings. 2020. vol. 140. pp. 105487. https://doi.org/10.1016/j.porgcoat.2019.105487
- Gingasu D., Mindru I., Patron L., Ianculescu A. et al. Synthesis and characterization of chitosan-coated cobalt ferrite nanoparticles and their antimicrobial activity. Journal of Inorganic and Organometallic Polymers and Materials. 2018. vol. 28. no. 5. pp. 1932-1941. https://doi.org/10.1007/s10904-018-0870-3
- Sofi S.A., Singh J., Rafiq S., Ashraf U. et al. A comprehensive review on antimicrobial packaging and its use in food packaging. Current Nutrition & Food Science. 2018. vol. 14. no. 4. pp. 305-312. https://doi.org/10.2174/1573401313666170609095732
- Mirabelli V., Majidi Salehi S., Angiolillo L., Belviso B.D. et al. Enzyme crystals and hydrogel composite membranes as new active food packaging material. Global Challenges. 2018. vol. 2. no. 1. pp. 1700089. https://doi.org/10.1002/gch2.201700089.
- Galante Y.M., Merlini L., Silvetti T., Campia P. et al. Enzyme oxidation of plant galactomannans yielding biomaterials with novel properties and applications, including as delivery systems. Applied microbiology and biotechnology. 2018. vol. 102. no. 11. pp. 4687-4702. https://doi.org/10.1007/s00253-018-9028z
- Avramescu S.M., Butean C., Popa C.V., Ortan A. et al. Edible and functionalized films/coatings-performances and perspectives. Coatings. 2020. vol. 10. https://doi.org/10.3390/coatings10070687
- Saadat S., Pandey G., Tharmavaram M., Braganza V. et al. Nano-interfacial decoration of Halloysite Nanotubes for the development of antimicrobial nanocomposites. Adv Colloid Interface Sci. 2020. vol. 275. https://doi.org/10.1016/j.cis.2019.102063.
- Arsenie L.V., Lacatusu I., Oprea O., Bordei N. et al. Azelaic acid-willow bark extract-panthenol-Loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Industrial Crops and Products. 2020. vol. 154. pp. 112658. https://doi.org/10.1016/j.indcrop.2020.112658.
- Realini C.E., Marcos B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014. vol. 98. https://doi.org/10.1016/j.meatsci.2014.06.031
- Feng K., Wen P., Yang H., Li N. et al. Enhancement of the antimicrobial activity of cinnamon essential oil-loaded electrospun nanofilm by the incorporation of lysozyme. RSC advances. 2017. vol. 7. no. 3. pp. 1572-1580. https://doi.org/10.1039/c6ra25977d.
- He S., Yang Q., Ren X., Zi J. et al. Antimicrobial efficiency of chitosan solutions and coatings incorporated with clove oil and/or ethylenediaminetetraacetate. Journal of Food Safety. 2014. vol. 34. no. 4. pp. 345-352. https://doi.org/10.1111/jfs.12134
- Mulla M., Ahmed J., Al-Attar H., Castro-Aguirre E. et al. Antimicrobial efficacy of clove essential oil infused into chemically modified LLDPE film for chicken meat packaging. Food Control. 2017. vol. 73. pp. 663-671. https://doi.org/10.1016/j.foodcont.2016.09.018
- Radulescu M., Popescu S., Ficai D., Sonmez M. et al. Advances in Drug Delivery Systems, from 0 to 3D superstructures. Curr Drug Targets. 2016. vol. 19. https://doi.org/10.2174/1389450117666160401122926
- Lopes F.A., de Fátima Ferreira Soares N., de Cássia Pires Lopes C., da Silva W.A. et al. Conservation of bakery products through cinnamaldehyde antimicrobial films. Packaging Technology and Science. 2014. vol. 27. https://doi.org/10.1002/pts.2033.
- Mihaly-Cozmuta A., Peter A., Craciun G., Falup A. et al. Preparation and characterization of active cellulose-based papers modified with TiO2, Ag and zeolite nanocomposites for bread packaging application. Cellulose. 2017. vol. 24. https://doi.org/10.1007/s10570-017-1383x
- Al-Naamani L., Dutta J., Dobretsov S. Nanocomposite zinc oxide-chitosan coatings on polyethylene films for extending storage life of okra (Abelmoschus esculentus). Nanomaterials. 2018. vol. 8. https://doi.org/10.3390/nano8070479
- Xing Y., Li X., Guo X., Li W. et al. Effects of different tio2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials. 2020. vol. 10. https://doi.org/10.3390/nano10071365
- Xing Y., Li W., Wang Q., Li X. et al. Antimicrobial nanoparticles incorporated in edible coatings and films for the preservation of fruits and vegetables. Molecules. 2019. vol. 24. https://doi.org/10.3390/molecules24091695
- Sun L., Yang S., Qian X., An X. High-efficacy and long-term antibacterial cellulose material: anchored guanidine polymer via double “click chemistry.” Cellulose. 2020. vol. 27. https://doi.org/10.1007/s10570-020-03374-5
- Jouneghani R.S., Castro A.H.F., Panda S.K., Swennen R. et al. Antimicrobial activity of selected banana cultivars against important human pathogens, including candida biofilm. Foods. 2020. vol. 9. https://doi.org/10.3390/foods9040435.
