Pathophysiology of Parkinson’s disease and the efficiency of its treatment
Автор: Jalalov Ravshanbek, Salieva Minara, Abdurahimov Abdukhalim, Nugmanov Ozodbek
Журнал: Re-health journal @re-health
Рубрика: Неврология
Статья в выпуске: 2,3 (6), 2020 года.
Бесплатный доступ
Parkinsonism is a secondary neurodegenerative disease characterized by degeneration of dopaminergic neurons, accompanied by motor and non-motor symptoms. Primary neurodegenerative disease Alzheimer’s is a syndrome of mental retardation and memory loss. Parkinson's is one of the most common diseases in the world. It is mainly found in people over 50 years of age. According to modern statistics, about 100 million people are infected with Parkinson's disease. Of these, 40% are in North Africa and the Arab world as a whole, and about 14% are Ashkenazi Jews. This article discusses the pathophysiology of Parkinsonism and its treatment.
Parkinsonism, dopaminergic, degeneration, neurodegenerative, alzheimer's syndrome, pathophysiology, parkinsonizm, dopaminergik, degeneratsiya, neyrodegenerativ, altsgeymer sindromi, patofiziologiya
Короткий адрес: https://sciup.org/14125584
IDR: 14125584 | DOI: 10.24411/2181-0443/2020-10083
Текст научной статьи Pathophysiology of Parkinson’s disease and the efficiency of its treatment
Introduction: Parkinsonism is a clinical syndrome characterized by tremor, bradykinesia, rigidity, and postural instability. These are the four motor symptoms found in Parkinson's disease (PD), after which it is named, dementia with Lewy bodies (DLB),
Parkinson's disease dementia (PDD), and many other conditions. A wide range of causes may lead to this set of symptoms, including neurodegenerative conditions, drugs, toxins, metabolic diseases, and neurological conditions other than PD.
We can say from the results of research [1-6] there is no cure for Parkinson's disease, but medications, surgery, and physical treatment can provide relief and are much more effective than treatments available for other neurological disorders like Alzheimer’s disease, motor neuron disease, and Parkinson plus syndromes. The main families of drugs useful for treating motor symptoms are levodopa (always combined with a dopa decarboxylase inhibitor and sometimes also with a COMT inhibitor) , dopamine agonists and MAO-B inhibitors. The stage of the disease and the age at disease onset determine which group is most useful. Braak staging of Parkinson's disease gives six stages, that can be used to identify early stages, later stages, and late stages. The initial stage in which some disability has already developed and requires pharmacological treatment is followed by later stages associated with the development of complications related to levodopa usage, and a third stage when symptoms unrelated to dopamine deficiency or levodopa treatment may predominate. Treatment in the first stage aims for an optimal trade-off between symptom control and treatment side-effects. The start of levodopa treatment may be postponed by initially using other medications such as MAO-B inhibitors and dopamine agonists instead, in the hope of delaying the onset of complications due to levodopa use. However, levodopa is still the most effective treatment for the motor symptoms of PD and should not be delayed in patients when their quality of life is impaired. Levodopa-related dyskinesias correlate more strongly with duration and severity of the disease than duration of levodopa treatment, so delaying this therapy may not provide much longer dyskinesia-free time than early use. In later stages the aim is to reduce PD symptoms while controlling fluctuations in the effect of the medication. Sudden withdrawals from medication or its overuse have to be managed. When oral medications are not enough to control symptoms, surgery, deep brain stimulation, subcutaneous waking day apomorphine infusion and enteral dopa pumps can be of use. Late stage PD presents many challenges requiring a variety of treatments including those for psychiatric symptoms particularly depression, orthostatic hypotension, bladder dysfunction and erectile dysfunction. In the final stages of the disease, palliative care is provided to improve quality of life.
