Oxidative stress markers and antioxidant potential of wheat treated with phytohormones under salinity stress
Автор: Barakat Nasser A.M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.7, 2011 года.
Бесплатный доступ
The interactive effects 0.5 mM indole acetic acid or 0.1 mM of salicylic acid as shoot spraying on NaCl wheat stressed plant organs (spike, shoot and root) grown in pot experiment under different salinity levels (0, 50, 100, 150 and 200 mM NaCl) were studied. The antioxidant enzymes as catalase, peroxidase and ascorbate peroxidase, photosynthetic pigments, reducing sugar, proteins, amino acids, and proline contents in spike, shoot and root of salinity stressed plants were the most affected parameters specially at high salinity levels (150-200 mM NaCl).Treatments with 0.5 mM indole acetic acid or 0.1 mM of salicylic acid as shoot spraying on NaCl wheat stressed plant organs mitigated the harmful effect of NaCl. To conclude the phytohormone acetic acid or salicylic acid salt tolerance in stressed wheat by significantly catalase, peroxidase, and ascorbate peroxidase, increased photosynthetic pigments and the accumulation of nontoxic metabolites (sugars, proteins, amino acid and free proline) as a protective adaptation mechanismin different wheat organs. However, the magnitude of increase was more pronounced in salicylic acid treated plants than in indole acetic acid treated ones, and the spike was more accumulator organ of non toxic metabolites compared to shoot and root. salicylic acid and/or indole acetic acid treatments prevents the deleterious effects of salinity stressed wheat and could be adopted as a potential growth regulator or antioxidant to improve growth particularly under moderate NaCl salinity levels, wheat plant respond positively to SA foliar application than IAA application.
Antioxidant enzymes, photosynthetic pigments, metabolic processes, indole acetic acid, salicylic acid, salinity, triticum
Короткий адрес: https://sciup.org/14323556
IDR: 14323556
Текст научной статьи Oxidative stress markers and antioxidant potential of wheat treated with phytohormones under salinity stress
List of abbreviations: A.A. : amino acids. ANOVA: analysis of variance. APX: ascorbate. CAT: catalase enzyme. Chl a: chlorophyll a. Chl b : chlorophyll b. d.m. : dry mass. f.m. : fresh mass. Hor*sal* : hormoes * salinity levels. IAA: indole acetic acid. POD : Peroxidase. Prol. : prolines. Prot.: proteins. R.S. : reducing sugars. SA : salicylic acid
Most of the crop species as wheat are glycophytes, and generally show limited growth and development due to soil salinity which mostly sodium and chloride that adversely diminishing the growth, many physiological, metabolic and eventually the yield of many plant species (Ashraf and Harris 2004). However, with increasing amounts of arable and undergoing salinization approximately 100 million ha-1 of the worldwide land has been adversely affected by salinity (Ghassemi et al. 1995) and increasing food demand from the growing human population, there is a need to ameliorate the harmful effect of salinity using various strategies (Szabolcs 1994).
Some researchers, (Antoline and Sauchez-Dais 1992) reported that in alfalfa soluble sugars, proteins and total free amino acids including proline were progressively accumulated as NaCl level increased, (Charparzadeh et al . 2004) in Calendula , reported that high salinity caused reduction in growth, lipid peroxidation and hydrogen peroxide accumulation and (Hassanein et al . 2009) in stressed wheat, found reduction in Chl a , b , carotenoids and total pigments. Glycophytic species employ different strategies to withstand salinity stress. The increase in salt resistance may involve protection of cell and organelle membranes (Mansour 2004), and accumulation of some protector components (Mansour 2000). One of the biochemical changes occurring in plants subjected to environmental stress conditions is the production of reactive oxygen species (ROS) (Farooq and Azam 2006).
Reactive oxygen species (ROS) attack proteins, lipids and nucleic acids, and the degree of damage depends on the balance between formation of ROS and its removal by the antioxidative scavenging systems and it appears to represent an important stress-tolerance trait. So, in the absence of any protective mechanism, plants can seriously disturb normal metabolism through oxidative damage toward pigments, lipids, proteins and nucleic acids (Molassiotis et al . 2006; Noreen and Ashraf. 2009).
Many reports indicated that antioxidant could be used as a potential growth regulator to improve salinity stress resistance in several plant species
(Shalata and Peter 2001, Gunes et al. 2007; Khan 2006). In order to avoid the harmful effects of ROS, plants evolved an effective scavenging system composed of enzymatic antioxidants, such as peroxidase (POD, EC1.11.1.7), catalase (CAT, EC 1.11.16) (Hajiboland and Hasani 2007), ascorabate (APX, EC: 1.11.1.11) (Sheteawi 2007) and others. Therefore, these enzymes are very good biochemical markers of stress and their increased activity may attest to a potential for remediation (Vangronsveld and Clijstrs 1994). Several studies, however, indicated that levels of ROS in plant cells are normally protective by antioxidant activity. Association between saline environment and endogenous level of water soluble antioxidant enzymes has been reported (Foyer et al . 1993; Tsugane et al. 1999; Sheteawi 2007). Phytohormones such as indole acetic acid, salicylic acid and others may act as modulator by suppressing or enhancing the stress responses of plants (Popova et al . 1995).
During the last 20 years, Phytohormones, drew the attention of researchers due to its ability to induce systemic acquired resistance (SAR) in plants to different stress types (Levent Tuna et al. 2007). Recently, considerable interest has been aroused by the ability of indole acetic acid, salicylic acid and others as modulator by suppressing or enhancing the stress responses of plants (Popova et al . 1995). However, little information seems to be available on the relationship between salinity stress and auxin levels in plants and the role of auxin in alleviating salt stress.
It has been reported that the exogenous application of IAA showed high stimulatory effects on the root and shoot growth of wheat seedling in saline condition (Egamberdieva 2009). Therefore, the reduction in plant growth under salinity stress conditions could be an outcome of altered hormonal balance. Hence, IAA exogenous application provides an attractive approach to counter the stress conditions (Javid et al. 2011).
Salicylic acid (SA) is an endogenous growth regulator of phenolic nature, which participates in the regulation of physiological processes in plants (Hayat et al . 2010) and also provides protection against biotic and abiotic stresses such as salinity (Kaya et al . 2002). The role of SA in defense mechanism to alleviate salt stress in plants was studied (Afzal et al . 2006; Hussein et al . 2007). The ameliorative effects of SA have been well documented including salt tolerance in many crops such as bean (Azooz 2009), tomato (Tari et al . 2002) and maize (Gunes et al . 2007).
