Phagocytic response and phenoloxidase activity of the hemocytes of Bellamya bengalensis exposed to synthetic fenvalerate
Автор: Mukherjee S., Mandal Ch.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.19, 2023 года.
Бесплатный доступ
Background: Bellamya bengalensis is an important bioresource of the freshwater ecosystem of India. This edible mollusc is filter feeder, indigenous diet of human, poultry and fishery. Natural habitat of this freshwater gastropod mollusc bears the risk of contamination by agricultural pesticide fenvalerate, a synthetic type II pyrethroid. Hemocytes are the immuno effector cells of molluscs which are affected by environmental toxins or pathogenic microorganisms. Hemocytes perform various types of immunological functions such as phagocytosis, cytotoxic response etc. Results: Experimental exposure of fenvalerate resulted in impairment of phagocytic efficacy and alteration in the generation of phenoloxidase in B. bengalensis. Conclusion: The alteration of phagocytic response and generation of phenoloxidase of hemocytes, exposed to experimental concentrations of fenvalerate under static laboratory condition have been determined to establish as biomarker of aquatic toxicity in toxin contaminated natural habitat.
Fenvalerate, mollusc, phagocytosis, phenoloxidase
Короткий адрес: https://sciup.org/143180561
IDR: 143180561
Текст научной статьи Phagocytic response and phenoloxidase activity of the hemocytes of Bellamya bengalensis exposed to synthetic fenvalerate
Bellamya bengalensis is a freshwater benthic gastropod in eastern part of India. This molluscan species is an important bio resource of India (Ray et al . 2013). Large scale consumption of B. bengalensis by human is reported in various states including West Bengal, Orissa, Bihar and Mizoram (Rao and Dey, 1989) and this species is also used in poultry and fishery (Baby et al . 2010). It has ethno-medicinal and ecological importance (Prabhakar and Roy, 2009). B. bengalensis has been encountering physiological adversity due to unrestricted contamination of its natural habitat by various environmental contaminants including fenvalerate, an agrotoxin. Fenvalerate, a cyanide group containing type II pyrethroid is prescribed to control insect pests of paddy, jute, cotton etc. (Ray and Forshaw, 2000; Singh and Narkhede, 2012). During monsoon and flood the residues of fenvalerate often contaminate the freshwater wetland and the natural habitat of B. bengalensis .
The molluscan immune system depends on circulating hemocytes present in the hemolymph. Hemocytes are actively migrating throughout the tissues in response to foreign substances including pathogens. The primary defence mechanism is involved with phagocytosis, encapsulation and release of various cytotoxic molecules (Carballal et al . 1997). Most of the multicellular organisms have mobile phagocytic cells that are capable of self-recognition and destruction of foreign toxic substances. Phagocytosis is an established immunological response and also reported as a biomarker of aquatic pollution (Oliver and Fisher, 1999). Upon treatment of various experimental concentrations of fenvalerate, the phagocytic indices of the hemocytes in vivo of large sized groups of both male and female B. bengalensis was decreased significantly in a dose dependent pattern. Treatment of fenvalerate in vitro resulted in an identical trend of decrease in the phagocytic index of hemocytes of both large sized male and female B. bengalensis .
In mollusc, phenoloxidase is reported as a vital defence enzyme and is an integral component of subcellular antimicrobial defence in oysters (Munoz et al. 2006). The production of active phenoloxidase is dependent upon a prophenoloxidase activating system which is initiated by the presence of PAMP, such as lipopolysaccharide, peptidoglycan, laminarin and β-1, 3 glucans (Cerenius and Soderhall, 2004). Activity of phenoloxidase has been altered by the exposure of fenvalerate in B. bengalensis. In this paper the toxic effects of fenvalerate on phagocytic response and in relation to cytotoxic enzyme phenoloxidase activity is studied in detail.
MATERIALS AND METHODS
Collection , laboratory acclimation and maintenance of B. bengalensis in static laboratory condition
Fresh live specimens of B. bengalensis with an average shell lengths 35-44 mm was collected from selected freshwater ponds of the district South 24 Parganas of West Bengal, India. The collected specimens were transported to the laboratory in rectangular plastic containers as moist heaps within 2 hours of collection. The collected specimens were acclimated in the borocilicate glass jars for 6-8 days. Replenishment of freshwater was done at every 24 hours to avoid residual toxicity. B. bengalensis were maintained according to the protocols and methods of Raut (1991).
