Oxidative stress tolerance by calcium and histidine in two tomato cultivars under nickel stress
Автор: Mozafari H., Asrar Z., Rezanejad F., Pourseyedi S., Yaghoobi M.M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.10, 2014 года.
Бесплатный доступ
We investigated calcium (Ca) and L-histidine (His) interaction on nickel (Ni)-induced oxidative stress tolerance in two tomato (Solanum lycopersicum Mill.) cultivars including Cal-J N3 and Petoearly CH. CaCl 2 (0 and 300 µM) and L-histidine (0 and 300 µM) effects on the oxidative responses in these cultivars cultured were compared in the hydroponic media under Ni stress (NiSO 4; 0,150 and 300 µM). The activities of antioxidative enzymes including catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), superoxide dismutase (SOD) and total content of proteins, malondialdehyde (MDA), other aldehydes, H 2O 2, Ca 2+, Ni 2+, ascorbate (ASC), dehydroascorbate (DHA) and electrolytes leakage (EL) were determined. The obtained results indicated that the application of Ca and His generally reduced oxidative markers such as the contents of EL, H 2O 2, MDA and activity of CAT as well as the Ni 2+content of root and shoot organs under nickel toxicity, while application of Ni treatment without Ca+His increased these oxidative parameters and accumulation of Ni 2+, compared to the control. Applying Ni without Ca and His has resulted in reduction of GPX, APX and SOD activities as well as concentrations of root and shoot Ca 2+and ASC in the two mentioned cultivars. Application of Ca and His lead to the elevated contents of Ca 2+ and ASC, increased activities of GPX, APX and SOD as well as inhibition of Ni 2+ accumulation differently in both cultivars. Ca and His also alleviated the adverse effects of Ni stress on the selected investigated parameters especially in Petoearly CH cultivar. Thus, interaction of Ca and His appeared to improve adaptive responses to Ni stress leading to decreasing Ni-induced oxidative stress in the tomato plants. Therefore, our results suggest that Ca+His alleviated nickel-induced oxidative stress by uptake and inhibition of translocation of Ni 2+ plus Ni chelating mechanism improvement in the tomato cultivars.
Cal-j n3, petoearly ch, antioxidative enzymes, malondialdehyde, electrolytes leakage
Короткий адрес: https://sciup.org/14323851
IDR: 14323851
Текст научной статьи Oxidative stress tolerance by calcium and histidine in two tomato cultivars under nickel stress
Soil pollution by heavy metals (HMs) including containing toxic heavy metals in environment
Cd, Cu, Ni, Pb, and Zn occur by industrial wastes specially cultivated fields. Most of the wastes contain heavy metals in an amount enough to cause toxicity to the crop plants (Whitby and Huchinson, 1974). Therefore, abiotic stresses like heavy metal stress, air pollutants stress and others negatively affect processes associated with biomass production and seed yield in almost all major field grown crops. Every metal and plant interacts in a specific way, which depends on several factors such as type of soil, growth conditions, and the presence of other ions (Felix-Henningsen, 2010; Ghani, 2010; Dalvi and Bhalerao, 2013).
Heavy metal toxicity as one of the major abiotic stresses induces hazardous effects in crop plants. A common consequence of HM toxicity is the excessive accumulation of reactive oxygen species (ROS) and methylglyoxal (MG), both of which can cause peroxidation of lipids, oxidation of protein, inactivation of enzymes, DNA damage and/or interact with other vital constituents of plant cells. Higher plants have evolved antioxidant defense systems to scavenge ROS and MG. In addition, HMs may be sequestered by organic acids, glutathione (GSH), or by specific metal-binding ligands in cells. GSH as a central molecule of antioxidant defense systems is involved in either direct or indirect control of ROS and MG and their reaction products in plant cells, thus protecting the plant from HM induced oxidative damage (Hossain et al ., 2012).
Nickel is naturally present in soil and water, usually in low concentrations. Most agricultural soils contain an average of 0.005 цд of Ni g-1 dry weight (DW), and symptoms of phytotoxicity often become apparent at Ni concentrations as low as 2530 μg g-l soil (Khalid and Tinsley, 1980). Growth inhibition, chlorosis and reduced water content of tissues are commonly observed in plants exposed to phytotoxics amounts of Ni (Pandolfini et al., 1992). Despite his harmful effect, several plants nutritionally require Ni for various metabolic activities (Welch, 1981) in very low concentrations. Physiological role of Ni and its toxic effects on higher plants have been observed (Temple and Bisessar, 1981).
At cellular and molecular levels, oxidative stress is widely studied as a key marker of plant stress especially metal toxicity. Studies have reported that toxic levels of Cu, Ni and Zn result in a stimulation of lipid peroxidation leading to a loss of membrane function. It is documented that excessive heavy metal such as Ni exposure may increase reactive oxygen species (ROS) accumulation in plants, and oxidative stress would arise if the balance between ROS generation and destruction were broken (Gajewska and Sklodowska, 2007). Membrane peroxidation has been observed in plants that treated with excess Ni amounts (Gajewska and Sklodowska, 2007). Cells have evolved an elaborate system of enzymatic and non enzymatic antioxidants which help to scavenge these indigenously generated ROS. Various enzymes involved in ROS-scavenging have been manipulated, over expressed or down regulated to add to the present knowledge and understanding the role of the antioxidant systems. (Ahmad et al ., 2010). At the time the first mechanisms of chloroplast redox regulation were being discovered, ROS were regarded as by-products of potentially beneficial reactions. Indeed, it remains the case that reduction of O2 to superoxide by the thylakoid electron transport chain can prevent over reduction (redox poising) and contribute to chloroplast ATP pools via pseudo cyclic phosphorylation. Nevertheless, as they became implicated in diverse stress responses, ROS subsequently gained the reputation as damaging molecules (Foyer and Noctor, 2012).
Superoxide Dismutase (SOD) and catalase (CAT) are enzymes that represent the first line of antioxidant defense in plant cells. Whereas SOD is responsible for the removal of superoxide radical (O 2 •-), CAT metabolizes H 2 O 2 into H 2 O and O 2 . Both enzymes not only prevent formation of more toxic ROS, but play an essential role in cellular H 2 O 2 signaling. Since there are a number of ROS producing sites, both enzymes were found to be present in different isoforms localized in ROS generating organelles. Whereas CAT has been identified in three different isoforms that could be localized in both cytosol and peroxisomes based on their amino-acid targeting sequence, SOD is present in three metallic forms each occupying a specified organelle. Fe 2 + SODs are located in the chloroplasts, the Mn2+ isoform in the mitochondria and Cu2+/Zn2+ isoforms are present in the chloroplast, cytosol and possibly in extracellular spaces as well (Skyba et al ., 2012).
Plants possess an enzymatic protective mechanism including peroxidases such as GPX and APX, besides CAT and SOD. These anti oxidative enzymes have an important role in radicals and peroxides control that are produced under metals stress such as Ni (Tanyolac et al ., 2007). Kumar et al . (2007) showed that nickel treatment, 100 μM NiSO 4 , caused increase in the H 2 O 2 concentration concomitantly with antioxidant enzyme activities in maize. Therefore, SOD catalyzes disproportion of superoxide radicals (O 2 •-) into H 2 O 2 for decreasing the hydroxyl radical (OH•) content (Foyer, 1994). CAT, GPX and APX are contributed in decreasing the content of plant H 2 O 2 . In leaf tissues, H 2 O 2 is transformed in chloroplasts through the ascorbate dependent H 2 O 2 scavenging pathway which involves oxidations and re-reductions of ascorbate, glutathione and NADPH (Baccouch et al ., 1998).
The prevention of toxic metal accumulation in the cytoplasm and organelles is one of the important mechanisms for plant tolerance to heavy metals. In tolerant plants, heavy metals as Ni2+, Cd2+ and Cu2+ are very often chelated or precipitated inside the cell vacuoles that this indicates a transport of heavy metals through the cytoplasm (Howden et al ., 1995). In plants with ability for Ni hyper accumulation, the low molecular chelators including histidine (Kramer et al ., 1996), malonate or malate (Brooks et al ., 1979) and citrate (Lee et al ., 1977) have been contributed for Ni detoxification. It is approved that yeast tolerance ( Saccharomyces cerevisiae ) to Ni2+ toxicity was depending on high cellular His concentrations (Joho et al ., 1992). Increasing of free histidine and the chelating of the heavy metals ions were found for the Ni transport by the imidazole nitrogen of the amino acid (Kramer et al ., 1996). In addition, metal chelators such as phytosiderophores (Von Wiren et al ., 1996) or organic acids (Ma et al ., 2001) regulate the transportable nutrients availability for metal uptake by plant cells. Phytosiderophores have been identified in the barley ( Hordeum vulgare ) xylem sap by Shah et al . (2001).