The aim of the current work was to investigate the ameliorative effect of foliar application of Indole acetic acid and salicylic acid on antioxidant enzymes, photosynthetic apparatus and metabolic constituents of salt stressed wheat.
MATERIALS AND METHODS
Plant material and experimental design:
Wheat ( Triticum aestivum L. Giza 186) was obtained from breeding program of seeds center, Beni Suef, Egypt. Wheat grains were surface sterilized by immersion in a mixture of ethanol 96% and H 2 O 2 (1:1) for 3 times, followed by several washings with sterile distilled water. Ten seeds were sown per pots. Each pot contained 2 kg of clay soil. All forty five pots (3 treatments X 5 levels X 3 replicates) were irrigated with normal tap water weekly basis to achieve soil water field capacity for three weeks. After that pots were randomly classified into three treatment groups with three replicate each.
Treatment sets were as follows:
-
I. Control and salt treatments: (reference group).
-
a. (0.0 NaCL). Control.
-
b. 50 mM NaCl.
-
c. 100 mM NaCl.
-
d. 150 mM NaCl.
-
e. 200 mM NaCL.
-
II. Indole acetic acid and salt treatments (IAA treatment): in this group treated plants were sprayed once a time with 0.5 mM IAA.
0.5 mM IAA+0.0 mM NaCL.
0.5 mM IAA+50 mM NaCL .
0.5 mM IAA+100 mM NaCL.
0.5 mM IAA+150 mM NaCL.
0.5 mM IAA+200 mM NaCL.
-
III. Salicylic acid and salt treatments (SA treatment): in this group treated plants were sprayed once a time with 0.1 mM SA.
0.1 mM SA+0.0 mM NaCL.
0.1 mM IAA+50 mM NaCL.
0.1 mM IAA+100 mM NaCL.
0.1 mM IAA+150 mM NaCL.
0.1 mM IAA+200 mM NaCL.
Plants of the three groups were harvested after 70 days and divided into spike, shoot and root, dried in an oven at 70 0C to constant mass to make plant extract for chemical constituent. The chlorophyll a, b and carotenoids of fresh leaves were determined using the spectrophotometeric method of (Metzner et al . 1965). Reducing sugars were determined by anthrone-sulphuric method (Fales 1951). Soluble proteins, amino acids and proline were determined according to Lowery et al . 1951; Moore and Stein 1948 ; Bates et al . 1973, respectively.
Enzyme extraction and assays for antioxidant enzyme activities
Enzyme extraction was carried out as described by Mukherjee and Choudhuri (1983). Catalase (EC 1.11.1.6) activity was assayed in a reaction solution
(3 ml) composed of phosphate buffer (50 mM, at pH 7), 30% (w/v) H 2 O 2 and 0.5 ml enzyme extract (Aebi 1984). The activity of catalase was estimated by the decrease of absorbance at 240 nm as a consequence of H 2 O 2 consumption and was expressed according to (Havir and Mellare 1987) as units per mg of proteins (unite = change in 0.1 absorbance min-1 mg-1 protein) . Total peroxidase (EC 1.11.1.6) was determined using guaiacol reaction solution containing 10 mM (KH 2 PO 4 ) at pH 7, 10 mM H 2 O 2 , 20 mM guaiacol and 0.5 ml crude extract in 3 ml (Maehly and Chance 1954). The
Barakat
Barakat 254
increase in absorbance due to formation of tetraguaiacol was recorded at 470 nm (Klapheck et al . 1990). The ascorbate peroxidase activity (APX, EC: 1.11.1.11) was determined from decrease in absorbance of ascorbic at 290 nm (Asada and Chen 1992). The enzymes activity was assayed in 3 ml containing 50 mM phosphate buffer (pH 7), 0.5mM ascorbic acid and 0.5 mM H 2 O 2 . The reaction was started by addition of H 2 O 2 .
Statistical analysis
Experimental data were subjected to analysis of variance using MINITAB software (version 14).
Table 1. Analysis of variance for antioxidant enzymes, photosynthetic pigments, some metabolic constituents, for spike, shoot and root of wheat plant.
|
Source |
Hormones |
Salinity |
Hor.*Sal. |
|
Catalase |
0.0001*** |
0.0001*** |
|
|
Peroxidase |
0.0001*** |
0.0061*** |
0.0001*** |
|
Ascorbate |
0.0001*** |
0.0001*** |
0.0005*** |
|
Chl a. |
0.0001*** |
0.0001*** |
0.0001*** |
|
Chl b. |
0.0001*** |
0.0001*** |
0.0001*** |
|
Carotene |
0.0001*** |
0.0004*** |
0.0001*** |
|
R.S. Spike |
0.000*** |
0.000*** |
0.000*** |
|
R.S. Shoot |
0.000*** |
0.000*** |
0.000*** |
|
R.S. Root |
0.000*** |
0.000*** |
0.000*** |
|
Prot. Spike |
0.000*** |
0.000*** |
0.000*** |
|
Prot. Shoot |
0.000*** |
0.000*** |
0.000*** |
|
Prot. Root |
0.000*** |
0.000*** |
0.000*** |
|
A.A. Spike |
0.000*** |
0.000*** |
0.000*** |
|
A.A. Shoot |
0.000*** |
0.000*** |
0.000*** |
|
A.A. Root |
0.000*** |
0.000*** |
0.000*** |
|
Prol. Spike |
0.000*** |
0.000*** |
0.000*** |
|
Prol. Shoot |
0.000*** |
0.000*** |
0.000*** |
|
Prol. Root |
0.000*** |
0.000*** |
0.000*** |
(***) significance at P<0.001
Table 2. Correlation between Salinity or Salinity andIAA and Salinity and SA with antioxidant, photosynthetic pigments, and some metabolites.
|
Treat. |
Salinity |
0.5 mM IAA+salinity |
0.1mM SA+salinity |
|
CAT. |
.765(**) |
-0.339 |
-.638(*) |
|
POD. |
.865(**) |
-.673(**) |
-.893(**) |
|
APX. |
0.468 |
-0.09 |
-0.068 |
|
Chl a |
.641(*) |
-.518(*) |
-.762(**) |
|
Chl b |
0.479 |
-0.393 |
-0.165 |
|
Carotenoid |
.520(*) |
-0.193 |
-.728(**) |
|
R.S. spike |
-.674(**) |
-.796(**) |
.805(**) |
|
R.S. shoot |
-.937(**) |
-.675(**) |
-.919(**) |
|
R.S. root |
0.221 |
.873(**) |
0.209 |
|
Prot. spike |
.880(**) |
.885(**) |
-.776(**) |
|
Prot. shoot |
-.878(**) |
-.820(**) |
0.036 |
|
Prot. root |
-.852(**) |
-.854(**) |
.577(*) |
|
A.A. spike |
-0.428 |
.856(**) |
-0.362 |
|
A.A. shoot |
0.214 |
.863(**) |
-0.154 |
|
A.A. root |
-.889(**) |
-.719(**) |
-0.285 |
|
Prol. spike |
.518(*) |
-.554(*) |
.640(*) |
|
Prol.shoot |
-0.486 |
0.022 |
0.416 |
|
Prol.root |
-.898(**) |
.755(**) |
-0.34 |
* Correlation is significant at the 0.05 level (2-tailed).