- Chaudhry Q., Scotter M., Blackburn J., Ross B. et al. Food Additives and Contaminants Applications and implications of nanotechnologies for the food sector. Taylor & Francis. 2008. vol. 25.
- Silvestre C., Duraccio D., Cimmino S. Food packaging based on polymer nanomaterials. Progress in polymer science. 2011. vol. 36. no. 12. pp. 1766-1782. https://doi.org/10.1016/j.progpolymsci.2011.02.003.
- Salleh E., Muhamad I.I. Starch‐based Antimicrobial Films Incorporated with Lauric Acid and Chitosan. AIP Conference Proceedings. American Institute of Physics, 2010. vol. 1217. no. 1. pp. 432-436. https://doi.org/10.1063/1.3377861
- Joerger R.D., Sabesan S., Visioli D., Urian D. et al. Antimicrobial activity of chitosan attached to ethylene copolymer films. Packaging Technology and Science. 2009. vol. 22. https://doi.org/10.1002/pts.822
- Jin T., Gurtler J.B. Inactivation of Salmonella in liquid egg albumen by antimicrobial bottle coatings infused with allyl isothiocyanate, nisin and zinc oxide nanoparticles. J Appl Microbiol. 2011. vol. 110. https://doi.org/10.1111/j. 1365-2672.2011.04938.x
- Jin T. Inactivation of Listeria monocytogenes in Skim Milk and Liquid Egg White by Antimicrobial Bottle Coating with Polylactic Acid and Nisin. J Food Sci. 2010. vol. 75. https://doi.org/10.1111/j. 1750-3841.2009.01480.x
- Makwana S., Choudhary R., Kohli P. Advances in Antimicrobial Food Packaging with Nanotechnology and Natural Antimicrobials. International Journal of Food Science and Nutrition Engineering. 2015. vol. 2015. pp. 169-175. https://doi.org/10.5923/j.food.20150504.02
- Nielsen P. V., Rios R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. International journal of food microbiology. 2000. vol. 60. no. 2-3. pp. 219-229. https://doi.org/10.1016/S0168-1605(00)00343-3
- Dahham S.S., Ali M.N., Tabassum H., Khan M. Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.). Am. Eurasian J. Agric. Environ. Sci. 2010. vol. 9. no. 3. pp. 273-281.
- Nirmala J.G., Narendhirakannan R.T. In vitro antioxidant and antimicrobial activities of grapes (Vitis vinifera L) seed and skin extracts-Muscat variety. Int J Pharm Pharm Sci. 2011. vol. 3. no. 4. pp. 242-249.
- Céspedes C.L., Avila J.G., Martínez A., Serrato B. et al. Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida). J Agric Food Chem. 2006. vol. 54. https://doi.org/10.1021/jf053071w
- Markín D., Duek L., Berdícevsky I. In vitro antimicrobial activity of olive leaves. Mycoses. 2003. vol. 46. https://doi.org/10.1046/j. 1439-0507.2003.00859.x
- Rai M., Ingle A.P., Gupta I., Pandit R. et al. Smart nano packaging for the enhancement of food shelf lifE. Environ Chem Lett. 2019. vol. 17. https://doi.org/10.1007/s10311-018-0794-8
- Vilas C., Mauricio-Iglesias M., García M. R. Model-based design of smart active packaging systems with antimicrobial activity. Food Packaging and Shelf Life. 2020. vol. 24. pp. 100446. https://doi.org/10.1016/j.fpsl.2019.100446
- Szabo K., Teleky B.E., Mitrea L., Călinoiu L.F. et al. Active packaging-poly (vinyl alcohol) films enriched with tomato by-products extract. Coatings. 2020. vol. 10. https://doi.org/10.3390/coatings10020141
- Motelica L., Ficai D., Oprea O.C., Ficai A. et al. Smart food packaging designed by nanotechnological and drug delivery approaches. Coatings. 2020. vol. 10. https://doi.org/10.3390/COATINGS10090806.