Calcium is an essential macronutrient with diverse functions in plants (Wu and Hendershot, 2010). Calcium plays an important regulatory role in cell division, cell extension, cell wall and membrane synthesis and function (Hanson, 1984; McLaughlin and Wimmer, 1999; Girija et al., 2002). Calcium also functions as a second messenger at low concentrations in the signal transduction between environmental factors and plant responses (Marschner, 1995). The concentration of Ca in the cytosol is very low as 1µM approximately, since Ca is cytotoxic in higher concentrations at milimolar range (Marschner, 1995). The various roles of Ca in plant systems depend on its unique chemical properties that allowed it to exist in a wide variety of binding states (Hepler and Wayne, 1985). In the cell wall, Ca is mainly bound to exchangeable sites in the middle lamella. By binding to carboxylic groups of the poly galacturonic acids (pectins) and cross linking the pectic chains of the middle lamella, Ca strengthens the cell wall and controls its rigidity (Demarty et al., 1984). The cell wall selectivity property correlates with optimum calcium content in plant tissues and rhizosphere (Girija et al., 2002). The cell wall is important chelator of toxic metals such as nickel, as far as the metal is transported apoplastically especially in root tissue. Pectic sites, histidyl groups and extracellular carbohydrates present in plants cell wall causes immobilization of heavy metals and prevent their uptake in to cytosol. The heavy metals are bound to carboxylic groups of poly galacturonic acids restricting the plant uptake. Recent studies indicated that the chemical properties of the cell wall might modulate metal uptake and consequently metal tolerance (Dalvi and Bhalerao, 2013). The cell walls inevitably come into direct contact with the heavy-metal-containing medium in soil and act as a cation exchanger in plant roots (Küpper et al., 2001). It has been described that calcium may decrease the uptake, translocation and accumulation of heavy metals in plants (Osteras and Greger, 2006).
There is little information about the sensitivity and physiological responses of different tomato cultivars to Ni toxicity, although tomato rank is the second important vegetables in terms of planting areas and production. Moreover, results from effect of Ni toxicity on the antioxidant enzymes are contradictory and both increase and decrease in their activities have been observed under different treatments. This warrants the further study of Ni- induced antioxidant systems in other plants such as tomato cultivars. The objectives of the present study are to evaluate the effects of Ca, His and Ni treatments on the activities of four anti oxidative enzymes (CAT, GPX, APX, SOD), and accumulation of protein, MDA, other aldehydes, H2O2, Ca2+, Ni2+, ASA, DHA and membrane ion leakage in the two probable Ni sensitive tomato cultivars in hydroponic culture. Thus, the aim of this research is to determine the Ca and His role in the alleviation of oxidative stress and improvements of the antioxidant enzyme activities in the two mentioned tomato cultivars from Iran. Moreover, we evaluated and compared Ni stress sensitivity and tomato cultivars response to Ca, His and Ni treatments between these two important tomato cultivars.
MATERIALS AND METHODS
Plant Growth: Two tomato (Solanum lycopersicum Mill.) cultivars including Cal-J N3 and Petoearly CH, being widely planted in southeastern of Iran, were used in this study. Seeds of recent tomato cultivars were placed on two sheets of filter paper in petri dishes (90 mm) containing Hoagland solution for optimum seedling growth (Hoagland and Arnon, 1950). After 7 d of culture, uniform seedlings of tomato were selected and transferred into dark polyethylene vessels (two plants per vessel), each supplied with 50 mL of a modified one-tenth-strength Hoagland solution containing 0.5 mM KNO3, 0.4 mM Ca(NO3)2, 10 µM Fe-EDTA, 0.2 mM MgSO4, 0.1 mM KH2PO4, 10 µM H3BO3, 2 µM MnCl2, 2 µM ZnSO4, 0.1 µM Na2MoO4 and 0.2 µM CuSO4 buffered pH 5.8±0.1. The growth medium was continuously aerated and nutrient solutions were exchanged once a day. Seedlings and plants were grown in a greenhouse with supplementary light provided by sodium vapor lamps at a 200 µmol m-2 s-1 during the day, a photoperiod of 16/8 h and temperature of 25°C and 22°C (day/night), respectively, and 60% constant relative humidity.
Experimental design: We evaluated several treatments (containing excess Ni, Ca and His) effects on seed germination and plantlet growth parameters at independent experiment in tomato cultivars for 20 d. Thus, we observed primitive response of tomato plants to the Ni, Ca and His concentrations and the final Ni, Ca and His levels were selected for main project (data not shown in results and discussion parts). After this stage, the optimum time (3 weeks) and concentrations of Ca, His and Ni resulted for treatments application on the tomato plants .
Therefore , tomato plants were grown in a modified one-tenth-strength Hoagland solution for 3 weeks after transplanting. After this period, the effect of Ni exposure and other compound (L-histidine and CaCl 2 ) on growth parameters, membrane properties and oxidative parameters were investigated by replacing the nutrient with a fresh solution containing the respective added compounds for 10 d. The indicated total treatments concentrations were supplied as NiSO 4 (0, 150 or 300 µM) and Ca as CaCl 2 (0 or 300 µM) and L-histidine (0 or 300 µM) (extra pure, Merck Co., Germany) that adjusted to pH 5.8±0.1 with 0.1 M KOH or 0.1 M HCl as required. The tomato plants under Hoagland media without adding NiSO 4 , CaCl 2 and L-histidine were presented as “Control” in our results description. At the end of the treatment period, 3rd leaf, whole root and shoot organs were fresh weighted (FW) and length per plant in both shoot and root organs were determined. After that, these organs were washed in deionized water, blotted dry with tissue paper, weighted, frozen in liquid nitrogen and stored at -70°C until analysis.
Electrolyte leakage: The electrolyte leakage was determined as described by Ben Hamed et al . (2007) in leaves tissue of the tomato plants. The leave samples (0.2 g) were placed in test tubes containing 10 ml of double distilled water. The leaves were cut into discs of uniform size (5 mm length). The tubes were incubated in a water bath at 32°C for 2 h and the initial electrical conductivity of the medium (EC 1 ) measured. The samples were autoclaved at 121°C for 20 min to release all the electrolytes, cooled to 25°C and the final electrical conductivity (EC 2 ) measured. The electrolyte leakage (EL) was calculated by using the formula: EL = 100 (EC 1 / EC 2 ).
H 2 O 2 content: For determination of H 2 O 2 concentration, leaf fresh tissue (0.1 g) was extracted with 3 ml TCA (0.1 %, w/v) in an ice bath and centrifuged at 12,000 x g for 15 min (Velikova et al ., 2000). The content of H 2 O 2 was expressed as μM g-1 FW using the extinction coefficient (ε=0.028 mM-1 mm-1) at 390 nm.
Analysis of ASC and DHA: ASC and DHA of leaves were measured as described by de Pinto (1999). Briefly, total ascorbate was determined after reduction of DHA to ASC with DTT, and the concentration of DHA was estimated from the difference between total ascorbate pool (ASC plus DHA) and ASC. A standard curve was developed based on ASC in the range of 0-20 mg L -1 .
MDA and other aldehydes assay: The level of lipid peroxidation in tomato leaves was determined as the amount of 2-thiobarbituric acid-reactive substances (TBARS) by means of malondialdehyde (MDA) and other aldehydes content formed as described by Heath and Packer (1969) and Meirs et al . (1992) respectively. The concentration of MDA and other aldehydes was calculated using an extinction coefficient of 15.5 mM-1 mm-1 and
0.00457 mM-1 mm-1 expressed as μ mol g -1 FW.
Enzyme activity determinations: The total protein content in leaves and roots was assayed according to the method of Bradford (1976) using bovine serum albumin as a standard. All spectrophotometric analyses of enzyme were conducted in a total volume of 3 mL in a Carry UV/visible light spectrophotometer at 25°C. CAT (E.C 1 .11.1.6) activity of leaves was measured according to the modified method of Dhindsa et al . (1981). The activity was calculated using an extinction coefficient of 4 mM-1mm-1. The unit (U) of CAT activity was defined as the amount of enzyme that decomposed 1 mM H 2 O 2 per minute per mg protein in 100 µL enzyme extract are given in U mg -1 protein. The activities of peroxidases were assayed using two different electron donors guaiacol and ascorbate. The GPX (EC 1.11.1.7) activity of leaves was determined by using method derived from Plewa et al . (1991). The increase of absorbance, due to tetra guaiacol formation, was recorded at 470 nm and enzyme activity was determined using an extinction coefficient of 2.55 mM-1mm-1. The unit (U) of GPX activity was defined as the amount of enzyme that produced 1 mM tetra guaiacol per minute per mg protein in 20 µL enzyme extract that given in U mg -1 protein. The APX (EC 1.11.1.11) activity of leaves was assayed by monitoring the decrease in absorbance at 290 nm due to ascorbate oxidation (Nakano and Asada, 1987). The activity of APX was calculated using the extinction coefficient of 0.28 mM-1mm-1. The unit (U) of APX activity was defined as the amount of enzyme that decomposed 1 mM ascorbate per minute per mg protein in 150 µL enzyme extract that given in U mg -1 protein.
Total SOD (EC 1.15.1.1) activity of leaves was determined by the ability of SOD to inhibit reduction of Nitro Blue Tetra zolium (NBT) to furmazone at pH=7.0 (Giannopolitis and Ries, 1977). The unit (U) of SOD activity was defined as the amount of enzyme that inhibits the NBT photo reduction by 50 %. SOD activity values that given in U mg-1 protein.
Ca and Ni accumulation Analysis: Samples of whole root and shoot were oven dried at 70°C for 72 h and after determination of dry biomass. Dry root and shoot biomass (0.5 g was digested in an acid mixture of nitric acid:sulphuric acid:perchloric acid (2:1:1, v/v). Acid mixture was allowed to evaporate and the metal residues were dissolved in 10 ml of 0.1 N HCl. Ni and Ca concentrations in the plant digest was measured using coupled plasma atomic emission spectroscopy (ICP, OES, Varian CO) on dry weight basis (Ganje and Page, 1974).