** Correlation is significant at the 0.01 level (2-tailed).
RESULTS
(a ) Antioxidant enzymes
CAT activity
Data in the table 1 showed that, phytohormones, salinity and their interactions significantly affected the studied antioxidant enzyme. Catalase activity ( P =0.0001). The salinity induced a marked increase in CAT activity in wheat plants, especially at high salinity level (150 and200 mM NaCL). Treatments with IAA in most salinization levels resulted in a pronounced stimulation of CAT activity as compared with only NaCl (Reference group) especially at lower salinity levels (50 mM NaCL). The same was true for plants sprayed with SA but at moderate salinity levels 100 mM NaCL) compared with untreated plants (0.0 Salinity), (Figure 1).
POD activity
Phytohormones, salinity and their interaction significantly affected peroxidase activity (Table 1). Salinity stress resulted a marked increase in POD activity of wheat plants, as compared with control plants. Spraying wheat plants with any of the two hormones result a marked increase in POD activity which was more prominent in plant treated with SA than those treated with IAA especially at no and low salinity levels ( 0.5 mM IAA+0.0 mM NaCL, and 0.5 mM IAA+ 50 mM NaCL, respectively., (Figure 1).
APX activity
Phytohormones, salinity and their interaction significantly affected ascorbate peroxidae activity ( P = 0.0005) (Table 1). Ascorbate peroxidase activity of salinized wheat plant was increase as compared with untreated plants (Figure 1). The highest value of APX activity (0.84 units per mg of proteins ) was recorded at the lower salinity levels with 0.1 mM SA was about 4-times than control plants (0.22 units per mg of proteins). Adding any of the two growth regulators resulted a considerable increase in APX activity in wheat plants. It is worthy to mention that
SA and IAA treatments induced this activation only at lower salinity levels (50 mM) as compared with control plants.
The correlation coefficient computed for the data revealed that among the antioxidant defense system, Peroxidase positively correlated with salinity, negatively with Salinity and IAA as well as salinity and SA whereas, Ascorbate non significant correlated with neither salinity, salinity and IAA nor salinity and SA. CAT positively correlated with salinity, negatively with salinity and SA, and no significant correlation with salinity and IAA (Table 2).
From the present data it was observed that among the antioxidant defense system POD was the higher, followed by CAT and APX was the lower. On the other hand, In spite of both selected phytohormones SA and IAA significantly diminish the effect of salinity, SA as phytohormone was more affective than IAA.
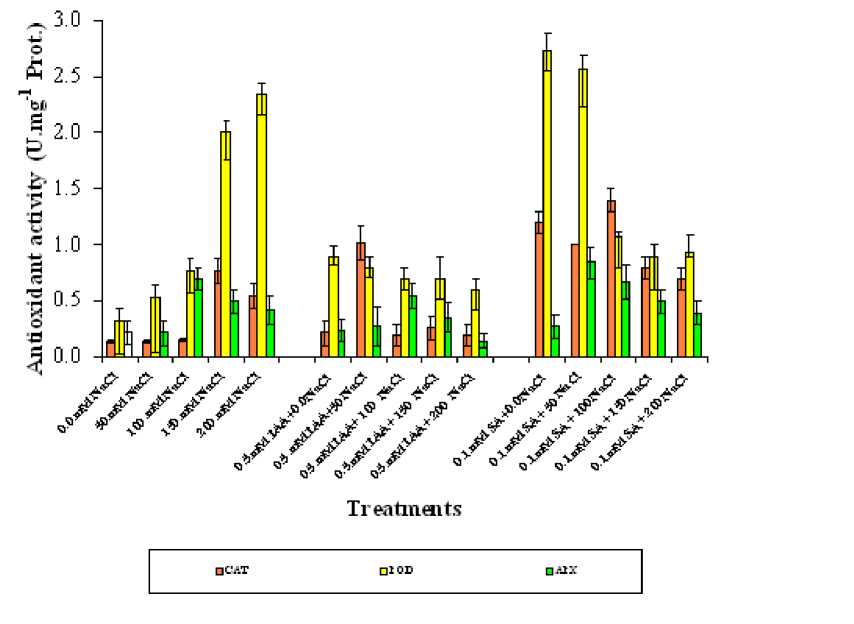
Figure 1. Effect of different salinity levels, combined effect of IAA and salinity or combined effect of SA and salinity on antioxidant activity of wheat. Vertical bars represent Standard deviation.
-
(b) Photosynthetic Pigments:
ANOVA table (1) showed that all treatments were highly significant affected both Chl a, b and carotenoid (P=0.000). These photosynthetic pigments tend to increase with increasing salinity.
Treatments of salinized wheat plant with either SA or IAA significantly increased the production of photosynthetic pigments in order of Chl a > chl b > carotenoids as compared with control plant. (Figure 2).
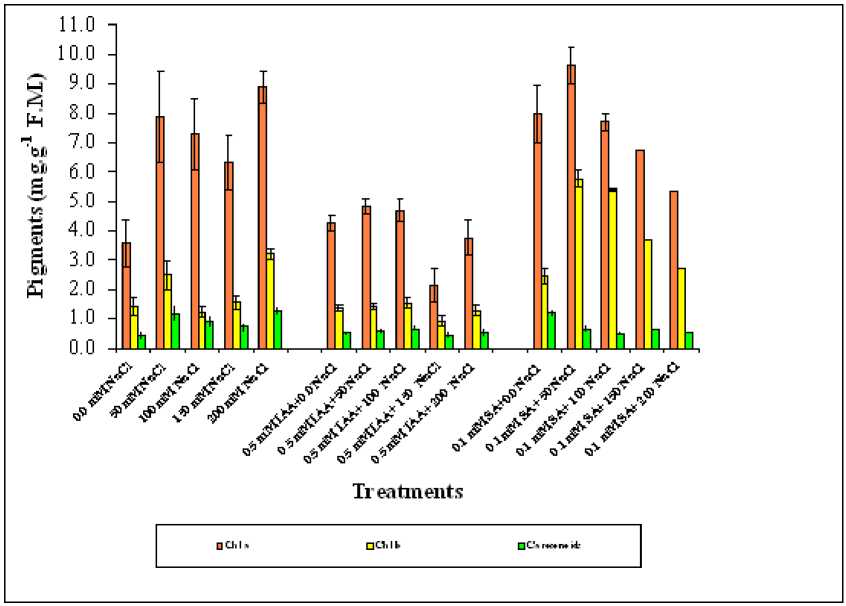
Figure 2. Effect of different salinity levels, combined effect of IAA and salinity or combined effect of SA and salinity on photosynthetic pigments of wheat. Vertical bars represent Standard deviation.