- Shruthy R., Jancy S., Preetha R. Cellulose nanoparticles synthesized from potato peel for the development of active packaging film for enhancement of shelf life of raw prawns (Penaeus monodon) during frozen storage. Int J Food Sci Technol. 2021. vol. 56. https://doi.org/10.1111/ijfs.14551
- Ramos M., Beltran A., Fortunati E., Peltzer M.A. et al. Controlled release of thymol from poly (Lactic acid) - based silver nanocomposite films with antibacterial and antioxidant activity. Antioxidants. 2020. vol. 9. https://doi.org/10.3390/antiox9050395.
- Settier-Ramírez L., López-Carballo G., Gavara R., Hernández-Muñoz P. PVOH/protein blend films embedded with lactic acid bacteria and their antilisterial activity in pasteurized milk. Int J Food Microbiol. 2020. vol. 322. https://doi.org/10.1016/j.ijfoodmicro.2020.108545.
- Surendhiran D., Li C., Cui H., Lin L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag Shelf Life. 2020. vol. 23. https://doi.org/10.1016/j.fpsl.2019.100439.
- Pan Y., Xia Q., Xiao H. Cationic polymers with tailored structures for rendering polysaccharide-based materials antimicrobial: An overview. Polymers (Basel). 2019. vol. 11. https://doi.org/10.3390/polym11081283
- Yildirim S., Röcker B., Pettersen M.K., Nilsen-Nygaard J. et al. Active Packaging Applications for Food. Compr Rev Food Sci Food Saf. 2018. vol. 17. https://doi.org/10.1111/1541-4337.12322
- Zhang Z., Wang X., Gao M., Zhao Y. et al. Sustained release of an essential oil by a hybrid cellulose nanofiber foam system. Cellulose. 2020. vol. 27. https://doi.org/10.1007/s10570-019-02957-1
- Vermeiren L., Devlieghere F., Debevere J. Effectiveness of some recent antimicrobial packaging concepts. Food Addit Contam. 2002. vol. 19. https://doi.org/10.1080/02652030110104852
- Brockgreitens J., Abbas A. Responsive Food Packaging: Recent Progress and Technological Prospects. Compr Rev Food Sci Food Saf. 2016. vol. 15. https://doi.org/10.1111/1541-4337.12174
- Ren G., Hu D., Cheng E.W.C., Vargas-Reus M.A. et al. Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents. 2009. vol. 33. https://doi.org/10.1016/j.ijantimicag.2008.12.004
- Ruparelia J.P., Chatterjee A.K., Duttagupta S.P., Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008. vol. 4. https://doi.org/10.1016/j.actbio.2007.11.006
- Wang R.H., Xin J.H., Tao X.M. UV-blocking property of dumbbell-shaped ZnO crystallites on cotton fabrics. Inorg Chem. 2005. vol. 44. https://doi.org/10.1021/ic0503176
- Kim B., Kim D., Cho D., Cho S. Bactericidal effect of TiO2 photocatalyst on selected food-borne pathogenic bacteria. Chemosphere. 2003. vol. 52. https://doi.org/10.1016/S0045-6535(03)00051-1
- Gamage G.R., Park H.J., Kim K.M. Effectiveness of antimicrobial coated oriented polypropylene/polyethylene films in sprout packaging. Food Research International. 2009. vol. 42. https://doi.org/10.1016/j.foodres.2009.03.012
- Kanmani P., Rhim J.W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr Polym. 2014. vol. 106. https://doi.org/10.1016/j.carbpol.2014.02.007
- Wang X.I.I., Song X.J., Zhang D.J., Li Z.J. et al. Preparation and characterization of natamycin-incorporated agar film and its application on preservation of strawberries. Food Packag Shelf Life. 2022. vol. 32. pp. 100863. https://doi.org/10.1016/j.fpsl.2022.100863.
- Iijima M., Kamiya H. Layer-by-layer surface modification of functional nanoparticles for dispersion in organic solvents. Langmuir. 2010. vol. 26. https://doi.org/10.1021/la1030747
- Gómez-Estaca J., López-de-Dicastillo C., Hernández-Muñoz P., Catalá R. et al. Advances in antioxidant active food packaging. Trends Food Sci Technol. 2014. vol. 35. https://doi.org/10.1016/j.tifs.2013.10.008
- Ha J.U., Kim Y.M., Lee D.S. Multilayered antimicrobial polyethylene films applied to the packaging of ground beef. Packaging Technology and Science. 2001. vol. 14. https://doi.org/10.1002/pts.537.