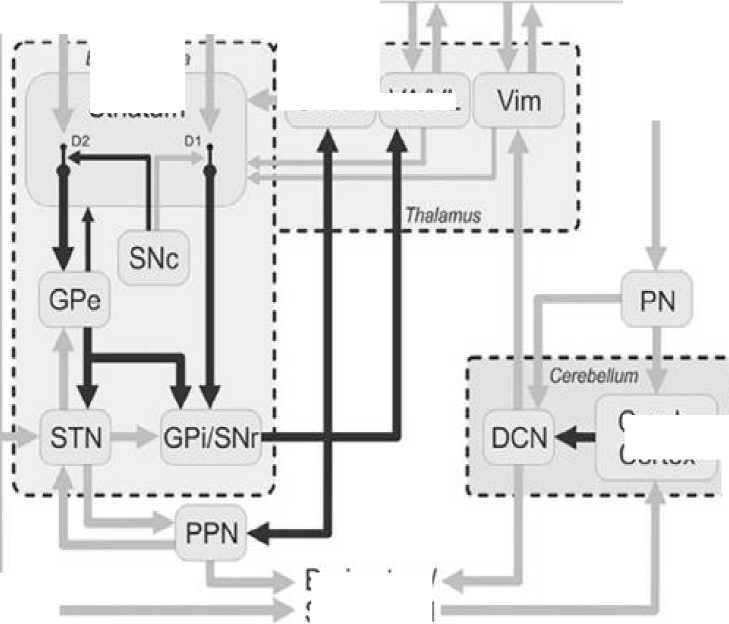
Pathophysiology. According to Wichmann. T [7] the term “parkinsonism” encompasses the classical motor signs of Parkinson’s disease (PD), that is, the combination of bradykinesia, akinesia, tremor, and rigidity (as well as gait/balance problems). These signs and symptoms are assumed to be triggered by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). Discussions of the pathophysiology of parkinsonism are therefore mostly discussions of the immediate and chronic brain circuit effects of the degeneration of dopaminergic neurons in the SNc. In the following paragraphs, a basic understanding of the anatomical structure of the basal ganglia and related brain areas (see Fig. 1) is assumed. We will first discuss past and current findings pertaining to a brain circuit-level understanding of the pathophysiology of parkinsonism, coming from experiments in parkinsonian animals and patients with PD. This will be followed by a discussion of recent conceptual changes that are beginning to shape our knowledge in this field. Most of the information presented here will focus on results obtained from studies in nonhuman primates and patients with PD. Most studies of brain dysfunction in parkinsonism assume that the primary brain activity changes that account for the parkinsonian state occur within the basal ganglia/ thalamocortical circuitry (Fig. 1). The fact that motor impairments dominate early stages of PD is understandable considering that dopamine loss in (early) PD preferentially affects the dopamine supply to the motor circuit of the basal ganglia, which is spatially segregated from nonmotor circuits within the basal ganglia/thalamocortical network of connections.