Experimental Design and statistical data analysis: The experimental design was a completely randomized design with 12 treatments, 2 cultivars and 4 replications per treatments. Samples were collected from two plants per culture vessel. Data were analyzed by using one-way analysis of variance (ANOVA). Differences between means were considers significant at confidence level of P≤0.05. All statistical analyses were done using the software SPSS package, Version 18.0. The Duncan test analysis was done to determine the significant difference between treatments.
RESULTS
In general, the interactive effects between Ca and His were significant to alleviation of most growth and oxidative stress markers under Ni 300 µM especially in Petoearly CH cultivar compared to Cal-J N3. Although, application of excess Ca or His in hydroponic media also improved some growth parameters and oxidative markers especially in Cal-J N3 under Ni stress. Whenever external histidine and calcium are low at each Ni2+ levels, an increase from 0 to 150 and 300 µM nickel concentrations significantly decreases length and FW of shoot and root in the two studied cultivars compared to the control plants. A further increase in Ca2+ and His concentration from 0 to 300 µM, separately or together, had positive effects on shoot and root length especially in Petoearly CH cultivar. However, histidine, root FW was more in the treatments containing only than calcium treatments in this Petoearly CH cultivar (Table 1).
Effect of exogenous Ca, His and Ni on total protein, ASC and DHA content
As it is shown in Table 2, nickel treatments without application of histidine and calcium, resulted in significant increase and decrease of total leaf protein in Cal-J N3 and Petoearly CH cultivars respectively, compared to the control, but the application of histidine adversely declined leaf protein content. Furthermore, addition of Ca plus Ni 150 µM decreased root total protein significantly and comparable to stress conditions.
Moreover, the interaction of Ca and His had significant effect on decreasing the total protein content markedly under high Ni 300 µM conditions at 0.05 levels in Cal-J N3 cultivar compared to Petoearly CH. Exogenous His without addition of Ca2+ was effective in tomato cultivar Petoearly CH (Table 2). Generally, exogenous application of Ca and His increased leave ASC content under non-Ni conditions compared to the control samples especially in Petoearly CH cultivar (Table 2). Utilization of Ca and His increased levels of ASC significantly under 300 µM Ni stress in Petoearly CH cultivar and we determined highest concentration of ASC in this condition. The interaction of Ca and His had no significant effect on ASC content in the absent of Ni. Under high Ni stress conditions (300 µM), histidine elevated ASC concentration in Cal-J
-
N3 cultivar.
Ni treatment (150 µM) led to asignificant decrease in DHA content by separate applications of Ca and His compared to this stress condition without Ca and His. 300 µM of nickel treatment along with 300 µM of Ca and His, DHA content was substantially reduced more than stress condition while this interactive effect of Ca and His was not observed in Petoearly CH cultivar under the mentioned toxic nickel level (Table 2).
-
H2O2, EL and TBA reactive substances
The applied toxic nickel levels increased EL percentage compared to the control in both cultivars of tomato. Exogenous application of His alone in Cal-J N3 cultivar and also Ca with or without His in Petoearly CH cultivar under 150 µM of Ni improved the membrane integrity. In 300 µM of nickel treatment containing both components, EL was significantly decreased compared to stress conditions in Petoearly CH cultivar (Table 3).
It is generally regarded that H 2 O 2 and other aldehydes content in both cultivars decreased at the concentrations of 150 and 300 μM of Ni containing Ca and His compared to the control. The Ni levels of 150 and 300 μM containing separate treatments of Ca and His decreased the MDA content only in Petoearly CH cultivar. The interactive effects between Ca and His was significant in decreasing of H 2 O 2 and other aldehydes contents in the Petoearly CH cultivar under 300 μM of Ni at the 95% confidence level (P ≤ 0.05) (Table 3).
Response of antioxidant enzymes
The results illustrate that both Ca and His could up-regulate the antioxidant mechanisms in the tomato cultivars under unstressed and Ni toxicity conditions. Nickel 150 µM treatment containing Ca and/or His increased CAT activity in both cultivars of tomato (Table 4). CAT activity decreased under 300 µM of Ni treatment with applications of both Ca and His but APX activity increased under similar conditions compared to the toxic level (300 µM) of Ni in both cultivars of tomato. Under high Ni stress condition (300 µM), SOD activity decreased comparing to the control in Cal-J N3 cultivar (Table 4). Excessive histidine increased the activity of GPX and SOD under Ni treatments (150 and 300 µM) compared to the control in Cal-J N3 cultivar. In this cultivar, the interaction effects of Ca and His stimulated APX activity in Ni applications that was significant statistically. In Petoearly CH cultivar, Ca increased GPX and SOD activities under Ni 150 µM (Table 4).
Ca and Ni accumulation in root and shoot of the cultivars
The results from ICP, OES showed that high concentrations of Ca in shoots and roots of the tomato cultivars under CaCl2 treatments and non stress conditions (Figures 1A-D). The treatments containing NiSO4 without Ca and His, increased Ni accumulation in root and shoot of the two cultivars similarly (Figures 2A-D). Figures 2A and 2B show that after treatment with Ni 150 μM, Ni content in the shoots was about 1.28-fold higher in cv. Cal-J N3 compared to cv. Petoearly CH (cv. Cal-J N3: approximately 0.068 %DW, cv. Petoearly CH: approximately 0.053 %DW). Furthermore, there were considerable differences between the cultivars in terms of Ni accumulation of shoots after treatment with 300 μM of nickel. Accumulation of Ni in the roots also differed between the cultivars. Assimilation of Ni induces a greater decrease in the Ca uptake of root and shoot in both cultivars of tomato (Figures 1A-D). The application of Ca and His in hydroponic media increased calcium content of shoot and root in Petoearly CH and Cal-J N3 cultivars compared with the control plants (Figures 1A-D). In root tissues, Ca uptake declined slightly with the increase in Ca and His concentration and Ca concentration increased in shoot under similar conditions. The relationship between Ca content of root and shoot was inversed with respect to Ca accumulation in the tomato plants especially in Petoearly CH cultivar under treatments with 300 μM of Ni (Figures 1A-D). However, Ca content of roots improved under both nickel toxic levels 150 µM and 300 µM containing CaCl2 without His in Cal-J N3 and Petoearly CH, respectively compared to same stress conditions (Figures 1C and D).
Our data showed that Ni accumulation decreased significantly in shoot of the tomato cultivars under 150 and 300 μM of nickel treatments containing Ca+His (Figures 2 A and B). Furthermore, the interactive effect of Ca and His on Ni content was observed in roots of the cultivars treated by 300 μM of Ni. We also observed this interaction effect only about decrease of Ni content of root only in Petoearly CH cultivar under 150 μM of Ni treatment (Figure 2D). The application of Ca with His led to high decreasing in shoot nickel accumulation in Cal-J N3 cultivar treated by150 μM toxic level. On other hand, our data from root and shoot Ni analysis showed that nickel translocation from roots to shoots was declined. This was observed specially under Ni stress (150 μM) in both cultivars and also in Cal-J N3 cultivar treated by Ni 300 μM (Figures 2A-D). According to Table 1, it seems that Petoearly CH cultivar was more Ni-sensitive compared to other tomato cultivar. Thus, we observed significant effects for interaction of Ca and His in Petoearly CH cultivar compared to Cal-J N3 under Ni stress at 0.05 level . In other hand, the data concerning growth parameters presented in
Tab. 1 clearly show that Ni-induced growth inhibition especially in the case of roots was more pronounced in cv. Petoearly CH. In the latter cultivar treatment with Ni 300 μM, root length and fresh weight were reduced by 94% and 66%, respectively, comparing to the control (length control: 141 mm,
Ni 300: 9 mm; FW control: 1.42 g, Ni 300: 0.49 g), while the same Ni dose decreased root length and weight by 20% and 31%, respectively (length control: 145.6 mm, Ni 300: 116.6 mm; FW control: 1.26 g, Ni 300: 0.87 g) in cv. Cal-J N3.
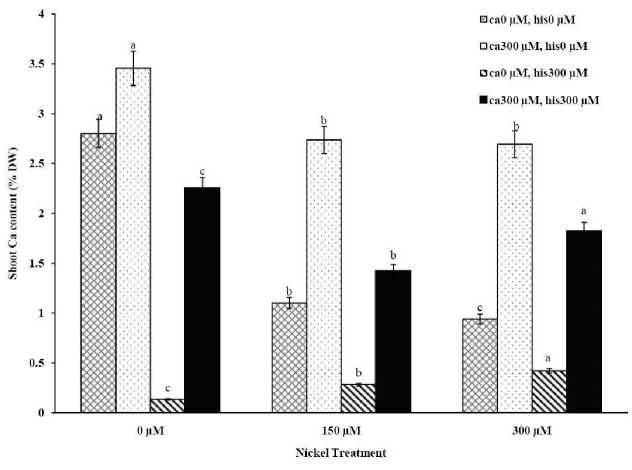
Figure 1A . The Mean of shoot calcium accumulation determined and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on Ca content changes in Cal-J N3 cultivar of tomato treated with a nutrient solution containing different concentrations of nickel, calcium and histidine. Vertical bars indicate the mean of four replications ± SD (n=4). Different letters indicate significantly different values at a particular treatment component containing calcium and histidinde ( P ≤ 0.05).