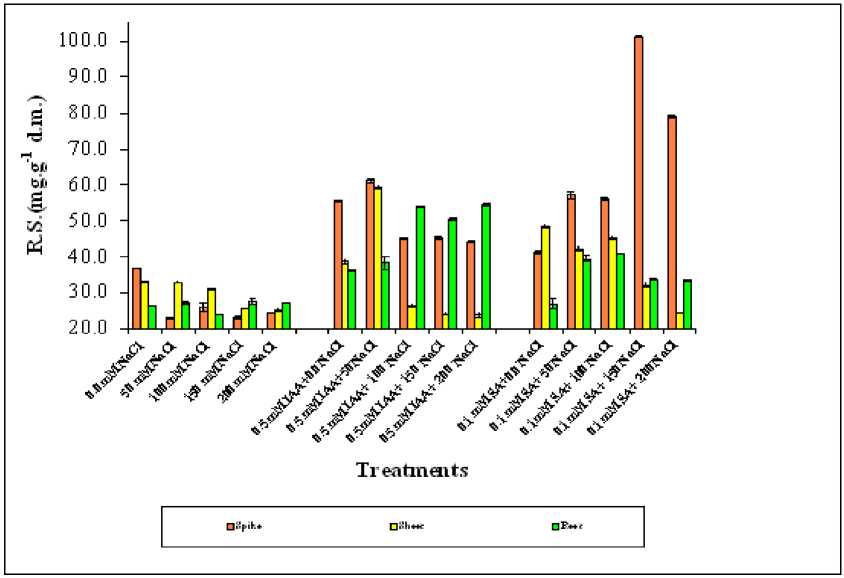
Figure 3. Effect of different salinity levels, combined effect of IAA and salinity or combined effect of SA and salinity on reducing sugar accumulation of different wheat organs. Vertical bars represent Standard deviation.
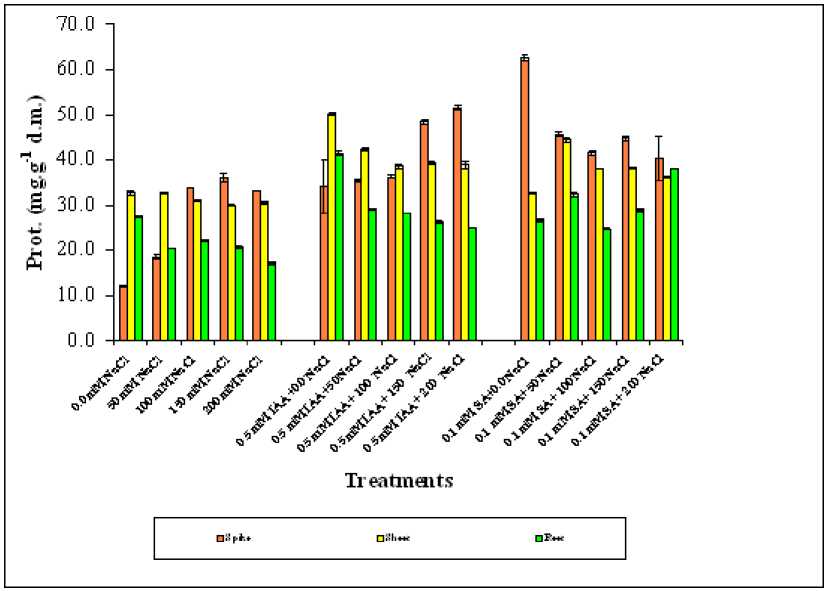
Figure 4. Effect of different salinity levels, combined effect of IAA and salinity or combined effect of SA and salinity on soluble proteins accumulation of different wheat organs. Vertical bars represent Standard deviation.
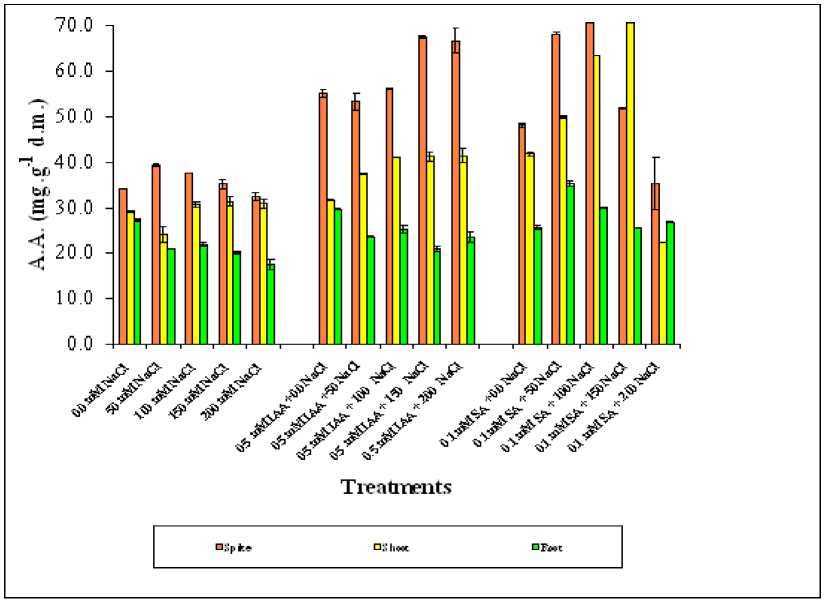
Figure 5. Effect of different salinity levels, combined effect of IAA and salinity or combined effect of SA and salinity on soluble amino acid accumulation of different wheat organs. Vertical bars represent Standard deviation.