Treatment with fenvalerate in vivo
After 6-8 days of laboratory acclimation, the animals were exposed to sublethal concentrations of 0.5, 1, 2 and 3ppm of fenvalerate (Fenvalerate, 20 % E.C., Cas Number- 51630-58-1) for various spans of time period i.e. 24, 48, 72, 96 hours and 15 days for determination of phagocytic index of hemocyte, cytotoxicity of hemocyte along with the control.
Treatment with fenvalerate in vitro
Hemocyte suspension of B. bengalensis were exposed to sublethal concentrations of 0.001, 0.002, 0.003 and 0.004 ppm of fenvalerate for 2 hours of span for the determination of phagocytic index.
Collection of hemolymph
Hemolymph was aseptically collected under laminar flow hood by foot prodding method (Sminia, 1972). The collected hemolymph was stored in prechilled vials to prevent hemocyte aggregation.
Yeast culture
Yeast was cultured in YM broth (Difco, E. Molesly, Surrey, UK) over night at 250c temperature in a shaking water bath. The cells were killed by boiling for 60 minutes and the cell suspension was washed thrice in pH7.4 TBS/Ca2+ by centrifugation at 650g for 10 mins. Then the washed cells were resuspended at the concentration of 107 cells/ ml Grace Insect Medium.
Cell viability test
The viability of hemocytes was examined by staining the cells with 0.4% (1:1) trypan blue (E. Merck, Germany) for 2-5 minutes.
Assay of phagocytic response of isolated hemocytes treated with fenvalerate in vivo
For phagocytosis assay, fixed number of hemocytes isolated from control and trated Bellamya bengalensis were challenged with freshly cultured yeast at an optimal phagocytic ratio (1:10). Yeast was used as foreign particle for determination of phagocytic response of hemocytes (Cima et al . 2000). Hemocytes were maintained in short term culture system for 6 hours to complete the phagocytosis. Cells were then processed, fixed and stained for microscopic observation. Phagocytic index (P.I) was determined, where P.I = (total no. of phagocytosed cells/total no. of cells x100 x total no. of yeast cells engulfed/total no. of phagocytosed cells) (Elssner et al . 2004).
Assay of phagocytic response of isolated hemocytes treated with fenvalerate in vitro
Hemocytes were treated with 0.001, 0.002, 0.003 and 0.004 ppm of fenvalerate for 2 hours in vitro along with a set of control. Fixed number of control and treated hemocytes were challenged with freshly cultured yeast at an optimal phagocytic ratio of (1:10). Cells which received in vitro exposure of fenvalerate were maintained in culture for 2 hours. After incubation of hemocytes were processed, fixed and stained in Giemsa and hematoxylin eosin stain for microscopic observation. Phagocytic index (P.I) was examined microscopically (Elssner et al . 2004).
Assay of phenoloxidase
Phenoloxidase activity was determined in the hemocyte lysate after Sung et al . (1998). 100µl of hemocyte lysate was pre-incubated at 300C for 15 min., after which 200 µl of L-DOPA (1.6mg/ml in cacodylate acid citrate buffer) was added and reacted for 1 min. Each reaction mixture was further diluted with 200µl of cacodylate acid citrate buffer and then absorbance was estimated spectrophotometrically (CECIL-CE 4002, Germany) at 490nm. The enzyme activity was expressed in terms of increase in absorbance as 0.001U/min/mg protein.
