Oxidation of tri( o-tolyl)antimony by tert-butyl hydroperoxide. Molecular structures of bis[µ 2-oxo-tri(o-tolyl)antimony] and µ 2-oxo- bis[( tert-butylperoxy)tri( o-tolyl)antimony]
Автор: Sharutin V.V., Sharutina O.K., Artemeva E.V., Makerova M.S.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Металлоорганическая химия
Статья в выпуске: 4 т.7, 2015 года.
Бесплатный доступ
Tri( o-tolyl)antimony oxidation by equimolar amount of tert-butyl hydroperoxide in diethyl ether led to the formation of bis[µ 2-oxo-tri( o-tolyl)antimony] (1). At the molar ratio of reactants 1:2 or 1:4 µ 2-oxo- bis[( tert-butylperoxy)tri( o-tolyl)antimony] (2) has been formed. According to the X-ray analysis data, antimony atoms are in the trigonal bipyramidal coordination in molecules 1 and 2. The bond lengths Sb-O vary within the ranges 1.937(2)-2.078(2) Å (1) and 1.975(17)-2.216(15) Å (2).
Tri(ortho-tolyl)antimony, tert-butyl hydroperoxide, oxidation, bis[µ2-oxo-tri(o-tolyl)antimony], µ2-oxo-bis[(tert-butylperoxy)tri(o-tolyl)antimony], molecular structures, x-ray analysis
Короткий адрес: https://sciup.org/147160326
IDR: 147160326 | УДК: 546.243+546.245+547.53.024+548.312.5 | DOI: 10.14529/chem150404
Текст научной статьи Oxidation of tri( o-tolyl)antimony by tert-butyl hydroperoxide. Molecular structures of bis[µ 2-oxo-tri(o-tolyl)antimony] and µ 2-oxo- bis[( tert-butylperoxy)tri( o-tolyl)antimony]
Synthesis of bis [ µ 2 -oxo-tri( o -tolyl)antimony] (1). Tri(o-tolyl)antimony (200 mg, 0.50 mmol) was dissolved in diethyl ether (20 mL). Then tert -butyl hydroperoxide (66 mg of 70 % aqueous solution, 0.50 mmol) was added. The solution was left to stand for 24 hours at temperature 20 °С. When the solvent evaporated, colourless cristalline substance 1 was obtained; the product yield was 199 mg (95 %), MP: 216 °C.
IR spectrum (ν, cm - 1): 3048, 2921, 2854, 1584, 1446,1280, 1202, 1160, 1120, 1031,935, 918, 890,764, 750, 740, 655, 636, 491, 471, 435.
Synthesis of µ 2 -oxo- bis [( tert -butylperoxy)tri( o -tolyl)antimony] 2. Tri(o-tolyl)antimony (200 mg, 0.50 mmol) was dissolved in diethyl ether (20 mL). Then tert -butyl hydroperoxide (132 mg of 70 % aqueous solution, 1.00 mmol) was added. The solution was left to stand for 24 hours at temperature 20 °С. Colorless crystals 2 were obtained; yield 230 mg (92 %), MP: 162 °C.
The reaction with the molar ratio 1:4 was carried out at the same conditons. The product yield of substance 2 was 87 %.
IR spectrum of the substunce 1 was recorded on the Bruker Tensor 27 FT-IR (KBr pellets; 4000 - 400 cm - 1).
The X-ray diffraction analyses of crystalline substances 1 and 2 were made on the Bruker D8 QUEST automatic four-circle diffractometer (Mo K α -emission, λ = 0.71073 Å, graphite monochromator). The data were collected and analyzed, the unit cell parameters were refined, and the absorption correction was applied using the SMART and SAINT-Plus programs [16]. All calculations for structure determination and refinement were performed using the SHELXL/PC programs [17]. The structures 1 and 2 were determined by the direct method and refined by the least-squares method in the anisotropic approximation for non-hydrogen atoms.