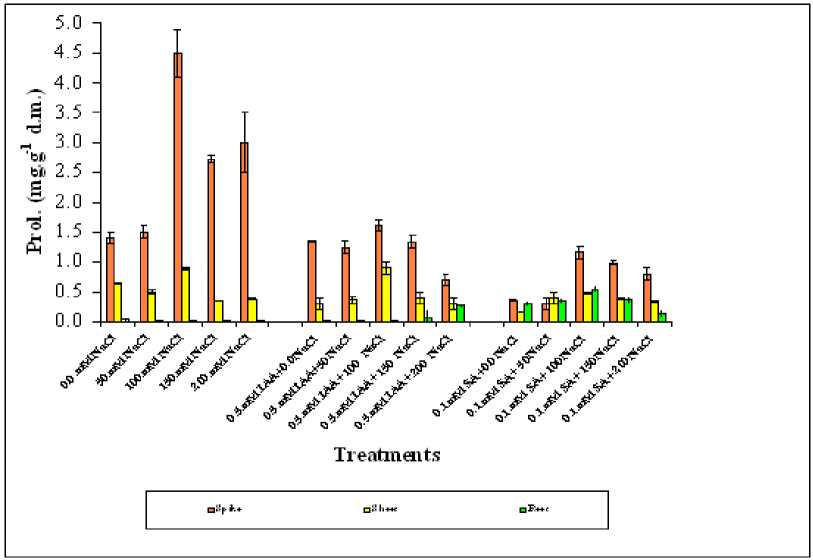
Figure 6. Effect of different salinity levels, combined effect of IAA and salinity or combined effect of SA and salinity on proline accumulation of different wheat organs. Vertical bars represent Standard deviation.
The correlation coefficient computed for the data revealed that among the Photosynthetic pigments, Chl b was none significantly correlated with both treatments. On the other hand Chl a significantly positively correlated with Salinity and Salinity and SA treatments, while with salinity and IAA treated plants was negatively correlated. Carotenoids were positively correlated with salinity, negatively with SA sprayed plants and none significantly correlated with IAA treated plants (Table 2). It was interesting to note that the concentration of Chl a was higher than Chl b and carotenoid was the lower in salinized wheat treated with SA than IAA especially at 50,100 and 150 mM NaCl.
-
(c) Metabolic constituents
ANOVA showed that phytohormones, salinity and their interaction had significant effects on reducing sugar P =0.000 (Table 1).
Among the three studied organs of salinity stressed wheat, spike showed more reducing sugar accumulation than both shoot and root respectively.
The reducing sugar of spike decreased as salinity increased in only NaCl stressed plants (Reference group). Foliar application with any of both phytohormones showed a marked and progressive increase in reducing sugar in the spike specially with SA treated plants at high salinity level (0.1mM SA+150 and 200 mM NaCl) than IAA treated one at no and low salinity dose (0.5 mM IAA+0.0 and50 mM NaCL). For reducing sugar of shoot and root, SA treated shoot was accumulator than IAA treated shoot, while in root vice versa was observed (Figure 3). As salinity increased the content of reducing sugars (R.S.) in the root become close to the value of those of control plant(0.0 mM NaCL) (Figure 3).
The correlation coefficient of the these selected organs (Table 2) showed the reducing sugar in spike and shoot were correlated with all treatments,while in root correlated only with IAA treated plants and none correlated with others.
Analysis of variance for soluble protein (Prot.) showed that all treatments were significantly affected the accumulation of soluble protein (Table 1), in all wheat organs (P=0.000). Salinity stress induced a pronounced increase in the soluble protein concentration in the spike, and gradually decreased it in both shoots and roots of wheat plants (Figure 4). The protein content in general was higher in spike than shoot and root organs especially at moderate and high salinity levels, regardless of the treatment. However it was higher in SA treated plants than IAA treated one. Slightly differences were observed between shoot and root protein content as a result of spraying with any of the studied hormones compared to the reference group (hormone non treated group). The correlation matrixes for protein showed that it was correlated in both shoot and root organs with all treatments. While in root it was only none significantly correlated with SA treated plant (Table 2).
For amino acid (A.A.), ANOVA showed that phytohormones, salinity and their interaction were highly significant effect on amino acids accumulation in spike, shoot and root organ P=0.000 (Table 1). General increase in amino acids contents was registered in spike than other studied organs and in ranks of spike>shoot > root. Exogenous application with any of the two studied phytohormones had significantly increased in amino acid content in spike, shoot and slightly in root. However, it was interesting to note that SA treated plants was more significant than IAA treated plants specially in spike and shoot organs, compared to root organ. (Figure 5).
Correlation matrix of IAA treated plants showed a significant correlation with all studied organs while SA treated plants showed none correlation, while salinity treated plants (Reference group) showed correlation only with root organ Analysis of variance showed that all treatments and their interaction significantly affect proline accumulation in both studied organs (P= 0.000) (Table 1). The proline concentration was higher in spike than shoot and root under all treatments. Exogenous application of both studied hormones significantly retarded the accumulation of proline in shoot and root organs of stressed wheat plants (Figure 6).
Correlation coefficient of the present data showed proline accumulation in spike was significantly correlated all treatments while in shoot it was none correlated. Roots on the other hand, correlated with salinity and IAA treated plants. (Table 2).
Generally, data observed that spike was more accumulator organs among the selected organs and SA was more significant phytohormone affecting the studied antioxidant, photosynthetic pigments and measured metabolities than IAA hormone.
DISSUSSION
Tolerance to salinity stress in higher plants correlates to the levels of antioxidant systems and substrates (Jahnke and White 2003). These changes in the levels of antioxidant molecules are signals of plant tolerance/adaptation to stress conditions. Therefore, changes in the activity of these enzymes are correlated into oxidative stress tolerance of plants (Lee et al . 2001; Sudhakar et al . 2001). Variations in the antioxidant levels can serve as a signal for the modulation of ROS scavenging mechanisms and ROS signal transduction (Mittler 2002).
In our results, the activity levels of antioxidant enzymes as catalase (CAT), peroxidase (POD) and ascorbate (APX) showed progressive significant increases with increasing concentration of NaCl specially at high salinity level (150 and 200 mM NaCl) compared to control plant (0.0 mM NaCl). These results were in agreement with those of (Lee et al. 2001). They observed that salt stress increased the activities of leaf mitochondrial and chloroplastic antioxidant enzymes, which are considered the primary scavenger in the detoxification of active oxygen species in plants and converts superoxide to H2O2 and O2, and offers protecting cells against superoxide induced oxidative stress. Also, our results showed a general decrease in catalase activity in SA treated plants especially at high salinity levels (150 and 200 mM NaCl), but still higher (about three times) than reference group, which led to the accumulation of toxic level of H2O2 (Lee et al. 2001). On the other hand, catalase activation by salt stress and IAA treated plants (set II) may be due to synthesis of new enzyme, (Feierabend and Dehne 1996), or catalase photo activation, (Polle 1997).