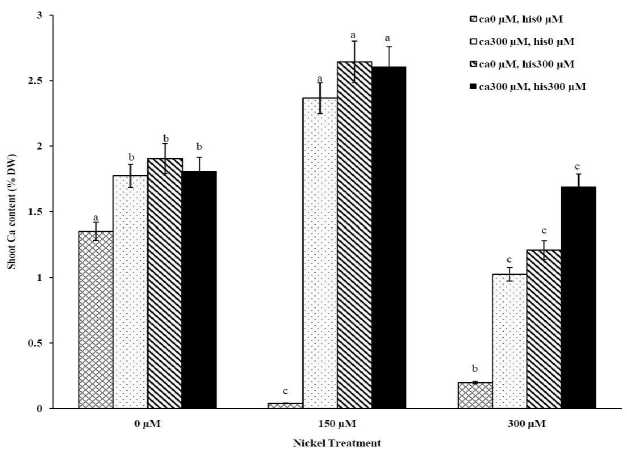
Figure 1B . The Mean of shoot calcium accumulation determined and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on Ca content changes in Petoearly CH cultivar of tomato treated with a nutrient solution containing different concentrations of nickel, calcium and histidine. Vertical bars indicate the mean of four replications ± SD (n=4). Different letters indicate significantly different values at a particular treatment component containing calcium and histidinde ( P ≤ 0.05).
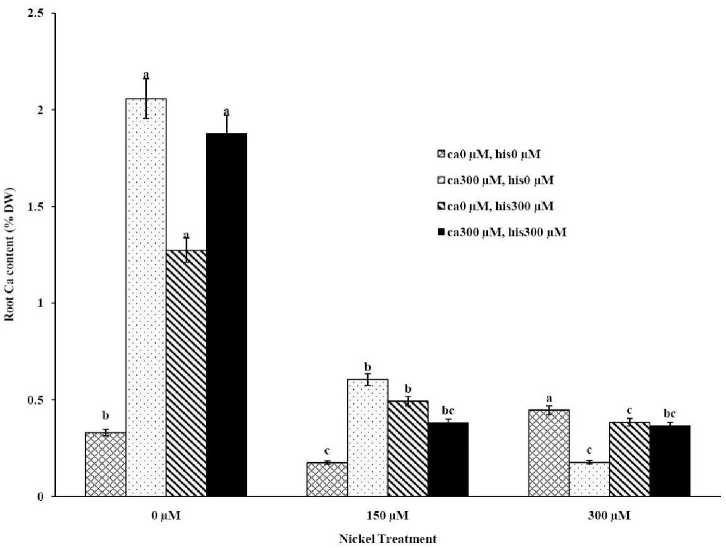
Figure 1C . The Mean of root calcium content determined and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on Root Ca content changes in Cal-J N3 cultivar of tomato treated with a nutrient solution containing different concentrations of nickel, calcium and histidine. Vertical bars indicate the mean of four replications ± SD (n=4). Different letters indicate significantly different values at a particular treatment component containing calcium and histidinde ( P ≤ 0.05).
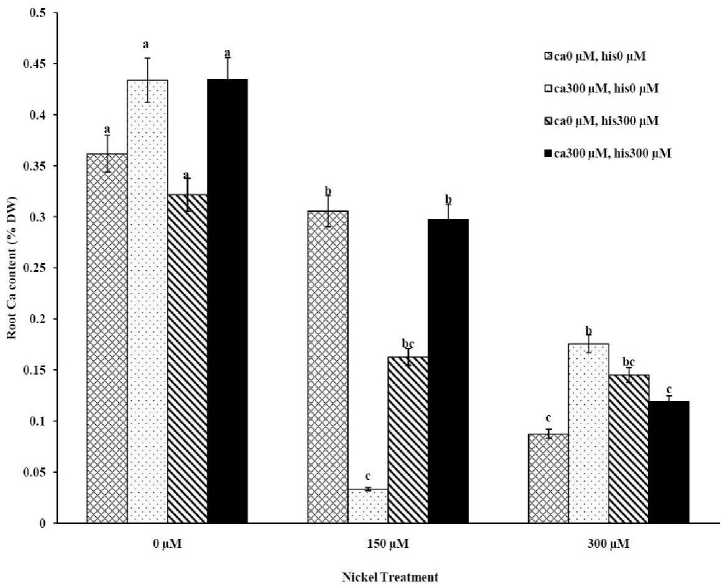
Figure 1D . The Mean of root calcium content determined and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on Root Ca content changes in Petoearly CH cultivar of tomato treated with a nutrient solution containing different concentrations of nickel, calcium and histidine. Vertical bars indicate the mean of four replications ± SD (n=4). Different letters indicate significantly different values at a particular treatment component containing calcium and histidinde ( P ≤ 0.05).
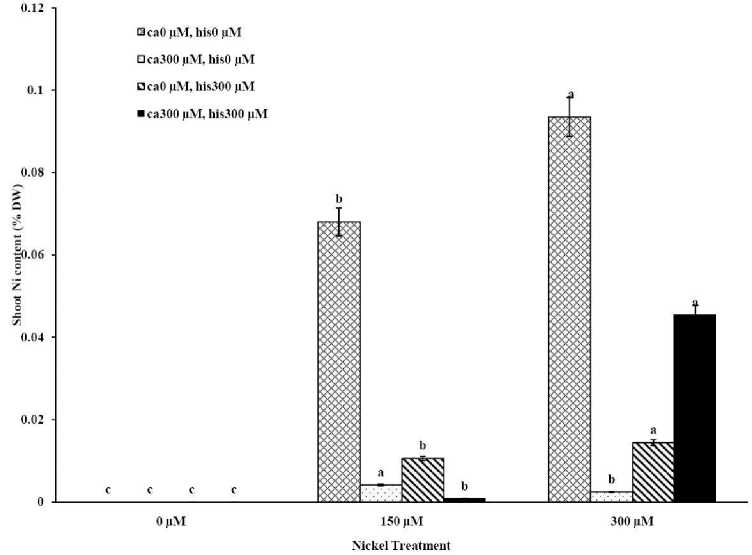
Figure 2A . The Mean of shoot nickel accumulation determined and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on Ni content changes in Cal-J N3 cultivar of tomato treated with a nutrient solution containing different concentrations of nickel, calcium and histidine. Vertical bars indicate the mean of four replications ± SD (n=4). Different letters indicate significantly different values at a particular treatment component containing calcium and histidinde ( P ≤ 0.05).
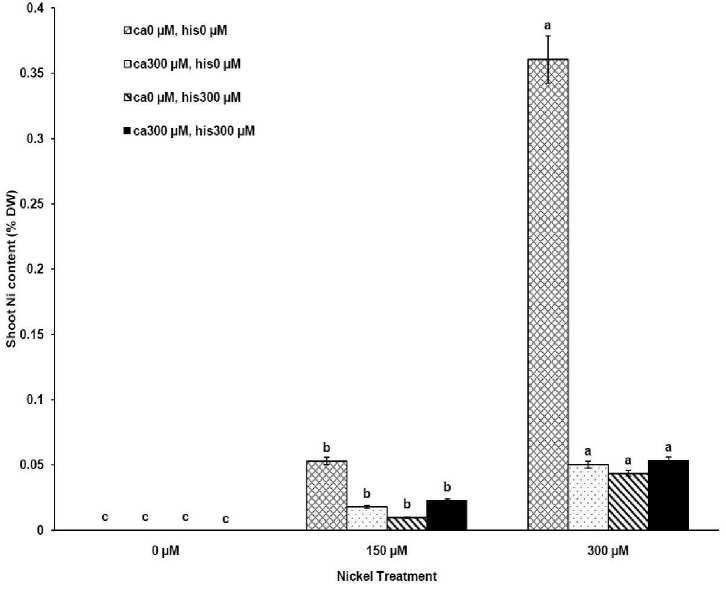
Figure 2B . The Mean of shoot nickel accumulation determined and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on Ni content changes in Petoearly CH cultivar of tomato treated with a nutrient solution containing different concentrations of nickel, calcium and histidine. Vertical bars indicate the mean of four replications ± SD (n=4). Different letters indicate significantly different values at a particular treatment component containing calcium and histidinde ( P ≤ 0.05).
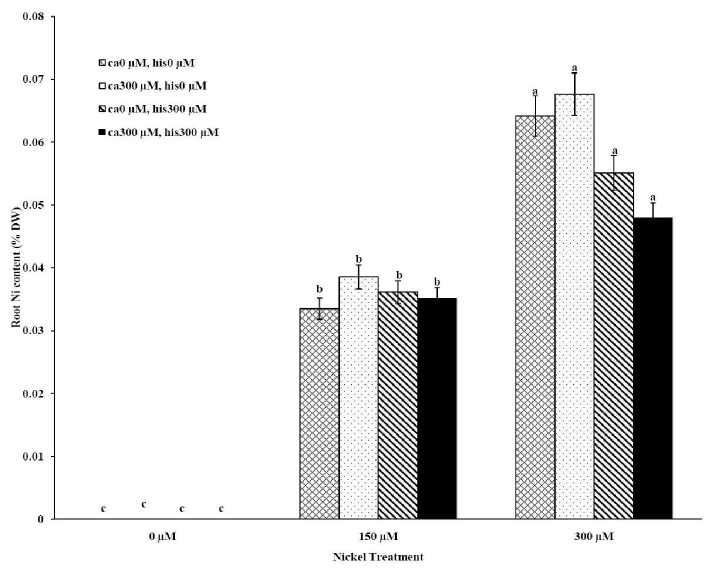
Figure 2C . The Mean of root nickel content determined and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on Root Ni content changes in Cal-J N3 cultivar of tomato treated with a nutrient solution containing different concentrations of nickel, calcium and histidine. Vertical bars indicate the mean of four replications ± SD (n=4). Different letters indicate significantly different values at a particular treatment component containing calcium and histidinde ( P ≤ 0.05).