The main crystallographic data and refinement results for structures 1 and 2 are listed in Table 1. The selected bond lengths and bond angles are given in Table 2.
Table 1
Crystallographic data and the experimental and structure refinement parameters for compound 1
|
Parameter |
Value |
|
|
1 |
2 |
|
|
Empirical formula |
C 42 H 42 O 2 Sb 2 |
C 50 H 60 O 5 Sb 2 |
|
Formula weight |
822.26 |
984.48 |
|
Т , К |
296(2) |
296(2) |
|
Crystal system |
Triclinic |
Triclinic |
|
Space group |
P-1 |
P1 |
|
a , Å |
11.0684(3) |
10.3355(4) |
|
b , Å |
11.1721(3) |
11.0049(5) |
|
c, Å |
17.0248(5) |
11.0848(4) |
|
α , deg |
80.7820(10) |
69.771(2) |
|
β, deg |
86.0600(10) |
84.636(2) |
|
γ , deg |
61.0370(10) |
81.907(2) |
|
V , Å3 |
1818.06(9) |
1169.88(8) |
|
Z |
2 |
1 |
|
ρ (calcd.), g/сm3 |
1.502 |
1.397 |
|
µ , mm–1 |
1.520 |
1.198 |
|
F (000) |
824.0 |
502.0 |
|
Crystal size, mm |
0.17×0.09×0.08 |
0.55×0.38×0.21 |
|
2 θ Range of data collection, deg |
7.38 - 58.28° |
3.98 - 47.5° |
|
Range of refraction indices |
- 15 ≤ h ≤ 15, - 15 ≤ k ≤ 15, - 23 ≤ l ≤ 23 |
- 11 ≤ h ≤ 11, - 12 ≤ k ≤ 12, - 12 ≤ l ≤ 12 |
|
Measured reflections |
32558 |
14775 |
|
Independent reflections |
9014 |
6981 |
|
R int |
0.0480 |
0.0245 |
|
Refinement variables |
421 |
521 |
|
GOOF |
1.030 |
1.159 |
|
R factors for F2 > 2 σ (F2) |
R 1 = 0.0314, wR 2 = 0.0551 |
R 1 = 0.0511, wR 2 = 0.1274 |
|
R factors for all reflections |
R 1 = 0.0564, wR 2 = 0.0611 |
R 1 = 0.0589, wR 2 = 0.1361 |
|
Residual electron density (min/max), e /Å3 |
0.49/ - 0.33 |
1.04/ - 2.17 |
Table 2
Selected bond lengthes and bond angles in the structures of compounds 1 - 2
|
Bond |
d , Å |
Angle \ |
ω , deg |
Bond |
d , Å |
Angle |
ω , deg |
|
1 |
2 |
||||||
|
Sb(1) - Sb(1a) |
3.1409(4) |
O(1a)Sb(1)C(1) |
165.14(10) |
Sb(1) - O(1) |
1.997(17) |
O(1)Sb(1)C(11) |
93.7(9) |
|
Sb(1) - O(1) |
1.9372(18) |
O(1)Sb(1)C(11) |
114.54(10) |
Sb(1) - C(11) |
2.145(14) |
O(1)Sb(1)O(2) |
167.6(6) |
|
Sb(1) - O(1a) |
2.0784(18) |
O(1a)Sb(1)C(11) |
89.40(9) |
Sb(1) - O(2) |
2.143(18) |
O(1)Sb(1)C(21) |
86.7(7) |
|
Sb(1) - C(1) |
2180(3) |
O(1)Sb(1)C(21) |
130.04(10) |
Sb(1) - C(21) |