The shoot spraying of phytohormones under the various levels of salinity caused marked stimulation in the catalase, peroxidase and slightly increase in ascorbate activity as antioxidant defense compound in wheat plant as compared with the values of reference controls. Therefore, treatments with any of the tested phytohormones alleviated the adverse effect of salinity on photosynthetic pigments, growth parameters and metabolic activities through decreasing the build-up of active oxygen species especially at high salinity levels and thereby increasing resistance to salt stress, (Hassanein et al . 2009).
These observations were confirmed by the correlation coefficient, which proved that Cat and POD were significantly positively correlated with salinity (table 2). These correlations were confirmed in many literary data as reported by Jebara et al . (2005). A rise in the peroxidase activity with salinity has also bean verified in Morus alba (Sudhakar et al . 2001) Glycine max (Ghorbanli et al . 2004) and Lycopersicon esculentum (Rahnama and Ebrahimzadeh 2005) .
The severe reduction in the photosynthetic pigments (Chl a, b and carotenoids) in Wheat plants in the present investigation of the control treatments may be attributed to the toxic action of NaCl on the biosynthesis of pigments, increasing their degradation and/or maintaining damage of the chloroplast thylakoid. These results are in harmony with those observed by (Quartacci and Navari-Izzo 1992; Rao and Rao 1981). Application of any of the two phytohormones in most cases, did not only alleviate the inhibitory effect of salinity stress on the biosynthesis of photosynthetic pigments, but also induced a significant stimulatory effect greater than observed in the corresponding controls, a response which may contribute directly to the effectiveness on photosynthetic apparatus and in some way can alter plant productivity.
Many authers, Shakirova et al . (2003) and Iqbal et al . (2006) on wheat plants and Abdel-Wahed et al . 2006; El-Mergawi and Abdel-Wahed 2007) on maize plants found that salicylic acid caused significant increased in chlorophyll content. This accumulation of photosynthetic pigments as a result of exogenous application of SA may be due to increase in photosynthetic efficiency as reflected by increasing in both chl a, chl. b and carotenoids content in the leaves of stressed wheat plants. Also, Tari et al . (2002) observed that SA provides a pool of compatible osmolyts in the presence of salinity. The increase in production of photosynthetic pigments in SA treated plants was concomitant with the accumulation of saccharides and growth yield of wheat plant under salinity levels as compared with control plants.
Regarding to metabolic constituents, carbohydrate plays a multiple roles in the acclimatization of roots by 1- production the precursors of most chemical synthases 2- production of metabolic energy 3-and consequently maintained osmoregulation in roots. Data of reducing sugar in the present investigation of the wheat plant organs grown under salinity stress showed significant decreases in reducing sugars. The significant decrease in reducing sugar of both organs led to conclude that the photosynthetic efficiency was decreased in response to salinity of NaCl and thus led to retard the biosynthesis of carbohydrates which are utilized in growth of wheat plants, (Patricia et al. 1992) and/or increased partial utilization of carbohydrates into other metabolic pathways (Singh and Dubey 1995).
Foliar Application of studied phytohormones generally stimulated the accumulation of carbohydrates in the salt-affected wheat plant organs by acting as activators of carbohydrates synthesis, (Kodandaramaiah 1983).
Moreover, accumulation of carbohydrate play a key role in alleviating the salinity stress, either via osmotic adjustment, as Ackerson (1985), or by conferring some desiccation resistance to plant cells according to (Srivastava et al . 1995).
Data of the present investigation observed that a variable response in the distribution of saccharides, proteins and amino acids in the three organs of wheat plant with increasing salinity stress. Protein content decrease in root and shoot but increase in spike under all treatments. Also, generally run parallel with this trend, the increasing of amino acid content in both shoot and spike, and decreasing in root (i.e. the root organ share with the reduction of both protein and amino acids). In opposite with this situation, the saccharides content decreased in shoot and spike. This means that the conversion of saccharides into amino acids and proteins increase in both shoot and spike which may increase the osmotic potential of these organs and increase the osmotic tolerant of wheat plants through shoot and spike organs. This was in agreement with findings of (Hamdia, 2004).
The recorded promotion in saccharides, proteins and amino acids after treatment with SA or IAA were linked with a great promotion in the growth yield. The data indicated that a variable response of the three organs under hormonal treatments, the observed high accumulation of saccharides, proteins and amino acids in spike organ.
The physiological significance of proline accumulation is controversial. While some researchers have reported that it is a sign of stress (Rai et al . 2003; Hamdia, 2004), others suggested that a high concentration act as a solute of intercellular osmotic adjustment (Silveira et al . 2003). According to our results, proline showed a variable strategy in different organs of the same plant. It was consider a sign of stress in spike, while in shoot organ behaves as a sign of sensitivity.
Since proline is one of the important components of defense reactions of plants to salinity (Kuznetsov and Shevyakova 1999), it might be expected that pretreatment with SA contributes to accumulation of this amino acid under salt stress.
The data of this study showed that salinity induced accumulation of proline in different plant organs (Fig. 6). These data suggests that proline is an important component in the spectra of SA-mediated protective reactions of wheat plants in response to salinity which contribute to a reduction of injurious effects of stress factors and acceleration of restoration processes during the period after action of stress, which might be a manifestation of the protective action of SA on wheat plants. These data in agreement with what was found by (Sakhabutdinova et al . 2003)
Proline was also interesting while it increased in shoot, it decreased in root. Theses problematic behavior of proline in our study decrease the physiological significance of proline especially we take into consideration that the absolute amount of proline was very rare to play any role in osmoregulation or the other roles which reported by many authors (Yordanov et al. 2003; Hamdia, 2004). This was recommended by the observable improvement of growth by phytohormones which were found to be associated with the observable retardation of protein and carbohydrate which might confirm that the salt tolerance was linked with the regulation of carbohydrate and nitrogen components while, saline injury disturbed both of them and also their conservation.