Another group of scientists explains the pathophysiology of parkinsonism [8]: The pathophysiology of dystonia in APD has not been directly investigated. Inferences on dystonia pathophysiology in APD may be drawn from clinical observations, such as in the relationship between dystonia and dopaminergic medications in these conditions. Clinical observations suggest that limb dystonia disappears after initiation of therapy in levodoparesponsive MSA patients, reflecting a prominent role of nigrostriatal dysfunction over postsynaptic striatal pathology [9,10]. On the other hand, the mechanisms must be different for the lower facial and jaw dystonia of MSA and for other dystonic features in PSP, CBS and DLB in which dystonia is induced rather than ameliorated by levodopa. Earlier clinicopathological studies provided clear evidence of basal ganglia involvement in dystonia caused by stroke and other focal lesions. Thus, neurodegenerative phenomena within the basal ganglia in APD likely represent the most important determinant of dystonia in these conditions. The appearance of dystonia in the same anatomical regions as parkinsonian features in APD, however, may be viewed as a paradox of the functional model of basal ganglia function, which predicts that basal ganglia inhibitory output toward the thalamus is increased in parkinsonism but decreased in dystonia. The cooccurrence of dystonia and parkinsonism in the same body part may reflect a greater disruption in the sensorimotor network, which, in addition to the basal ganglia includes the motor cortex, the brainstem and the cerebellum, and their connections. Primary dystonia is characterized by enhanced excitability and loss of inhibition at several anatomical levels, including sensorimotor cortex, brainstem and spinal cord. Notably, loss of intracortical inhibition has been demonstrated also in secondary dystonia due to basal ganglia lesions, which share pathological topography with dystonia in APD. However, there is lack of neurophysiological studies on dystonia in APD. Neurophysiological studies based on transcranial magnetic stimulation and other techniques in APD have revealed enhanced excitability at cortical and brainstem levels without controlling for presence of dystonia. Increased cortical excitability in APD may result from the loss of inhibitory input from non-primary motor areas, similarly as in PD, or loss of somatosensory-motor cortex input, as in CBS due to parietal lobe involvement. Alternative mechanisms may include the loss of inhibitory interneurons in the cortex or thalamus, as demonstrated in PSP [11-17,18-26]. Another neurophysiological finding in primary dystonia is an abnormally enhanced plasticity [21]. While enhanced plasticity has been documented in PSP patients, abnormally reduced plasticity have been observed in MSA and CBS patients; all these studies, however, did not control for dystonic features [24,25]. Finally, neurophysiological assessments in PSP and MSA support the hypothesis of cerebellar dysfunction as a plausible mechanism involved in the generation of dystonia in these disorders. Data are lacking for CBD and DLB [27]. In summary, despite the scarcity of dedicated studies, neurodegenerative mechanisms along with neurophysiological changes at cortical, brainstem and cerebellar levels are all putative pathophysiological mechanisms involved in dystonia generation in APD. Either the dysfunction of specific nodes (basal ganglia, motor cortex and cerebellum) or an abnormal interaction between these systems through the networks in which they operate may explain the variable phenomenology of dystonia in APD.
Treatment. A group of scientists [28] commented on their research with the following points: Out of 245,958 patients treated with APDs, a total of 200 cases of severe APD-induced parkinsonism were identifed between 2001 and 2016. In more than 20% of these cases, the syndrome was very serious, with afected patients being completely immobile and/or sufering from harmful complications, such as pneumonia or severe injuries due to falls. The frst generation high-potency APDs were associated with the highest rates, confrming their outstanding risk of inducing parkinsonism. The first- generation lowpotency APDs were associated with the lowest risk, even falling signifcantly below that of the second-generation APDs. Among the individual second-generation APDs, amisulpride and risperidone ranked highest followed by aripiprazole and ziprasidone. The high risk associated with amisulpride and aripiprazole compared to the other second-generation and to the frst-generation low-potency APDs is a new and important fnding, because both drugs are generally associated with low EPS-risks and because comparative data are missing so far. Closely refecting the naturalistic characteristics of psychiatric inpatient treatment, the present AMSP results provide useful supplementary information for clinicians with respect to APD-induced parkinsonism.
Cortex
Basal ganglia
Striatum
Brain stem/ Spinal cord
Figure 1. Corticosubcortical motor circuits involved in the pathophysiology of parkinsonism.
CM/Pf VAA/L
Cereb. Cortex
Dark arrows indicate inhibitory connections; gray arrows indicate excitatory connections. CM/Pf, centromedian and parafascicular nuclei of the thalamus; Cereb. Cortex, cerebellar cortex; CMA, cingulate motor area; DCN, deep cerebellar nuclei; DP, direct pathway; D1, D1-like dopamine receptor subtype; D2, D2-like dopamine receptor subtype; HP, hyperdirect pathway; IP, indirect pathway; MC, motor cortex; PMC, premotor cortex; PN, pontine nuclei; SMA, supplementary motor area; VA/VL, ventral anterior and ventral lateral nuclei of the thalamus. Figure published in a previous work,285 here shown with permission.