2.166(17) |
O(1)Sb(1)C(1) |
95.3(8) |
|
Sb(1) - C(11) |
2.135(3) |
C(11)Sb(1) C(1) |
103.30(11) |
Sb(1) - C(1) |
2.18(2) |
С(11)Sb(1)C(21) |
116.3(10) |
Table 2 (end)
|
Bond |
d , Å |
Angle |
го , deg |
Bond |
d , Å |
Angle |
го , deg |
|
1 |
2 |
||||||
|
Sb(1) - C(21) |
2.150(3) |
C(11)Sb(1)C(21) |
111.93(11) |
Sb(2) - O(1) |
1.951(18) |
С(11)Sb(1)C(1) |
124.4(12) |
|
Sb(2) - Sb(2b) |
3.1441(3) |
C(21)Sb(1)Sb(1a) |
110.58(8) |
Sb(2) - C(41) |
2.150(17) |
O(2)Sb(1)C(11) |
87.2(9) |
|
Sb(2) - O(2) |
2.0585(17) |
C(21)Sb(1)C(1) |
96.05(11) |
Sb(2) - C(61) |
2.114(12) |
O(2)Sb(1)C(21) |
81.9(7) |
|
Sb(2) - O(2b) |
1.9473(17) |
O(2b)Sb(2)C(31) |
89.87(9) |
Sb(2) - C(51) |
2.114(12) |
O(2)Sb(1)C(1) |
94.3(8) |
|
Sb(2) - C(31) |
2.186(3) |
O(2)Sb(2)C(31) |
163.65(9) |
Sb(2) - O(4) |
2.129(17) |
С(1)Sb(1)C(21) |
118.9(10) |
|
Sb(2) - C(41) |
2.131(3) |
O(2b)Sb(2)C(41) |
108.70(10) |
О(2) - О(3) |
1.337(17) |
Sb(2)O(1)Sb(1) |
169.2(6) |
|
Sb(2) - C(51) |
2.151(3) |
O(2)Sb(2)C(41) |
92.51(10) |
O(4) - O(5) |
1.356(17) |
C(22)C(21)Sb(1) |
127.0(19) |
|
O(1) - Sb(1a) |
2.0784(18) |
O(2b)Sb(2)C(51) |
130.20(9) |
O(5) - C(35) |
1.51(2) |
C(46)C(41)Sb(2) |
114.2(15) |
|
O(2) - Sb(2b) |
1.9473(17) |
O(2)Sb(2)C(51) |
87.24(9) |
O(3) - C(31) |
1.451(17) |
C(42)C(41)Sb(2) |
124.6(15) |
|
Symmetry relation: a) 1 - x, - y, 2 - z; b) 2 - x, 1 - y, 1 - z |
|||||||
The full tables of atomic coordinates, bond lengths, and bond angles for the substance 1 was deposited with the Cambridge Crystallographic Data Centre (№ 1052677; ; .
Results and Discussion
It has been found that the oxidation of tri( o -tolyl)antimony by tert -butylhydroperoxide at the molar ratio 1:1 in diethyl ether goes with the formation of tri( o -tolyl)antimony oxide with dimeric structure: bis [μ 2 -oxo-tri( o -tolyl)antimony] ( 1 ):
2 ( o -Tol^Sb + 2 t -BuOOH ^ [( o -Tol^SbOh + 2 t -BuOH
According to X-ray diffraction data the crystal of compound 1 contains two types of crystallographically independent molecules ( А , B ). The antimony atoms have intermediate coordination between tri-gonal-bipyramidal and square-pyramidal coordination (Fig. 1).