RESULTS
Phagoc ytosis was reported as an important immune response of hemocytes of mollusc (Adema et al . 1991) under the challenge of pathogen and toxin. Phagocytosis was the cellular response offered by the hemocytes involving multiple stages of surface attachment, internalisation and ultimate engulfment (Figure-1). Bright field images of phagocytic hemocytes of untreated and treated B. bengalensis presented the phase of yeast engulfment (Figure-1). The control B. Bengalensis exhibited a relatively high degree of phagocytic index in comparison to the treated sets ( in vivo ). The phagocytic index exhibited by the control hemocytes of male B. bengalensis ranged between 118.45 ± 1.52 to 131.76 ± 4.08 under different spans of experiment (Figure-2).However, upon treatment with different experimental concentrations of fenvalerate, the phagocytic indices of hemocytes were decreased significantly in a dose dependent pattern. The highest inhibition in the phagocytic response of male B. bengalensis was recorded as 56.6 ± 3.73 against the concentration of 3ppm fenvalerate for 15 days of exposure in vivo (Figure-2). The phagocytic index exhibited by the control hemocytes of female B. bengalensis ranged between 135.5 ± 5.76 to 155.7 ± 8.00 under different spans of experiment. Upon treatment with different experimental concentrations of fenvalerate, the phagocytic indices of hemocytes were decreased significantly in a dose dependent manner. The highest inhibition in the phagocytic response of female B. bengalensis was recorded as 68.17 ± 2.18
against the concentration of 3ppm fenvalerate for 15 days of exposure in vivo (Figure 3). Treatment of isolated hemocytes of male B. bengalensis with 0.001, 0.002, 0.003 and 0.004 of fenvalerate in vitro exhibited a dose dependent decrease in phagocytic index in comparison to control (Figure-4). The highest inhibition of phagocytic index as 79.60 ± 3.40 was recorded in isolated hemocytes of male B. bengalensis under the exposure of 0.004 ppm fenvalerate in vitro . Treatment of isolated hemocytes of female B. bengalensis with 0.001, 0.002, 0.003 and 0.004 of fenvalerate in vitro exhibited a dose dependent decrease in phagocytic index in comparison to control (Figure-5). The highest inhibition of phagocytic index as 78.62 ± 1.87 was recorded isolated hemocytes of female B. bengalensis under the exposure of 0.004 ppm fenvalerate in vitro .
Activity of phenoloxidase in the hemocytes of both sex of B. bengalensis was determined under all experimental concentrations i.e. 0.5,1, 2 and 3ppm of fenvalerate exposure in vivo along with the respective control. The activity of phenoloxidase in the respective sets of control male B. bengalensis ranged from 26.25 ±
2.39 U/µg protein/ min to 33.25 ± 4.56 U/µg protein/min under different spans of exposure (Figure-6). A non directional, significant fluctuation in the activity of phenoloxidase was found against all experimental concentrations of fenvalerate for all spans of exposure in comparison to the respective control. The maximum inhibition in the activity of phenoloxidase was recorded as 22.77 ± 4.49 U/µg protein/min against 1ppm of fenvalerate for 24 hours of exposure (Figure-6).
The activity of phenoloxidase in the respective sets of control female B. bengalensis ranged from 28.52 ± 2.45 U/µg protein/min. to 32.22 ± 3.57 U/µg protein/min under different spans of exposure (Figure-7). The lowest activity of phenoloxidase was recorded as 24.77 ± 2.46 U /µg protein /min against the concentration of 1ppm of fenvalerate for 24hours of exposure. The maximum increase of phenoloxidase activity was estimated as108.75 ± 4.99 U/µg protein/min against 0.5ppm of fenvalerate for 72hours of exposure (Figure-7). Pattern of fenvalerate induced alteration in the activity of phenoloxidase in the hemocytes of male and female B. bengalensis exhibited a similar pattern.
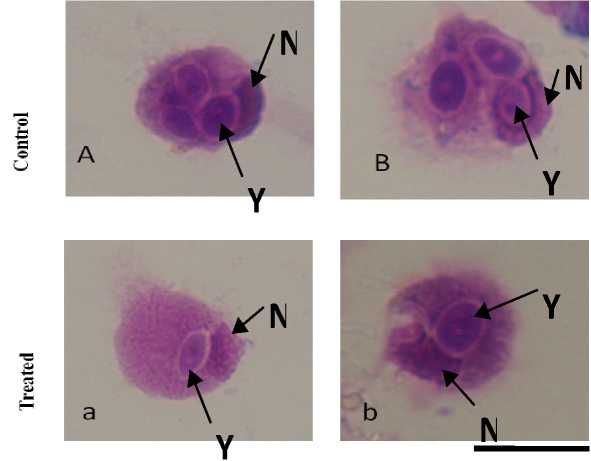
Figure 1. (A, B): Control hemocytes of B. bengalensis with engulfed yeast particles. (a-b): Fenvalerate exposed (3 ppm/ 96hours) hemocytes with engulfed yeast particles. N: Nucleus; Y: Yeast particles. x 1000.