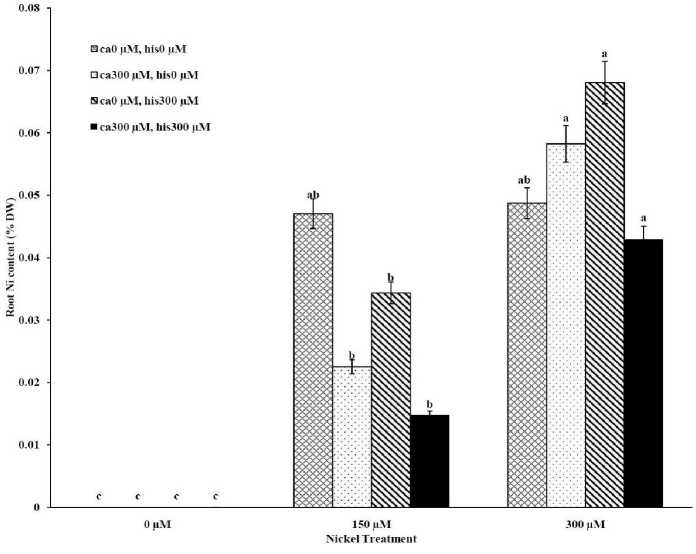
Figure 2D . The Mean of root nickel content determined and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on Root Ni content changes in Petoearly CH cultivar of tomato treated with a nutrient solution containing different concentrations of nickel, calcium and histidine. Vertical bars indicate the mean of four replications ± SD (n=4). Different letters indicate significantly different values at a particular treatment component containing calcium and histidinde ( P ≤ 0.05).
Table 1. Mean±SE of the Length and FW from shoot and root organs determined in our research and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on parameters changes in the two cultivars of tomato including Cal-J N3 and Petoearly CH . Means followed by different letter within columns are significantly different at P ≤ 0.05 according to Duncan’s Multiple Range Test.
|
Petoearly CH cultivar |
Cal-J N3 cultivar |
Treatments components (µM) |
|||||
|
Shoot FW (g / plant) |
Root Length (mm / plant) |
Shoot Length (mm / plant) |
Root FW (g/ plant) |
Shoot FW (g per plant) |
Root Length (mm / plant) |
Shoot Length (mm/ plant) |
Ni +His+ Ca |
|
1.76±0.02 a |
141.0±0.76 bc |
158.3±0.32 a |
1.26±0.04 ab |
1.18±0.06 ab |
145.6±0.60 ab |
143±0.70 ab |
0+0+0 |
|
0.77±0.01 cd |
123.0±6.5 d |
119.6±0.35 cd |
0.81±0.01 c |
0.55±0.02 e |
99.3±0.44 d |
136.6±0.66 ab |
150+0+0 |
|
0.61±0.05 d |
90.0±0.04 e |
112.0±0.65 d |
0.87±0.03 c |
0.50±0.03 e |
116.6±0.55 cd |
101.6±0.41 d |
300+0+0 |
|
1.77±0.05 a |
178.3±0.48 a |
138.3±0.28 ab |
1.03±0.02 bc |
0.94±0.05 cd |
165.9±0.74 ab |
139.6±0.80 ab |
0+0+300 |
|
0.77±0.06 cd |
135.3±0.54 c |
108.3±0.45 d |
1.25±0.02 ab |
1.22±0.05 ab |
120.0±0.59 cd |
116.6±0.51 cd |
150+0+300 |
|
0.59±0.03 e |
140.0±0.62 bc |
138.3±0.65 ab |
0.81±0.03 c |
0.84±0.03 d |
118.3±0.52 d |
116.6±0.62 cd |
300+0+300 |
|
1.06±0.01 b |
178.0±0.22 a |
146.6±0.48 ab |
1.29±0.04 a |
1.21±0.06 ab |
165.4±0.78 ab |
152.6±0.81 ab |
0+300+0 |
|
0.91±0.02 bc |
173.3±0.52 ab |
140.0±0.68 ab |
1.32±0.04 a |
0.98±0.04 bc |
149.0±0.74 ab |
148.3±0.33 ab |
150+300+0 |
|
1.13±0.05 b |
164.6±0.46 ab |
157.6±0.45 a |
1.00±0.05 c |
0.93±0.03 cd |
123.0±0.61 cd |
132.1±0.32 bc |
300+300+0 |
|
0.77±0.02 cd |
106.6±0.44 d |
121.6±0.77 bc |
0.52±0.01 d |
0.45±0.01 e |
108.0±0.52 cd |
100.1±0.59 d |
0+300+300 |
|
1.01±0.02 bc |
36.6±0.16 f |
118.3±0.19 c |
0.87±0.03 c |
1.14±0.05 ab |
133±0.04 d |
146.6±0.68 ab |
150+300+300 |
|
0.66±0.04 d |
123.3±0.61 d |
123.6±0.64 b |
0.86±0.03 c |
0.77±0.03 d |
133.3±0.67 bc |
133.3±0.64 bc |
300+300+300 |
|
0.0 |
0.0005 |
0.0231 |
0.01015 |
0.006 |
0.018 |
0.016 |
ANOVA Sig. (a ≤ 0.05) |
Table 2. Mean±SE of the total protein, ASC and DHA parameters determined in our research and threeway ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on parameters changes in the two cultivars of tomato including Cal-J N3 and Petoearly CH . Means followed by different letter within columns are significantly different at P ≤ 0.05 according to Duncan’s Multiple Range Test.
|
Petoearly CH cultivar |
Cal-J N3 cultivar |
Treatments components (µM) |
|||||
|
Leaf ASC (mg/ g FW) |
Root protein (mg/ g FW) |
Leaf protein (mg/ g FW) |
Leaf DHA (mg/ g FW) |
Leaf ASC (mg/ g FW) |
Root protein (mg/ g FW) |
Leaf protein (mg/ g FW) |
Ni +His+ Ca |
|
2.08±0.12 f |
1.73±0.04 de |
7.51±0.31 a |
4.57±0.25 b |
3.93±0.19 d |
2.51±0.05 cd |
5.61±0.24 d |
0+0+0 |
|
2.25±0.11 f |
3.64±0.13 bc |
7.14±0.32 a |
1.70±0.04 c |
3.55±0.16 d |
4.04±0.16 b |
5.16±0.23 d |
150+0+0 |
|
8.15±0.41 bc |
5.09±0.24 a |
4.51±0.22 c |
4.63±0.21 b |
5.04±0.20 cd |
7.16±0.34 a |
8.62±0.74 ab |
300+0+0 |
|
2.71±0.11 f |
0.55±0.01 f |
4.86±0.23 c |
4.18±0.26 b |
8.76±0.26 a |
1.22±0.04 d |
5.57±0.15 d |
0+0+300 |
|
2.48±0.15 f |
0.52±0.02 f |
6.13±0.28 ab |
0.93±0.01 de |
2.89±0.10 d |
5.47±0.27 b |
9.01±0.44 ab |
150+0+300 |
|
2.33±0.14 f |
3.11±0.12 c |
7.70±0.29 a |
1.48±0.02 d |
4.18±0.18 d |
7.64±0.31 a |
6.39±0.31 cd |
300+0+300 |
|
9.27±0.34 bc |
2.37±0.13 cd |
5.50±0.11 bc |
2.83±0.13 c |
7.05±0.28 b |
0.75±0.01 e |
1.70±0.26 f |
0+300+0 |
|
2.62±0.16 f |
5.80±0.24 a |
7.26±0.23 a |
0.56±0.01 e |
3.51±0.21 d |
3.19±0.12 bc |
5.09±0.11 d |
150+300+0 |
|
5.76±0.24 d |
1.19±0.03 ef |
4.97±0.24 c |
1.00±0.04 c |
6.36±0.29 c |
2.01±0.13 cd |
2.58±0.10 f |
300+300+0 |
|
1.61±0.01 g |
0.76±0.01 f |
5.36±0.26 bc |
4.40±0.17 b |
4.26±0.17 d |
4.08±0.24 b |
4.73±0.18 de |
0+300+300 |
|
16.20±0.74 a |
2.89±0.12 cd |
6.48±0.31 ab |
7.66±0.28 a |
2.09±0.11 d e |
7.70±0.25 a |
6.16±0.27 c |
150+300+300 |
|
4.76±0.26 d e |
4.83±0.21 ab |
3.65±0.11 d |
0.69±0.01 e |
1.63±0.04 e |
2.80±0.15 cd |
7.43±0.32 c |
300+300+300 |
|
0.026 |
0.015 |
0.00 |
0.011 |
0.004 |
0.013 |
0.00014 |
ANOVA Sig. (a ≤ 0.05) |
Table 3. Mean±SE of the some of leafs biochemical parameters determined in our research and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on parameters changes in the two cultivars of tomato including Cal-J N3 and Petoearly CH . Means followed by different letter within columns are significantly different at P ≤ 0.05 according to Duncan’s Multiple Range Test.