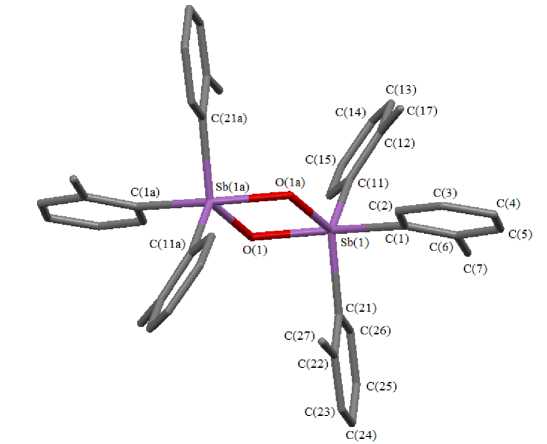
Fig. 1. The structure of compound 1А (hydrogen atoms aren’t shown)
Two carbon atoms of the aryl substituents and μ2-bridging oxygen atom are placed in equatorial plane, the second μ2-bridging oxygen atom and carbon atom are in axial positions. The sum of equatorial OSbC and CSbC angles is 356.57(10)º for А and 350.83(10)º for B. The axial OSbC angles are significantly distorted, they are equal to 165.14(10)° and 163.65(9)°. The OSbO and SbOSb angles in the flat cyclic fragment [Sb2O2] equal 77.14(8)°, 102.86(8)° (А) and 76.62(8)°, 103.13(7)° (B). The Sb-Ceq bond lengths (2.135(3), 2.150(3) А А; 2.131(3), 2151(3) A B) and Sb-Oeq (1.937(2) А А; 1.943(2) A B) are less than Sb-Cax (2.180(3) А А; 2.186(3) A B) and Sb-Oax (2.078(2) А А; 2.058(2) A B). The distances between antimony atoms in the cycle (3.1409(5) (А), 3.1441(3) Å (B)) are considerably less than the double Van der Waals radius of antimony atom (4.4 Å [18]). The о-Tol3Sb fragments in compound 1 are in staggered conformation with respect to each other. Geometrical parameters of complex 1 are close to geometrical parameters of such compounds as (Ph3SbO)2 [19] and [(2-MeOC6H4)3SbO]2 [20].
When the concentration of tert -butylhydroperoxide has increased (1:2 or 1:4) the single organoan-timony product in the reaction mixture is μ 2 -oxo- bis [( tert -butylperoxo)tri( o -tolyl)antimony] ( 2 ), the product yield is 92 %:
2 ( o -Tol^Sb + 4 t -BuOOH ^ [( o -Tol^SbOOBu- t ]2O + 2 t -BuOH + H2O
The coordination polyhedron of antimony atoms in binuclear molecule 2 is an insignificantly distorted trigonal bipyramid (Fig. 2). The bipyramid distortion is characterized by deflection of Sb(1) and Sb(2) atoms from their respective equatorial planes by 0.02 Å and 0.08 Å to the direction of the bridging oxygen atom O(1), which leads to angle deviation between axial and equatorial bonds from the theoretical value 90 ° . The axial OSb(1,2)O angles are equal to 167.6(6) ° and 159.5(5) ° .
The equatorial CSb(1,2)C angles are changed in the range of 116.3(8) °- 124.4(12) ° . The Sb(1)O(1)Sb(2) angle is 169.2(6) ° . The SbOSb fragment has linear structure in the centrosymmetric molecule of μ 2 -oxo- bis [( tert -butylperoxo)triphenylantimony] [21].
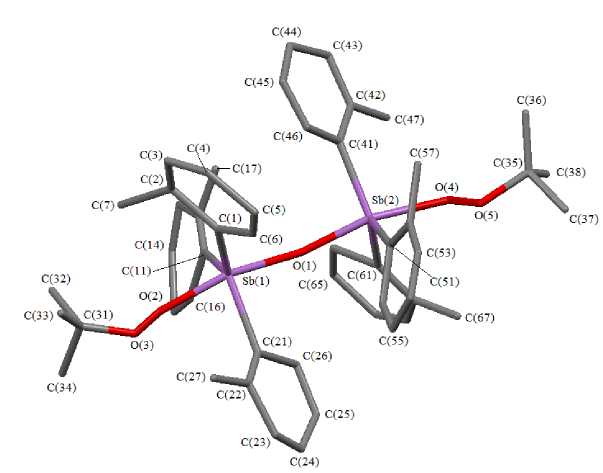
Fig. 2. The structure of compound 2 (hydrogen atoms aren’t shown)
The equatorial bonds Sb(1)-Ceq and Sb(2)-Ceq are changed in the range of 2.14(1) - 2.18(2) A and 2.10(1) - 2.15(1) A.