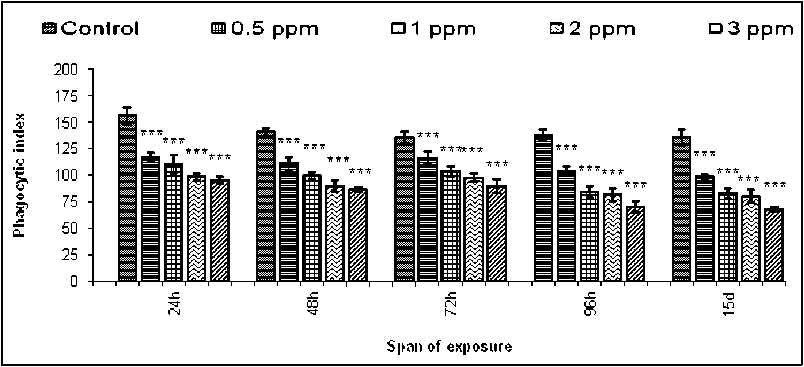
Figure 2. Phagocytic response of the hemocytes of male B. bengalensis exposed to fenvalerate in vivo . Data represented as mean ± S.D. (n=5) *p<0.05, **p<0.01, ***p<0.001.
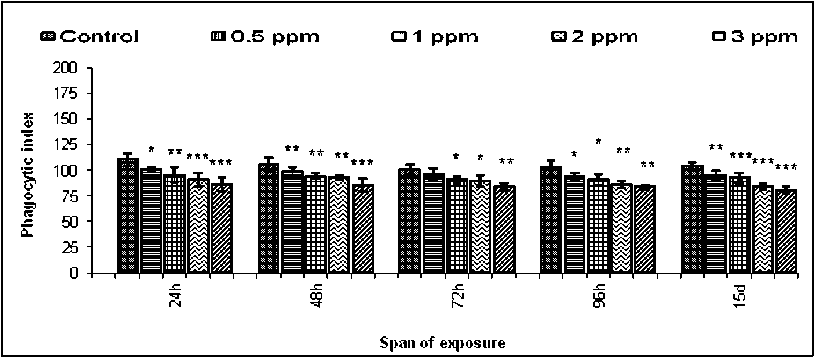
Figure 3. Phagocytic response of the hemocytes of female B. bengalensis exposed to fenvalerate in vivo . Data represented as mean ± S.D. (n=5) *p<0.05, **p<0.01, ***p<0.001.
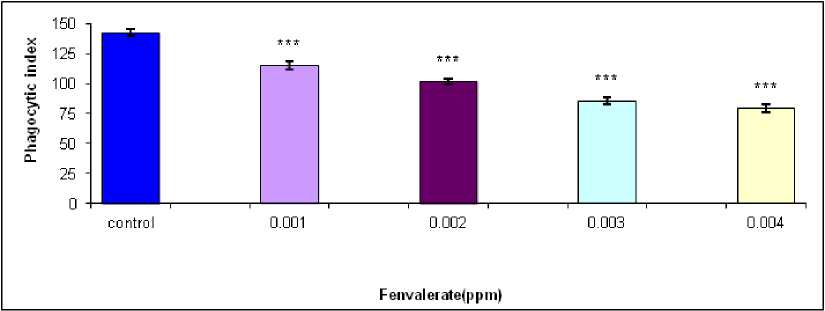
Figure 4. Phagocytic response of the hemocytes of male B. bengalensis exposed to fenvalerate in vitro . Data presented as mean ± S.D. (n = 5) *p<0.05, **p<0.01, ***p<0.001.