|
Petoearly CH cultivar |
Cal-J N3 cultivar |
Treatments components (µM) |
|||||
|
Leaf MDA (µM/ g FW) |
Leaf EL (%) |
Leaf H 2 O 2 content (µM/ gFW) |
Leaf aldehydes (µM/ g FW) |
Leaf MDA (µM/ g FW) |
Leaf EL (%) |
Leaf H 2 O 2 content (µM/ g FW) |
Ni +His+ Ca |
|
0.16±0.02 cd |
6.86±0.24 e |
13.92±0.64 g |
2.22±0.12 c |
0.07±0.01 f |
8.11±0.42 d |
61.10±2.88 e |
0+0+0 |
|
0.43±0.02 b |
27.59±1.24 bc |
191.36±5.14 a |
2.80±0.11 a |
0.19±0.01 e |
27.69±1.28 ab |
127.0±5.54 a |
150+0+0 |
|
0.49±0.03 a |
41.38±2.36 a |
152.13±6.24 b |
3.08±0.13 b |
0.28±0.01 c |
27.14±1.33 ab |
101.1±4.11 b |
300+0+0 |
|
0.13±0.01 f |
31.19±1.08 b |
128.54±5.88 d |
1.69±0.02 d |
0.27±0.01 c |
10.97±0.54 c |
87.26±3.21 c |
0+0+300 |
|
0.27±0.01 c |
23.04±1.14 c |
165.72±8.47 b |
1.60±0.01 d |
0.22±0.01 d |
33.69±1.66 ab |
78.03±2.19 d |
150+0+300 |
|
0.20±0.01 d |
40.15±2.99 a |
140.08±7.24 c |
4.31±0.18 a |
0.40±0.02 b |
25.41±0.97 ab |
86.74±2.45 c |
300+0+300 |
|
0.16±0.01 e |
14.51±0.48 d |
40.85±2.08 f |
2.18±0.19 bc |
0.28±0.01 c |
18.95±0.95 ab |
70.85±3.02 d |
0+300+0 |
|
0.16±0.01 e |
45.43±2.54 a |
42.38±2.56 f |
2.19±0.21 bc |
0.28±0.01 c |
19.52±1.05 ab |
82.38±1.02 c |
150+300+0 |
|
0.21±0.02 d |
42.20±1.26 a |
43.67±2.33 f |
2.62±0.10 a |
0.38±0.02 b |
37.74±1.98 a |
53.92±2.05 f |
300+300+0 |
|
0.19±0.01 d |
41.55±2.17 a |
48.54±2.47 f |
2.39±0.12 a |
0.27±0.01 c |
15.31±0.74 bc |
79.05±3.54 d |
0+300+300 |
|
0.28±0.03 c |
21.85±1.45 c |
193.67±6.75 a |
3.34±0.14 ab |
0.26±0.01 c |
33.74±1.44 ab |
124.1±4.56 a |
150+300+300 |
|
0.30±0.02 c |
32.08±1.67 b |
82.90±4.12 e |
2.81±0.16 a |
0.54±0.03 a |
26.41±1.11 ab |
61.10±3.21 e |
300+300+300 |
|
0.004 |
0.0 |
0.001 |
0.005 |
0.026 |
0.028 |
0.004. |
ANOVA Sig. (a ≤ 0.05) |
Table 4. Mean±SE of the antioxidative enzymes activity of leaf determined in our research and three-way ANOVA with multiple but equal number of observations per test tube for the effects of individual treatments and their interactive effects on parameters changes in the two cultivars of tomato including Cal-J N3 and Petoearly CH . Means followed by different letter within columns are significantly different at P ≤ 0.05 according to Duncan’s Multiple Range Test.
|
Petoearly CH cultivar Leaf |
Cal-J N3 cultivar Leaf |
Treatments components (µM) |
|||||
|
APX (U/mg protein) |
CAT (U/mg protein) |
GPX (U/mg protein) |
SOD (U/ mg protein) |
APX (U/ mg protein) |
CAT (U/mg protein) |
GPX (U/mg protein) |
Ni +His+ Ca |
|
3.50±0.14 d e |
10.97±0.42 e |
18.39±0.87 d |
8.67±0.34 c |
1.30±0.03 d e |
2.46±0.13 d |
6.55±0.28 g |
0+0+0 |
|
2.11±0.16 e |
7.19±0.34 f |
17.85±0.56 e |
8.46±0.41 c |
0.98±0.02 e |
7.44±0.28 c |
9.76±0.51 f |
150+0+0 |
|
9.05±0.33 c |
16.26±0.75 c |
68.95±2.77 a |
5.81±0.31 e |
1.93±0.06 d |
13.05±0.1 b |
17.72±0.32 d |
300+0+0 |
|
0.97±0.02 f |
26.21±1.04 b |
21.26±0.99 d |
8.52±0.34 c |
4.42±0.26 a |
2.92±0.17 d |
14.70±0.65 de |
0+0+300 |
|
12.91±0.24 b |
1.51±0.03 h |
70.40±2.11 a |
5.37±0.24 e |
1.76±0.03 d |
9.17±0.37 bc |
9.84±0.39 f |
150+0+300 |
|
1.76±0.04 f |
5.50±0.18 f |
17.55±0.64 e |
7.77±0.34 d |
3.84±0.25 a |
9.85±0.15 bc |
8.38±0.33 f |
300+0+300 |
|
4.06±0.15 d e |
5.53±0.24 f |
34.61±1.33 b |
28.08±1.5 a |
2.27±0.06 c |
2.03±0.13 d |
52.36±1.13 b |
0+300+0 |
|
1.49±0.03 f |
14.47±0.41 c |
27.20±1.24 c |
13.35±0.54 b |
2.19±0.05 c |
24.34±1.3 a |
26.35±1.02 c |
150+300+0 |
|
1.66±0.03 f |
10.13±0.62 e |
19.44±0.57 d |
26.70±1.24 a |
4.03±0.14 a |
6.51±0.22 c |
58.50±2.34 a |
300+300+0 |
|
0.41±0.01 g |
41.83±1.24 a |
18.11±0.84 d |
11.61±0.64 bc |
1.98±0.05 d |
2.97±0.17 d |
7.79±0.28 g |
0+300+300 |
|
6.44±0.28 c d |
4.85±0.19 g |
26.09±1.29 c |
8.70±0.4 6c |
2.85±0.05 ab |
9.09±0.45 b c |
24.86±1.18 c |
150+300+300 |
|
16.88±0.71 a |
12.14±0.55 d |
27.85±1.18 c |
6.60±0.34 d |
2.81±0.06 ab |
6.88±0.52 c |
19.42±0.88 d |
300+300+300 |
|
0.0311 |
0.0 |
0.0003 |
0.001 |
0.0 |
0.003 |
0.02 |
ANOVA Sig. (a ≤ 0.05) |
DISCUSSION
Although the Ca and Ni accumulation in roots are usually inversely correlated, root Ca accumulation is more often accounted for a higher share of variability in root elongation compare with Ni accumulation in root tissue at high concentration of Ca compared with the control conditions (Wu and Hendershot, 2010). Therefore, the evaluation of root elongation requires including Ni and Ca as predictor whenever environmental conditions (low Ca concentrations) significantly affect the amount of Ca accumulated. Root elongation is highly positively correlated with total Ca content in the roots (Wu and Hendershot, 2010). In this study our data showed that Ca plus Ni increased length and FW of root in the tomato cultivars significantly in comparison to Ni treatments without excess CaCl 2 . Thus, the treatments containing excess Ca (300 µM) has positive effects on shoot and root length elongation (Table 1, Figures 1A-D). However, Ca and His interaction effect on alleviation of growth and Ca accumulation of tomato plants were significant especially in Petoearly CH cultivar under Ni stress. Additionally, Ca plus His alleviated calcium content of shoots and roots, more than control plants in both cultivars (Figures 1A-D). In root tissue of the cultivars, Ca uptake declined slightly with the increase in Ca and His concentration and Ca concentration increased in shoot under similar conditions. Therefore, the relationship between root and shoot Ca was inversed because of higher Ca accumulation in shoot especially in Petoearly CH cultivar under Ni 300 μM treatments (Figures 1A-D). However, Ca content of roots increased under nickel levels 150 µM and 300 µM containing CaCl 2 without His in Cal-J N3 and Petoearly CH, respectively which is compared to same stress conditions (Figures 1C and D).
In this investigation, the MDA and other aldehydes as lipid peroxidation markers were higher than the control under Ni toxic levels (Table 3). Membrane degradation was reported as a consequence of Ni stress in roots of Triticum aestivum (Pandolfini et al ., 1992). Highly reactive oxygen species could be responsible for Ni-induced membrane damage that increasing ROS content has been reported for roots and leaves of wheat, hairy roots of Alyssum bertolonii and Nicotiana tabacum (Gajewska and Sklodowska, 2007). Thompson et al . (1987) documented that lipoxygenase activation precedes lipid peroxidation. This mechanism might underlie Ni induced peroxidation as seen in primary leaves of Phaseolus vulgaris under Cu and Zn stress (Weckx et al ., 1997). In addition, Pandolfini et al . (1992) showed that Ni causes membrane degradation leading to a marked K+ and other cation leakage probably. In our research, electrolytes leakage percentage (as an important membrane damage index) increased in both cultivars under nickel stress compared to the control plants (Table 3). It has been suggested that the extent of lipid peroxidation and membrane permeability were closely related to higher levels of ROS during either senescence or under stress conditions (Weckx and Clijsters, 1997).
Our findings shown that Ca and His interaction inhibited MDA and other aldihydes generation compared to stress condition in the tomato cultivars especially in Petoearly CH compare with other cultivar (Table 3).
Application of His alone in Cal-J N3 cultivar and also Ca and/or His in Petoearly CH cultivar during 150 µM Ni, declined EL percentage. EL was significantly decreased, compared to stress conditions in Petoearly CH cultivar that treated with
300 µM of nickel treatment containing both Ca and His (Table 3).