The Sb(1,2)–О(1) distances are equal to 1.997(7) and 1.951(18) Å, and they are less than terminal distances Sb(1)–О(2) (2.143(18) Å) and Sb(2)–О(4) (2.129(17) Å) like in the molecule of μ 2 - oxo- bis [( tert -butylperoxo)triphenylantimony].
Conclusions
Thus, tert -butylhydroperoxide oxidizes tri( o -tolyl)antimony at the molar ratio of the reactants 1:1 into tri( o -tolyl)antimony oxide, which dimerizes into bis [μ 2 -oxo-tri( o -tolyl)antimony]. With tert butylhydroperoxide in excess (1:2 and 1:4) the reaction proceeds with the formation of the single orga-noantimony compound: q 2-oxo- bis [( tert -butylperoxo)tri( o -tolyl) antimony].
Список литературы Oxidation of tri( o-tolyl)antimony by tert-butyl hydroperoxide. Molecular structures of bis[µ 2-oxo-tri(o-tolyl)antimony] and µ 2-oxo- bis[( tert-butylperoxy)tri( o-tolyl)antimony]
- Bhattacharya, S.N. Oxidative Addition Reactions of Triarylarsines and Triarylstibines with Copper (II) and Mercury(II) Salts/S.N. Bhattacharya, M. Singh//Indian J. Chem. -1979. -V. 18A, N. 6. -P. 515-516.
- Metal Derivatives of Organoantimony Compounds; Reactions of Anhydrous Ferric Chloride with Arylantimony Compounds/H.K. Sharma, S. Singh, S.N. Dubey, D.M. Puri//Indian J. Chem. -1982. -V. 21A, N. 6. -P. 619-621.
- Alberola, A. The Reaction of p-Quinones with Triphenylstibine/A. Alberola, A.M. Gonzaleer, F.G. Pulido//Rev.Roum. Chim. -1984. -V. 29, N. 5. -P. 441-446.
- Oxidative Addition Reaction of o-Quinones to Triphenylantimony. Novel Triphenylantimony Catecholate Complexes/V.K. Cherkasov, E.V. Grunova, A.I. Poddel’sky et al.//J. Organomet. Chem. -2005. -V. 690. -N. 5. -P. 1273-1281.
- Goel, R.G. Organoantimony Сompounds/R.G. Goel, D.R. Ridlej//J. Organomet. Chem. -1979. -V. 182. -N. 2. -P. 207-212.
- Bajpai, K. Synthesis and Reactions of o-Triorganoantimony Dioximates/K. Bajpai, R.S. Srivastava//Synth. Inorg. Met.-Org. Chem. -1981. -V. 11, N. 1. -P. 7-13.
- Chang, M.-M.Y. Some New Organoantimony (V) Compounds/M.-M.Y. Chang, S. Kai, J.I. Musher//Isr. J. Chem. -1974. -V. 12, N. 5. -P. 967-970.
- Domagala, M. Triorganoantimon-und Triorganobismutderivate von Carbonsauren funfgliedriger Heterocyclen Kristall-und Molekulstruktur von (C6H5)3Sb(O2C-2-C4H3S)2 und (CH3)3Sb(O2C-2-C4H3S)2/M. Domagala, F. Huber, H. Preut//Z. Anorg. Allg. Chem. -1989. -Bd. 574. -S. 130-142.
- Domagala, M. Triorganoantimon-und Triorganobismutderivate von 2-Pyridincarbonsaure und 2-Pyridinlessigsaure. Kristall-und Molekulstrukturen von Ph3Sb(O2C-2-C5H4N)2 und Me3Sb(O2CCH2-2-C5H4N)2/M. Domagala, F. Huber, H. Preut//Z. Anorg. Allg. Chem. -1990. -Bd. 582. -S. 37-50.