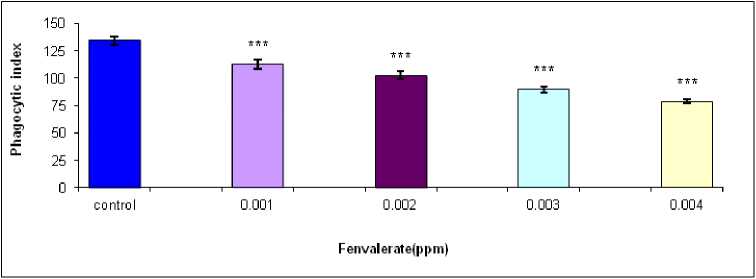
Figure 5. Phagocytic response of the hemocytes of female B. bengalensis exposed to fenvalerate in vitro . Data presented as mean ± S.D. (n = 5) *p<0.05, **p<0.01, ***p<0.001.
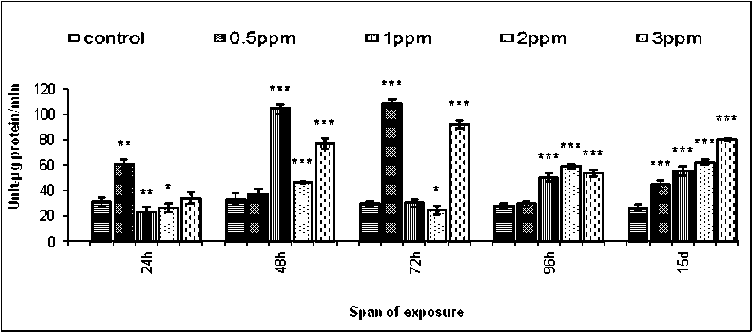
Figure 6. Intrahemocyte phenoloxidase activity in male B. bengalensis exposed to fenvalerate in vivo . Data presented as mean ± S.D. (n = 5) *p<0.05, **p<0.01, ***p<0.001.
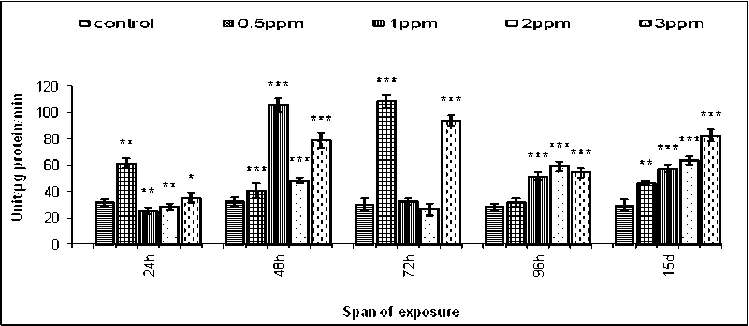
Figure 7. Intrahemocyte phenoloxidase activity of female B. bengalensis exposed to fenvalerate in vivo . Data presented as mean ± S.D. (n = 5) *p<0.05, **p<0.01, ***p<0.001.
DISCUSSION
Hemocytic phagocytosis is the endocytic process of ingestion of foreign micro-organisms, such as viruses, bacteria, fungi and protozoa by certain subpopulation of hemocytes. Most of the multicellular organisms have mobile phagocytic cells which are capable of selfrecognition and destruction of foreign microorganisms.
Phagocytosis is an established immunological response and reported as a biomarker of aquatic pollution (Chakraborty et al . 2009). Upon treatment with different experimental concentrations of fenvalerate, the phagocytic indices of the hemocytes in vivo, of both male and female B. bengalensis was decreased significantly in a dose dependent pattern (Figure 2-3).
Treatment of fenvalerate in vitro resulted in an identical trend of decrease in the phagocytic index of hemocytes of both male and female B. bengalensis (Figure 4-5). The experimental concentration of in vitro treatment was several times lower than the in vivo one. Data suggests that in vitro treatment had impaired acutely the phagocytic efficiency of hemocytes in both sexes of B. bengalensis . Various types of xenobiotics are reported to influence the functional attributes of hemocyte such as phagocytosis (Auffret et al . 2002), aggregation response and the ability to generate cytotoxic reactive oxygen species utilised for protection against invading bacteria.