- Solano A.C.V., de Gante C.R. Two Different Processes to Obtain Antimicrobial Packaging Containing Natural Oils. Food Bioproc Tech. 2012. vol. 5. https://doi.org/10.1007/s11947-011-0626-3
- Torlak E., Nizamlioǧlu M. Antimicrobial effectiveness of chitosan-essential oil coated plastic films against foodborne pathogens. Journal of Plastic Film and Sheeting. 2011. voi. 27. https://doi.org/10.1177/8756087911407391
- Muriel-Galet V., Cerisuelo J.P., López-Carballo G., Aucejo S. et al. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Control. 2013. vol. 30. https://doi.org/10.1016/j.foodcont.2012.06.032.
- Ye M., Neetoo H., Chen H. Control of Listeria monocytogenes on ham steaks by antimicrobials incorporated into chitosan-coated plastic films. Food Microbiol. 2008. vol. 25. https://doi.org/10.1016/j.fm.2007.10.014
- Ferrari M.C., Carranza S., Bonnecaze R.T., Tung K.K. et al. Modeling of oxygen scavenging for improved barrier behavior: Blend films. J Memb Sci. 2009. vol. 329. https://doi.org/10.1016/j.memsci.2008.12.030
- Tan C., Han F., Zhang S., Li P. et al. Molecular Sciences Novel Bio-Based Materials and Applications in Antimicrobial Food Packaging: Recent Advances and Future Trends. Int J Mol Sci. 2021. vol. 22. pp. 9663. https://doi.org/10.3390/ijms.
- Anitha S., Brabu B., Thiruvadigal D.J., Gopalakrishnan C. et al. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydrate polymers. 2012. vol. 87. no. 2. pp. 1065-1072. https://doi.org/10.1016/j.carbpol.2012.12.020.
- Gharoy Ahangar E., Abbaspour-Fard M.H., Shahtahmassebi N., Khojastehpour M. et al. Preparation and Characterization of PVA/ZnO NanocompositE. J Food Process Preserv. 2015. vol. 39. https://doi.org/10.1111/jfpp.12363.
- Paisoonsin S., Pornsunthorntawee O., Rujiravanit R. Preparation and characterization of ZnO-deposited DBD plasma-treated PP packaging film with antibacterial activities. Appl Surf Sci. 2013. vol. 273. https://doi.org/10.1016/j.apsusc.2013.03.026
- Li X., Feng, X.Q., Yang S., Fu G.Q. et al. Chitosan kills Escherichia coli through damage to be of cell membrane mechanism. Carbohydrate Polymers. 2010. vol. 79. no. 3. pp. 493-499. https://doi.org/10.1016/j.carbpol.2009.07.011
- Shi L.E. Li Z.H., Zheng W., Zhao Y.F. et al. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review. Food Additives & Contaminants: Part A. 2014. vol. 31. no. 2. pp. 173-186. https://doi.org/10.1080/19440049.2013.865147.
- Bassani A., Montes S., Jubete E., Palenzuela J. et al. Incorporation of waste orange peels extracts into PLA films. Chem Eng Trans. 2019. vol. 74. https://doi.org/10.3303/CET1974178
- Moyssiadi T., Badeka A., Kondyli E., Vakirtzi T. et al. Effect of light transmittance and oxygen permeability of various packaging materials on keeping quality of low-fat pasteurized milk: Chemical and sensorial aspects. Int Dairy J. 2004. vol. 14. https://doi.org/10.1016/j.idairyj.2003.09.001
- Han G., Guo R., Yu Z., Chen G. Progress on biodegradable films for antibacterial food packaging. E3S Web of Conferences. 2020. vol. 145. https://doi.org/10.1051/e3sconf/202014501036
- Byun Y., Kim Y.T., Whiteside S. Characterization of an antioxidant polylactic acid (PLA) film prepared with α-tocopherol, BHT and polyethylene glycol using film cast extruder. J Food Eng. 2010. vol. 100. https://doi.org/10.1016/j.jfoodeng.2010.04.005
- Sobhan A., Muthukumarappan K., Wei L. Biosensors and biopolymer-based nanocomposites for smart food packaging: Challenges and opportunities. Food Package Shelf Life. 2021. vol. 30. https://doi.org/10.1016/j.fpsl.2021.100745
- Motelica L., Ficai D., Ficai A., Oprea O.C. et al. Biodegradable antimicrobial food packaging: Trends and perspectives. Foods. 2020. vol. 9. https://doi.org/10.3390/foods9101438
- Youssef A.M., El-Sayed S.M. Bio nanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr Polym. 2018. vol. 193. https://doi.org/10.1016/j.carbpol.2018.03.088
- Sanuja S., Agalya A., Umapathy M.J. Synthesis and characterization of zinc oxide-neem oil-chitosan bionanocomposite for food packaging application. Int J Biol Macromol. 2015. vol. 74. https://doi.org/10.1016/j.ijbiomac.2014.11.036