According to Armstong and Okun [29] there are many medication options for treating PD, including carbidopa/levodopa, monoamine oxidase–B inhibitors (eg, rasagiline, selegiline), dopamine agonists (eg, pramipexole, ropinirole), adenosine A2A receptor antagonists, and amantadine. For disability due to PD symptoms, such as difficulty typing that is affecting one’s job, carbidopa/levodopa typically helps most. There is no evidence that delaying levodopa or dopaminergic therapy has any benefit or that levodopa is toxic. Different PD medications have different adverse effects that can affect treatment decisions. Most people with PD use more than 1 medication so as to receive combined benefits while avoiding adverse effects from high doses of a single medicine. An overlooked aspect of PD medical therapy is the timing of medication doses. Symptoms of PD can get worse immediately before the next medication dose is due (“wearing off”), and this worsening can affect day-to-day life. Dyskinesias are involuntary dancelike movements often occurring at peak (high) medication levels, midway between doses. Clinicians should be prepared to adjust medication timing and dose to maximize the time when the medicines are working well without dyskinesias. Deep Brain Stimulation and Other Surgical Approaches Deep brain stimulation (DBS) and other surgical approaches are considered when individuals with PD experience wearing off or dyskinesiasan dad justing medication does not help. These option smay also be offered when medication does not help a person’s tremor. DBS typically targets parts of the brain called the subthalamicnucleus and globuspallidus internusand can help one side of the body or both sides. DBS can help tremor, slowness, wearing off, and dyskinesia. Another option is focused ultrasound, which targets the part of the brain called the thalamus. This is used only for tremor and only helps one side of the body. Another surgical option involves placinga tube through the skin of the abdomen to the intestines. A gel form of carbidopa/levodopa is then pumped through the tube.This allows a person with PD to receive a consistent amount of levodopa throughout the dayandavoid the upsand downs that happen when taking pills every few hours. All surgical therapies require a detailed screening by a team of specialists (neurologists, surgeons, psychiatrists,and therapists). The health care team can estimate expected benefits and risks for an individual. Surgical therapies do not help nonmotor PD-related problems such as with speaking, memory, and thinking.
Conclusion: In conclusion, the pathophysiology and treatment of parkinsonism syndrome manifested in Parkinson's disease is very complex. It is a dangerous neurodegenerative disease that is widespread around the world. We also know a number of famous people who suffer from this disease. In the article we have given the brightest ideas on the pathophysiology of this disease. We concluded that levadopa, carbidopa, amisulpride, risperidone, aripiprazole, and ziprasidone were effective in treatment. We think research on this will continue.
Список литературы Pathophysiology of Parkinson’s disease and the efficiency of its treatment
- Connolly BS, Lang AE (23-30 April 2014). "Pharmacological treatment of Parkinson disease: a review". JAMA. 311 (16): 1670-83.
- The non-motor and non-dopaminergic fratures of PD. Parkinson's Disease: Non-Motor and Non-Dopaminergic Features. Olanow, C. Warren., Stocchi, Fabrizio., Lang, Anthony E. Wiley-Blackwell. 2011. 978-1405191852.
- ISBN: 978-1-4051-9185-2
- The National Collaborating Centre for Chronic Conditions, ed. (2006). "Symptomatic pharmacological therapy in Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 59-100. 978-1-86016-283-1. Archived from the original on 24 September 2010.
- ISBN: 978-1-86016-283-1
- Zhang J, Tan LC (2016). "Revisiting the Medical Management of Parkinson's Disease: Levodopa versus Dopamine Agonist". Current Neuropharmacology. 14 (4): 356-63. PMC 4876591.
- Pedrosa DJ, Timmermann L (2013). "Review: management of Parkinson's disease". Neuropsychiatric Disease and Treatment. 9: 321-40. PMC 3592512. PMID 23487540. RE-HEALTH JOURNAL 2.3-2020 55.