Anderson (1993) reported the effects of toxic chemical compounds on the phagocytic ability of hemocytes of molluscs. Chakraborty et al . (2009) reported the inhibitory effect of sodium arsenite on the phagocytic potency of hemocytes of bivalve Lamellidens marginalis at various sublethal concentrations. The result was suggestive to a state of fenvalerate induced immune suppression in B. bengalensis. Data is indicative to a possible state of immune alteration in B. bengalensis exposed to environmentally realistic concentration of fenvalerate. Such a situation may lead to a state of immune compromise in B. bengalensis distributed in the polluted habitat which may lead to decline in the population of the same species in the aquatic environment.
In mollusc, phenoloxidase is reported as a vital defence enzyme. Both in mollusc and arthropod, it has been associated with diverse physiological functions such as sclerotization, host defence and wound healing (Siddiqui, 2006). Phenoloxidase is an integral component of subcellular antimicrobial defence in oysters (Munoz et al . 2006). The production of active phenoloxidase is dependent upon a prophenoloxidase-activating system which is initiated by the presence of PAMP, such as lipopolysaccharide, peptidoglycans, laminarin and ß-1, 3-glucans (Cerenius, 2004). Different types of immunological functions like self-nonself recognition, phagocytosis, cytotoxicity have been reported to be functionally associated with the activity of phenoloxidase.
Phenoloxidase activity was significantly increased in both male and female B. bengalensis under the exposure of 0.5, 1, 2 and 3 ppm fenvalerate with time span of 48, 72, 96 hours and 15 days (Figure 6 and 7). Phenoloxidase activity was significantly decreased under the exposure 1 and 2 ppm fenvalerate with time span of 24 hours exposure (Figure 6 and 7). Maximum phenoloxidase activity in hemocytes of both male and female B. bengalensis were recorded against 0.5 ppm of fenvalerate exposure for 72 hours (Figure 6 and 7). Data was indicative to fenvalerate induced shift in the cellular homeostasis of B. bengalensis. Other studies showed that phenoloxidase can be released from circulating hemocytes into hemolymph when the animals experience physical injury or pathogenic infestation (Gonzalez et al. 2003). Melanin is the enzymatic end product of phenoloxidase which retards microbial growth (Nappi and Christensen, 2005). Sensitivity of phenoloxidase activity to environmental toxicity suggests that this enzyme might be a vital biological marker of “stress” (Sinderman, 1984). Lacoste et al. (2001) reported a specific physiological mechanism behind the stress related immuno-deficiency in mollusc Crassostrea gigas. This result is suggestive to a possible state of immunological shift due to fenvalerate induced alteration in the generation phenoloxidase in B. bengalensis.
ACKNOWLEDGEMENTS
The authors thankfully acknowledge the grant support received from University Grants Commission (DRS-SAP) and the Department of Science and Technology (FIST), Government of India for development of instrument section of Zoology department of Calcutta University.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
Список литературы Phagocytic response and phenoloxidase activity of the hemocytes of Bellamya bengalensis exposed to synthetic fenvalerate
- Adema C.M., Vander Knaap W.P.W. and Sminia, T. (1991) Molluscan hemocytes mediated cytotoxicity: role of reacting oxygen intermediates. Crit. Rev. Aquat.Sci, 4, 201- 223.
- Anderson R. S. (1993) Modulation of nonspecific immunity by environmental stressors. In: Advances in Fisheries Science J. A. Couch and J. W. Fournie (ed.), Pathobiology of Marine and Estuarine Organisms London: CRC Press, pp. 483-510.
- Auffret M., Mujdzic, N., Corporeau C. and Moraga D. (2002) Xenobiotic induced immunomodulation in the European flat oyster, Ostrea edulis. Mar. Environ. Res., 54, 585-589.
- Baby R.L., Hasan I., Kabir K.A. and Naser, M.N. (2010) Nutrient analysis of some commercially important molluscs of Bangladesh. J. Sci. Res., 2(2), 390396.