CONCLUSION
From the preceding results and discussion, it can be concluded that foliar application of Wheat ( Triticum aestivum L. Giza 186) with both IAA and SA individually or in combined with salinity induced plants stimulate the salt tolerance of wheat plants via improved the antioxidant enzymes, enhancement of the biosynthesis of photosynthetic pigments and thereby increasing the carbohydrate contents and the general growth rate; as well as enhancing the accumulation of nontoxic metabolites (sugars, protein, free amino acids and proline) which reflect an increase the production in spike, than shoot and root organ. Thus salicylic acid and/or indole acetic acid treatments prevents the deleterious effects of salinity stressed wheat and could be adopted as a potential growth regulator or antioxidant to improve growth particularly under moderate NaCl salinity levels, wheat plant respond positively to SA foliar application than IAA application.
Список литературы Oxidative stress markers and antioxidant potential of wheat treated with phytohormones under salinity stress
- Abdel-Wahed, M.S.A., Amin, A.A.and El-Rashad, S.M. (2006) effect of some bioregulators on vegetative growth, yield and chemical constituents of yellow maize plants. World J. Agric. Sci.2(2): 149-155.
- Ackerson, R. C. (1985) Osmoregulation of cotton in response to water stress III. Effects of phosphorus fertility. Journal of Plant Physiology. 77: 309-312.
- Aebi, A. (1984) Catalase in vitro.Meth. Enzymol105: 121-126.
- Afzal, I., Basara, S.M.A., Faooq, M. and Nawaz, A. (2006) Alleviation of salinity stress in spring wheat by hormonal priming with ABA, salicylic acid and ascorbic acid. Int J Agric Biol. 8: -28.
- Antoline, M.C. and Sauchez-Dais, M. (1992) Photosynthetic nutrient use deficiency, nodule activity and solute accumulation in drought stressed alfalfa plants. Photosynthetica 27: 595-604.
- Asada, K. and Chen, G.(1992) Interaction of ascorbate peroxidase by thoils requires hydrogen peroxide. Plant Cell Physiol. 33: 117-123.
- Ashraf, M.and Harris, P. J. C. (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci.166:-6.
- Azooz, M.M. (2009) Salt stress initigation by seed priming with salicylic acid in two faba bean genotypes differing in salt tolerant. Int J Agric Biol11: 343-350.
- Bates, L. S., Waldren R. P. and Tear, L. D.(1973) Rapid determination of free proline for water-stress studies. Plant and Soil. 39: 205-207.
- Charparzadeh, N., Lucia, M., Negad, R., Izzo, R. and Izzo, F. (2004) Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiol. Biochem., 42: 695-701.
- Egamberdieva, D. (2009) Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol Plant 31:861-864
- El-Mergawi, R. and Abdel-Wahed, M. (2007) Diversity in salicylic acid effects on growth criteria and different acetic acid forms among bean and maize Plant Growth Substances Association.19th Annual meeting, Puerto Vallarta, Mexico, July 21-25: 2007.
- Fales, D. R. (1951) The assimilation and degradation of carbohydrates of yeast cells. J. Biol. Chem. 193: 113-118.
- Farooq, S.and Azam, F.(2006) The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J. Plant Physiol., 163 -637.
- Feierabend, J. and Dehne, S. (1996) Fate of the porphyrin cofactors during the light-dependent turnover of catalase and of the photosysem II reaction center protein DI in mature rye leaves. Planta 198:413-422.
- Foyer, C. H., Alschei, R. C.and Hess, J.L.(1993) Ascorbic acid; an Antioxidants in Higher Plants. pp. 31-58. CRC Press, Boca Raton
- Ghassemi, F., Jakeman, A. J. and Nix, H. A. (1995) Salinisation of land and water resources.Wallingford, UK: CAB International.
- Ghorbanli, M., Ebrahimzadeh, H. and Sharifi, M. (2004) Effects of NaCl and mycorrizal fungi on antioxidative enzymes in soybean. Biol. Plant. 48: 575-581.
- Gunes, A., Inal, A., Alpaslam, M., Erslan, F., Bagsi, E. G. and Cicek, N. (2007) Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maiz (Zea mays L.) grown under salinity. J. Plant physiol 164: 728-736.
- Hajiboland, R. and Hasani, B. (2007) Responses of antioxidant defense capacity and photosynthesis of bean (Phaseolus vulgaris L.) plant to copper and manganese toxicity under different light intensities. Acta Biol Szeged 51:93-106.
- Hamdia, M. A. (2004) Mechanisms of salt tolerance and interactive effects of Azospirillum brasilenseinoculation on maize cultivars grown under salt stress conditions. Plant Growth regulation. 44: 165-174.
- Hassanein, R. A., Bassuony, F. M., Baraka, D. M. and Khalil, R. R. (2009) Effects of Nicotinamide and Ascrobic Acid on Zea mays. Grown Under Salinity Stress. I-Changes in Growth, Some Relevant Metabolic Activities and Oxidative Defense Systems. Research Journal of Agriculture and Biological Sciences5(1): 72-81
- Havir, E. A.and Mellare, N. A. (1987) Biochemical and developmental characterization of multiple forms of catalase in Tobacco leaves. Plant Physiology.84: 450-455.
- Hayat, Q., Hayat, S., Irfan, M. and Ahmad, A. (2010) Effect of exogenous salicylic acid under changing environment: A review. Environ Exp Bot 68: 14-25.
- Hussein, M. M., Balbaa, L. K. and Gaballah, M. S.(2007) Salicylic acid and salinity effect on growth of maiz plants. J Agr Biol Sci. 3: 321-328.
- Iqpal, M., Ashraf, M., Jamil, U.and Shafeq, A. (2006) Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plant under salt stress. J. of Integrative Plant Biology48(2): 181-189.
- Jahnke, L. S. and White, A. L.(2003) Long-term hyposaline and hypersaline stresses produce distinct antioxidant responses in the marine algae Dunaliella tertiolecta. J. Plant Physiol.160: 1193-1202.
- Javid, G. M., Sorooshzadeh, A., Moradi, F., Mohammad, S. A., Sanavy, M. Allahdadi, I. (2011) The role of phytohormones in alleviating salt stress in crop plants Australian J Crop Science 5(6):726-734.
- Jebara, S., Jebara, M., Liman, F. and Aquani, M. E. (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxidase dismutase activities in common bean (Phaseolus vulgaris L.) nodules under salt stress. J.plant. physiol.162:929-936.
- Kaya, C., Kirnak, H., Higgs, D. and Saltali, K. (2002) Supplementary calcium enhances plant growth and fruit yield in strawberry cultivars grown at high salinity. Sci Hortic 93:65-74.
- Khan, M. (2006) Effect of sea salt and L-ascorbic acid on the seed germination of halophytes. J. Arid Environ. 67: 535-40
- Klapheck, S., Zimmer, I. and Cosse, H.(1990) Scavenging of hydrogen peroxide in endosperm of Ricinus communisby ascorbate peroxidase. Plant Cell Physiology. 31: 1005-1013.