We evaluated the activities of SOD, CAT, GPX and APX that involved in free radicals and H 2 O 2 detoxification under the experiment treatments. Higher activity of the SOD led to production of H 2 O 2 . Subsequently, increasing in activity of CAT, GPX and APX can remove high H 2 O 2 significantly in the tomato plants (Table 4).
In the present study, a significant increase in leaf SOD activity was observed in both tomato cultivars treated with Ca and His treatments during different levels of nickel (Table 4), suggesting that SOD may function as a ROS scavenger (Alscher et al ., 2002). Recent studies have demonstrated that over expression of chloroplastic Cu/Zn-SOD in transgenic Nicotiana tabacum (Badawi et al ., 2004) can provide enhanced ion toxicity tolerance. Even though a high SOD activity (compared in equal total protein content of tissue) protects plants against the superoxide radical, it cannot be considered solely responsible for membrane protection against peroxidation. This ROS should be then scavenged by other enzymes such as catalase and peroxidases.
The fact that leave CAT activity of the Cal-J N3 cultivar in the control and Ni stress treatments was lower than Petoearly CH cultivar could be the result of genetic differences and higher Ni sensitivity of Cal-J N3 cultivar to Ni toxicity. However, when the tomato plants were subjected to NiSO 4 , the increases in CAT activity were higher under Ni stress 300 μM than the control but Ca and His stimulated its activity during Ni stress (Table 4).
It seems that up regulation of CAT is a requirement for the removal of H2O2 in roots and leaves of stressed plants or decreasing in CAT activity may result from enzyme deactivation (Dat et al., 2000). The contradiction between CAT activation and suppression in the presence of metals may depend upon the element, its concentration and the plant species and cultivars (Gratao et al., 2005).
Higher activity of APX was recorded after application of Ni, Ca and His treatments in the tomato cultivars. The higher level in APX activity was observed in the tomato cultivar Petoearly CH than other cultivar (Table 4). Further more GPX activity stimulation was considerably higher than APX in our research (Table 4). Consequently, APX may remain in a saturated state for its substrate. In addition, the increase in activities of APX and GPX has been found by Jocsak et al., (2008) in barley seedlings. However, the activities of APX and GPX in both tomato cultivars were also affected by Ca and His treatments compared to Ni 150 µM lacking of Ca and His. The similar results were found for other plant species by Mittova et al. 2002 and also elevated APX activity in plants exposed to toxic levels of Ni has been reported earlier in wheat and rice (Gajewska and Skłodowska, 2008). It has been speculated that under stress conditions, H2O2 acts as a systemic intracellular signal for the induction of APX (Hernandez et al., 2004). Therefore, H2O2 is considered to playing a central role in physiological functions such as plant signaling, regulating plant development and adaptation to biotic and abiotic stresses. Increased availability of H2O2 is a commonly observed feature of plant stress response signature. Stresses such as high light, heat, salinity, cold and pathogen attack, besides others, are all considered to trigger H2O2 accumulation that contributes to the regulation the expression of many genes. H2O2-mediated regulation of cell division, differentiation and growth must be viewed not only within the context of the basic redox chemistry of the plant cell that is governed by photosynthesis and respiration but also within the bigger picture of the physiological environment. The physiological context involves a continuous supply of environmental stimuli that can trigger intracellular H2O2 accumulation or modulate the response to such accumulation (Foyer and Noctor, 2012; Chakraborty and Pradhan, 2012).
These antioxidative changes could be related to complexity of the ascorbate-glutathione cycle that have enzymes coded by multigenic families whose products are localized in different cell compartments and are regulated differently by stress conditions. For instance, in the cytosolic fraction of sorghum leaves subjected to water stress there was a decrease in monodehydro ascorbate reductase (MDHAR) activity, and increased dehydro ascorbate reductase (DHAR) activity (Mittova et al ., 2002). Our data also showed that under high Ni stress conditions (300 µM), application of histidine resulted in more increased concentration of ASC than the control in Cal-J N3 cultivar while Ca and His interaction on increasing ASC content was significant at 0.05 Level in Petoearly CH cultivar plants (Table 2).
Generally, the obtained data (Figures 2A-D) indicated that Ca and His inhibited Ni accumulation and translocation in root and shoot in the tomato cultivars under Ni stress via ion chelating mechanisms. However, heavy metal detoxification in plants is linked to the synthesis of cysteine-rich poly- peptides, so called phytochelatins (PCs) (Grill et al., 1985). Similar to these compounds, L-histidine are able to chelate Ni2+ in different parts of plants (Howden et al., 1995). The function of calcium in preventing toxic effects of HMs during the transport across the cell wall is controversially discussed, very often the optimum Ca content in plant tissue correlate with HM tolerance (de Knecht et al., 1994).
The tomato plants accumulated appreciable amounts of Ni in both roots and shoots under Ni stress without Ca and His. Our results shown that inhibition of shoot and root Ca uptake was detected after lipid peroxidation began (Figures 1A-D and Table 3). This latter phenomenon appears to be a primary effect of Ni stress that may result in inhibition of tomato plants growth and nutrition especially in Cal-J N3 cultivar that this cultivar was less Ni sensitive compared to the other cultivar in our work (Table 1). Similar to the results obtained by the earlier studies (Madhava and Sresty, 2000), we found that the Ni uptake in the two cultivars (especially Petoearly CH cultivar) is dependent upon nickel concentration in treatments (Figures 2A-D). Accumulated calcium in root tissue increased tolerance of plants to Ni toxicity and also decrease Ni uptake and its accumulation especially in tomato cultivar Petoearly CH (Figures 1A-D and 2A-D).
The Ni and Ca internalization process within the root is physiologically controlled and root growth is a complicated process (Table 1). Our study showed that the measured accumulations of Ni and Ca correlate best with the treatments whenever data collected under varied concentration Ca and His treatments. This suggests that uptake of Ni and Ca is affected by influence of Ca and His on Ni uptake and translocation under the stress conditions (Wu and Hendershot, 2010).
Generally, based on the obtained results it can be suggested that Ni toxicity induced oxidative stress in the Ni sensitive cultivars of tomato resulting in increased generation of H 2 O 2 and activation of antioxidant enzymes. The different increases in CAT, APX, SOD and GPX activities under the treatments suggest their roles in scavenging
ROS in tomato plants especially in Petoearly CH cultivar. The data shown that Ca and His elevated antioxidative responses in studies on tomato cultivars under Ni toxicity especially Petoearly CH through inhibition the Ni toxic effects by decreasing nickel uptake and its translocation. For the first time our results showed that the interaction effects between Ca and His could alleviate nickel stress and growth promoting in sensitive tomato cultivar plants such as Petoearly CH. Finally, exogenous Ca+His improved tolerance of this Ni-sensitive tomato cultivar under oxidative stress and higher accumulated Ni of shoots compared to Cal-J N3 cultivar. Furthermore, new researches to understand the basic mechanism under Ca interaction effects are essential in cell wall with heavy metal ligands such as His for alleviation of oxidative stress. Also, determination of the differences in activity among the isoforms of SOD, CAT, GPX and other antioxiative enzymes is our next aim in future researches through the PAGE under Ca, His and Ni-induced oxidative stress in the tomato plants. Thus, field experiments are necessary at contaminated soils with nickel pollution for oxidative stress decreasing and plants tolerance in these conditions. In conclusion, the application of Ca+His as chelators was beneficial to higher oxidative stress tolerance in the tomato cultivars (especially Petoearly CH comparing to Cal-J N3) and increasing growth of the tomato plants under Ni stress conditions.
ACKNOWLEDGMENTS
This Original research Paper is a part of research project in Ph.D thesis (for Ph.D candidate) that funded by Biology Department, Shahid Bahonar University of Kerman, Iran. We thank MSc Elnaz Ghotbi for her coordination in text English editing of this MS.