- Carballal M. J., Lopez C., Azevedo C. and Villalba A. (1997) In vitro study of phagocytic ability of Mytilus galloprovincialis Lmk. hemocytes. Fish Shellfish Immunol., 7, 403-416.
- Cerenius L. and Soderhall K. (2004) The prophenoloxidase activating system in invertebrates. Immunol. Rev., 198, 116-126.
- Chakraborty S., Ray M. and Ray, S. (2009) Evaluation of phagocytic activity and nitric oxide generation by molluscan hemocytes as biomarkers of inorganic arsenic exposure. Biomarkers., 14(8), 539-546.
- Cima, F., Matozzo, V., Marin, M. G. and Ballarin, L. (2000) Hemocytes of the clam Tapes philippinarum (Adams and Reeve, 1850): morphofunctional characterization. Fish Shellfish Immunol., 10, 677693.
- Elssner A., Carter J.E., Yunger T.M. and Wewers, M.D. (2004) HIV-1 infection does not impair human alveolar macrophage phagocytic function unless combined with cigarette smoking. Chest., 125, 1071-1076.
- Gielens C. (2006) Location of intrinsic and inducible phenoloxidase activity in molluscan hemocyanin. Biochem. Biophy. Res. Com., 348, 1138-1144.
- Gonzalez A.L., Martinez M.A.N., Albores F.V., Valle F.A., and Mungaray M.R. (2003) Phenoloxidase activity in larval and juvenile homogenates and adult plasma and hemocytes of bivalve molluscs. Fish Shellfish Immunol., 15, 275-282.
- Lacoste A., Malham S.K., Cueff A., Jalabert F., Gelebart F. and Poulet S.A. (2001) Evidence for a form of adrenergic response to stress in the mollusc Crassostrea gigas. J. Exp. Biol., 204, 1247-1255.
- Muñoz P., Meseguer J. and Ángeles Esteban M. (2006) Phenoloxidase activity in three commercial bivalve species. changes due to natural infestation with Perkinsus atlanticus. Fish Shellfish Immunol., 20, 12-19.
- Nappi A. J. and Christensen B. M. (2005) Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. Biol., 35, 443 - 459.
- Oliver L.M. and Fisher W.S. (1999) Appraisal of prospective bivalve immunomarkers. Biomarker., 4, 510-530.
- Prabhakar A.K. and Roy, S.P (2009) Ethnomedicinal uses of some shellfishes by people of Kosi river basin in North Bihar, India. Ethno. Med., 3(1), 1-4.
- Rao N.V.S. and Dey A. (1989) Freshwater molluscs in aquaculture. In: Director (ed.), Handbook: Freshwater molluscs of India. Zool. Surv. India., pp, 225-82.
- Raut S.K. (1991) Laboratory rearing of medically and economically important molluscs. In M. S. Jalpaiguri (ed.), Snails, flukes and man.. Z. S. I. Pub, pp.79-83.
- Ray D.E. and Forshaw P.J. (2000) Pyrethroid insecticides: poisoning syndromes, synergies, and therapy. Clin. Toxicol., 38 (2), 95-101.
- Ray M., Bhunia A.S., Bhunia N.S. and Ray, S. (2013) Density shift, morphological damage, lysosomal fragility and apoptosis of hemocytes of Indian molluscs exposed to pyrethroid pesticides. Fish Shellfish Immunol., 35, 499-512.
- Sinderman C.J. (1984) Fish and environmental impacts. Arch. Fisch Wiss. Berlin., 35, 125-160.
- Singh M. and Narkhede S.D. (2012) Role of fenvalerate on growth and yield of cotton (Gossypium hirsutum L.). Sc. Res. Re., 2(3), 281-285.
- Sminia T. (1972) Structure and function of blood and connective tissue cells of the freshwater pulmonate Lymnaea stagnalis studies by electron microscopy and enzyme histochemistry. Z. Zellforsch. Mikrosk. Anat., 130, 497-526.
- Sung H.H., Chang H.J., Her C.H., Chang, J.C. and Song, Y.L. (1998) Phenoloxidase activity of hemocytes derived from Penaeus monodon and Macrobrachium rosenbergii. J.Invertebr.Pathol.,7, 26-33.