- Kodandaramaiah, J. (1983) Physiological studies on the influence of B -vitamins on leaf and fruit metabolism in cluster beans Cyamopsis tetragonoloba (L.) Tanb.Ph.D. Thesis submitted to Srivenkateswera Univ. Tirupati, India
- Kuznetsov, V. V. and Shevyakova, N. I. (1999) Proline under stress conditions: Biological role, metabolism, and regulation. Russ. J. Plant Physiol 46: 321-336.
- Lee, D. H., Kim, Y. S. and L, C. B.(2001) The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa.). J. Plant Phyiol. 158: 737-745.
- Levent Tuna, A., Cengiz, K., Mura, D., Ibrahim, Y., Betul, B. and Hakan, A.(2007) Comparative effects of various salicylic acid derivatives on key growth parameters and some enzyme activities in salinity stressed maize (zea maysL.) Plants. Pak. J. Bot. 39(3): 787-798.
- Lowery, O. H., Rosebrough, N. J., Fail A. l. and Randall, R. J. (1951) Protein measurements with foline phenol reagent. J. Bio. Chem.193: 265-275.
- Maehly, A. C. and Chance, B. (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1: 357-424.
- Mansour, M. M. F. (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 43: 491-500.
- Mansour, M. M. F. (2004). Cellular basis of salinity tolerance in plants. Environ. Exp. Bot.,52: 113-22.
- Metzner, H., Rau, H. and Senger, H.(1965) Untersuchungen zur Synchronisierbarkeit einzenlner Pigment-Mungel Mutanten von Chlorella. Planta. 65: 186-194.
- Mittler, R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7: 405-410.
- Molassiotis, A., Sotiropoulos, T., Tanou, G., Diamantidis, G. and Therios, I. (2006) Boron-induced oxidative damage and antioxidant and nucleolytic responses in shoot tips culture of the apple rootstock EM9 Malus domestica Borkh),. Environ. Exp. Bot.,56: 54-62.
- Moore, O. S. and Stein, W. (1948) Photoetric ninhydrine method for use in the chromatography of amino acids. J. Biol. Chem.,17: 367-388.
- Mukherjee, S. P. and Choudhuri, M. A. (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna. Plant Physiol.58: 166-170.
- Noreen, Z. and Ashraf, M. (2009) Assessment of variation in antioxidative defense system in salt treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J.Plant Physiol., 166: 1764-1774.
- Patricia, A., Thomas, J. C., V, D. M., B,t H. J. J, R. G.() Distinct cellular and organismic responses to salt stress. Plant Cell Physiol., 33 -1223.
- Polle, A. (1997) Defense against photooxidative damage in plants. In: Scendalios J. (ed): Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor laboratory Press, NY, PP. 785-813.
- Popova, L. P., Stoinova, Z. G. and Maslenkova, L. T. (1995) Involvement of abscisic acid in photosynthetic process in Hordeum vulgare. during salinity stress. J. Plant Growth Regul. 14: 211-218.
- Quartacci, M. E. and Navari-Izzo, F. (1992) Water stress and free radical mediaed changes in sunflower seedlings. J. Plant. Physiol., 139: 621-625.
- Rahnama, H. and Ebrahimzadeh, H. (2005) The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol. Plant., 49 -97.
- Rai, S. P., Luthra, R. and Kumar, S.(2003) Salt-tolerant mutants in glycophytic salinity response (GRS) genes in Catharanthus roseus. Theor. Appl. Genet.106 -230.
- Rao, G. G. and Rao, G. R. (1981) Pigment composition and chlorophylase activity in pigeon pea (Cajanus indicus Sperng) and gingelley (Sesamum indicum) under NaCl salinity,. Indian, J. Exp. Biol.,19: 768-770.
- Sakhabutdinova, A. R., Fatkhutdinova, D. R., Bezrukova, M. V. and Shakirova, F. M. (2003) Salicylic acid prevents the damaging action of stress factors on winter wheat leaves. -Phytochemistry 67: 710-715.
- Shakirova, F. M., Sakhabudinova, A. R., Bezrukova, M. V., Fatkhutdinova, R. A. and Fatkhutinova, D. R.(2003) Changes in the hormonal status of wheat seedlings induced by salicylic and salinity. Plant Science. 164: 317-322.
- Shalata, A. and Peter, M. N. (2001) Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J. Exp. Bot.52: 2207-2211.
- Sheteawi, A. S. (2007) Improving Growth and Yield of Salt-stressed Soybean by Exogenous Application of Jasmonic Acid and Ascobin. journal of agriculture and biology 9(3): 473-478.
- Silveira, J. A., Viegas Rde, A., Darocha, I. M., moreira, A. C., Moreira Rd,e A. and oliveira, J. T.(2003). Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J. Plant Physiol.160 -123.
- Singh, A. K.and Dubey, R. S. (1995) Changes in chlorophyll a and b contents and activities of photosystems 1 and 2 in rice seedling induced by NaCl. Photosnthetica, 31: 489-499.
- Srivastava, D. K., Gupta, V. K. and S, D.R.(1995) In vitro and characterization of water stress tolerance callus cultures of tomato (Lycopersicum esculentum). Indian J. Plant Physiol2: -104.
- Sudhakar, C., Lakshmi, A. and G, S. (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba.) under NaCl salinity. Plant Sci. 161: 613-619.
- Szabolcs, I. (1994) Soils and salinization. In: Pessarakli, M. (ed.), Hand-book of Plant and Crop Stress. pp. 3-11. Marcel Dekker, New York
- Tari, I., Csisaa, J., Szalai, G., Horath, F., Kiss, G., Szepesi, G., Szabl, M. and Erdei, L. (2002). Acclimation of tomato plants to salinity stress after a salicylic acid pre-treatmen. Acta Biol Szegediensis 46: 55-56
- Tsugane, K., Koboyashi, K., Niwa, Y., Ohba, Y., Wada, K. and Koboyashi, H. (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell, 11: 1195-206
- Vangronsveld, J. and Clijsters, H. (1994) Toxic effects of metals. In: Plants and the Chemical Elements. Biochemistry, Uptake, Tolerance and Toxicity (FARAGO M. E., Ed.), pp. 150.177, VCH Verlagsgesellschaft, Weinheim.
- Yordanov, I., Velikova, V. and Tsonev, T. (2003) Plant responses to drought and stress tolerance. Bulg. J. Plant Physiol., Special Issue, 187-206.