Список литературы Oxidative stress tolerance by calcium and histidine in two tomato cultivars under nickel stress
- Ahmad, P., Abdul Jaleel, C., Salem, M.A., Nabi, G., Sharma, S. (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotech., 30(3), 161-175
- Alscher, R.G., Erturk, N., Heath, L.S. (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot., 53, 1331-1341
- Baccouch, S., Chaoui, A., Ferjani, E. (1998) Nickel-induced oxidative damage and antioxidant responses in Zea mays shoots. Plant Physiol. Biochem., 36, 689-694
- Badawi, G.H., Yamauchi, Y., Shimada, E. (2004) Enhanced tolerance to salt stress and water deficit by over expressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci., 166, 919-928
- Ben Hamed, K., Castagna, A., Salem, E. (2007) Sea fennel (Crithmum maritimum L.) under salinity conditions: a comparison of leaf and root antioxidant responses. Plant Growth Regul., 53, 185-194
- Bradford, M.M. (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Bioch., 72, 248-254
- Brooks, R.R., Morrison, R.S., Reeves, R.D. (1979) Hyperaccumulation of nickel by Alyssum Linnaeus (Cruciferae). Proceedings of the Royal Society. London Ser. B, Biol Sci., 203, 387-403
- Dalvi, A.A., Bhalerao, S.A. (2013) Response of Plants towards Heavy Metal Toxicity: An overview of Avoidance, Tolerance and Uptake Mechanism. Annal. Plant Sci., 2(9), 362-368
- Dat, J., Vandenabeele, S., Vranova, E. (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci., 57, 779-795
- de Knecht, J.A., van Dillen, M., Koevoets, P.L. (1994) Phytochelatins in cadmium-sensitive and cadmium-tolerant Silene vulgaris. Plant Physiol., 104, 255-261
- de Pinto, M.C., Francis, D., De Gara, L. (1999) The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma., 209, 90-97
- Demarty, M., Morvan, C., Thellier, M. (1984) Calcium and the cell wall. Plant Cell. Environ., 7, 441-448
- Dhindsa, R.S., Plumb-Dhindsa, P., Thorpe, T.A. (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp Bot., 32, 93-101
- EL-beltagi, H.S., Mohamed, A.A. (2010) Changes in non Protein Thiols, some Antioxidant Enzymes Activity and Ultrastructural Alteration in Radish Plant (Raphanus sativus L.) Grown under Lead Toxicity. Not Bot Hort Agrobot., 38(3), 76-85
- Felix-Henningsen, P., Urushadze, T., Steffens, D., Kalandadze, B., Narimanidze, E. (2010) Uptake of heavy metals by food crops from highly-polluted Chernozem-like soils in an irrigation district south of Tbilisi, eastern Georgia. Agri Res., 8(1), 781-795
- Foyer, C.H., Descourvibres, P., Kunert, K. (1994) Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell. Environ., 17, 507-523
- Foyer, C.H., Noctor, G. (2012) Managing the cellular redox hub in photosynthetic Organisms. Plant, Cell. Environ 35, 199-201
- Gajewska, E., Sklodowska, M. (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. BioMetals., 20, 27-36
- Gajewska, E., Sklodowska, M. (2008) Differential biochemical responses of wheat shoots and roots to nickel stress: antioxidative reactions and proline accumulation. Plant Growth Regul., 54, 179-188
- Ganje, T.J., Page, A.L. (1974) Rapid acid dissolution of plant tissue for cadmium determination by atomic absorption spectrophotometry. At Absorption News Lett., 13, 131-134
- Ghani, A. (2010) Toxic Effects of Heavy Metals on Plant Growth and Metal Accumulation in Maize (Zea mays L.). Iranian J. Toxic., 3(3), 35-41
- Giannopolitis, C.N., Ries, S.K. (1977) Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol., 59, 309-314
- Girija, C., Smith, B.N., Swamy, P.M. (2002) Interactive effects of sodium chloride and calcium chloride on the accumulation of proline and glycinebetaine in peanut (Arachis hypogaea L.). Environ. Exp Bot., 47, 1-10
- Gratao, P.L., Gomes-Junior, A.L., Medici, L.O., Azevedo, R.A. (2005) Response of Crotalaria juncea to nickel exposure. Braz J. Plant Physiol., 17, 267-272
- Grill, E., Winnacker, E.L., Zenk, M.H. (1985) Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science., 230, 674-676
- Hanson, J.B. (1984) The functions of calcium in plant nutrition. In Tinke, P B, Läuchli, A (ed), Advances in Plant Nutrition. Praeger, New York, pp.149-208
- Heath, R.L., Packer, L. (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys., 125, 189-198
- Hepler, P.K., Wayne, R.O. (1985) Calcium and plant development. Annu Rev. Plant Physiol., 36, 397-439
- Hernandez, J.A., Escobar, C., Creissen, G., Mullineaux, P.M. (2004) Role of hydrogen peroxide and the redox state of ascorbate in the induction of antioxidant enzymes in pea leaves under excess light stress. Funct Plant Biol., 31, 359-368
- Hoagland, D.R., Arnon, D.I. (1950) The water-culture method for growing plants without soil. Calif Agric Exp Sta Circ., 347, 1-32
- Hossain, M.A., Piyatida, P., da Silva, J.A.T., Fujita, M. (2012) Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 55, 872-875
- Howden, R., Goldsbrough, P.B., Andersen, C.R., Cobbett, C.S. (1995) Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol., 107, 1059-1066
- Jocsak, I., Vegvari, G., Rabnecz, G., Droppa, M. (2008) Investigation of nickel stress induction in terms of metal accumulation and antioxidative enzyme activity in barley seedlings. Acta Biol Szeged., 52, 167-71
- Joho, M., Ishikawa, Y., Kunikane, M., Inouhe, M., Tohoyama, H., Murayama, T. (1992) The sub cellular distribution of nickel in Ni-sensitive and Ni resistant strains of Saccharomyces cerevisiae. Microbios., 71, 149-159
- Khalid, B.Y., Tinsley, J. (1980) Some effects of nickel toxicity on rye grass. Plant. Soil., 55, 139-144
- Kramer, U., Cotter-Howells, J.D., Charnock, J.M. (1996) Free histidine as a metal chelator in plants that accumulate nickel. Nature., 379, 635-638
- Kumchai, J., Huang, J.Z., Lee, C.Y., Chen, F.C., Chin, S.W. (2013) The Induction of Antioxidant Enzyme Activities in Cabbage Seedlings by Heavy Metal Stress. World Acad. Sci Engin. Techno., 73, 25-39
- Kumar, P., Tewari, R.K., Sharma, P.N. (2007) Excess nickel-induced changes in antioxidative processes in maize leaves. J. Plant Nutr. Soil Sci., 170, 796-802
- Küpper, H., Lombi, E., Zhao, F.J. (2001) Cellular compartmentation of nickel in the hyper accumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J. Exp Bot., 52, 2291-2300
- Lee, J., Reeves, R.D., Brooks, R.R., Jaffre, T. (1977) Isolation and identification of a citrato-complex of nickel from nickel-accumulating plants. Phytochem., 16, 1503-1505
- Ma, J.F., Ryan, P.R., Delhaize, E. (2001) Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci., 6, 273-278
- Madhava Rao, K.V., Sresty, T.V. (2000) Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci., 157, 113-128
- Marschner, H. (1995) Mineral nutrition of higher plants. 2nd edition, Academic Press, London
- McLaughlin, S. B., Wimmer, R. (1999) Calcium physiology and terrestrial ecosystem processes. New Phytol., 142, 373-417
- Meirs, S., Philosoph-hadas S., Aharoni N. (1992) Ethylene increased accumulation of fluorescent lipid peroxidation products detected during senescence of parsley by a newly developed method. Journal of the American Society for Hort Sci., 117(1), 128-132
- Mittova, V., Tal, M., Volokita, M., Guy, M. (2002) Salt stress induces up-regulation of an efficient chloroplast antioxidant system in the salt-tolerant wild tomato species Lycopersicon pennellii but not in the cultivated species. Physiol Plant., 115, 393-400
- Nakano, Y., Asada, K. (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell Physiol., 22(5), 867-880
- Osteras, A.H., Greger, M. (2006) Interactions between calcium and copper or cadmium in Norway spruce. Biol Planta., 50, 647-652
- Pandolfini, T., Gabbrielli, R., Comparini, C. (1992) Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L. Plant Cell. Environ., 15, 719-725
- Plewa, M.J., Smith, S.R., Wanger, E.D. (1991) Diethyldithiocarbamate suppresses the plant activation of aromatic amines into mutagens by inhibiting tobacco cell peroxidase. Mutat Res., 247, 57-64
- Salt, D.E., Rauser, W.E. (1995) MgATP-dependent transport of phytochelatins across the tonoplast of oat roots by Cd2+/H+ and Mn2+/H+ exchange activities. Plant Physiol, 107, 1293-1301
- Schickler, H., Caspi, H. (1999) Response of antioxidative enzymes to nickel and cadmium stress in hyper accumulator plants of the genus Alyssum. Physiol Plant., 105, 39-44
- Shah, A., Kamei, S., Kawai, S. (2001) Metal micronutrients in xylem sap of iron-deficient barley as affected by plant-borne, microbial and synthetic metal chelators. J. Soil Sci. Plant Nutr., 47, 149-156
- Skyba, M., Petijov, L., Kosuth, J., Koleva, D.P., Ganeva, T.G., Kapchina-Toteva, V.M., Cellrov, E. (2012) Oxidative stress and antioxidant response in Hypericum perforatum L. plants subjected to low temperature treatment. J. Plant Physiol., 169, 955-964
- Tanyolac, D., Ekmekci, Y., Unalan, S. (2007) Changes in photochemical and antioxidant enzyme activities in maize (Zea mays L.) leaves exposed to excess copper. Chemosphere., 67, 89-98
- Temple, P. J., Bisessar, S. (1981) Uptake and toxicity of nickel and other metals in crops grown on soil contaminated by a nickel refinery. J. Plant Nutr., 3, 473-482
- Thompson, J.E., Legge, R.L., Barber, R.J. (1987) The role of free radicals in senescence and wounding. New Phytol., 105, 317-344
- Tiffin, L.O. (1971) Translocation of nickel in xylem exudate of plants. Plant Physiol., 48, 273-277
- Velikova, V., Yordanov, I., Edreva, A. (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Science., 151, 59-66
- Von Wiren, N., Marschner, H., Romheld, V. (1996) Roots of iron-efficient maize also absorbs phyto siderophore-chelated zinc. Plant Physiol., 111, 1119-1125
- Weckx, J.E.J., Clijsters, H.M.M. (1997) Zn phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol. Biochem., 35, 405-410
- Welch, R.M. (1981) The biological significance of Nickel. J. Plant Nutr., 3, 345-356
- Whitby, L.M., Hutchinson T.C. (1974) Heavy-metal Pollution in the Sudbury Mining and Smelting Region of Canada, II. Soil Toxicity Tests. Environ Conserv., 1(3), 191-200
- Wu, Y., Hendershot, W.H. (2010) The effect of calcium and pH on nickel accumulation in and rhizotoxicity to pea (Pisum sativum L.) root-empirical relationships and modeling. Environ pollut., 158(5), 1850-1856