- Ruther, R. Triorganoantimon-und Triorganobismutdisulfonate Kristall-und Molekulstrukturen von (C6H5)3M(O3SC6H5)2 (M=Sb, Bi)/R. Ruther, F. Huber, H. Preut//Z. Anorg. Allg. Chem. -1986. -Bd. 539. -S. 110-126.
- Westhoff, T. Syntesis of Tris(2,4,6-trimetylphenyl)hydroxoantimony Carboxylates. Crystall Structure of Tris(2,4,6-trimetylphenyl)hydroxoantimony 1-Adamantylcarboxylate/T. Westhoff, F. Huber, H. Preut//J. Organomet. Chem. -1988. -V. 348, N. 2. -P. 185-191.
- Hiatt, R. The Reaction of Hydroperoxides with Triphenylarsine and Triphenylstibine/R. Hiatt, C. McColeman, G.R. Howe//Canad. J. Chem. -1975. -V. 53, N. 4. -P. 559-563.
- Термохимия реакции трифенилфосфора, -мышьяка и -сурьмы с гидроперекисью третичного бутила/В.Г. Цветков, Ю.А. Александров, В.Н. Глушакова и др.//Журн. общ. химии. -1980. -T. 50. -№ 2. -С. 256-258.
- Reactions of Organometallic Compounds with Organic peroxides/G.A. Razuvaev, V.A. Shushunov, V.A. Dodonov, T.G. Brilkina//Organic Peroxides. -N.Y.: J. Willey and Sons. -1972. -V. 3. -P. 141-270.
- Пероксидные соединения трифенилсурьмы, их синтез и строение/И.Е. Покровская, В.А. Додонов, З.А. Старикова и др.//Журн. общ. химии. -1981. -T. 51, № 6. -С. 1247-1253.
- Bruker (2000) SMART. Bruker Molecular Analysis Research Tool, Versions 5.625 Bruker AXS, Madison, Wisconsin, USA.
- Bruker (2000) SAINTPlus Data Reduction and Correction Program Versions 6.02a, Bruker AXS, Madison, Wisconsin, USA.
- Бaцaнов, С.С. Атомные радиусы элементов/С.С. Бацанов//Журн. неорган. химии. -1991. -T. 36, № 12. -С. 3015-3037.
- Bordner, J. Crystal Structure of 2,2,2,4,4,4-Tetrahydro-2,2,2,4,4,4-hexaphenyl-1,3,2,4-dioxadistibetane (Triphenylstibine Oxide Dimer) and Related Compaunds/J. Bordner, G.O. Doak, T.S. Everett//J. Am. Chem. Soc. -1986. -V. 108, N. 14. -P. 4206-4213.
- Diverse Structures and Remarkable Oxidizing Ability of Triarylbismuthane Oxides. Comparative Study on the Structure and Reactivity of a Series of Triarylpnictogen Oxides/Y. Matano, H. Nomura, T. Hisanaga et al.//Organometallics. -2004. -V. 23, N. 23. -P. 5471-5480.
- Кристаллическая и молекулярная структура окса-бис(трет.бутилперокситрифенилсурьмы)/З.А. Старикова, Т.М. Щеголева, В.К. Трунов и др.//Кристаллография. -1978. -Т. 23, № 5. -С. 969-973.

![Oxidation of tri( o-tolyl)antimony by tert-butyl hydroperoxide. Molecular structures of bis[µ 2-oxo-tri(o-tolyl)antimony] and µ 2-oxo- bis[( tert-butylperoxy)tri( o-tolyl)antimony] Oxidation of tri( o-tolyl)antimony by tert-butyl hydroperoxide. Molecular structures of bis[µ 2-oxo-tri(o-tolyl)antimony] and µ 2-oxo- bis[( tert-butylperoxy)tri( o-tolyl)antimony]](/file/cover/147160326/oxidation-of-tri-o-tolyl-antimony-by-tert-butyl-hydroperoxide-molecular.png)
