Phenotypic profile of monocyte-macrophage lineage cells as a function of respiratory epithelium status
Автор: Fedorov A.A., Ermak N.A., Topolnitskiy E.B., Shefer N.A., Rodionov E.O., Pankova O.V., Cherdyntseva N.V., Stakheyeva M.N.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Лабораторные и экспериментальные исследования
Статья в выпуске: 4 т.22, 2023 года.
Бесплатный доступ
The mechanism of the relationship between pretumor changes in the bronchial respiratory epithelium and the risk of progression of non-small cell lung cancer (NSCLC) remains unclear. It has been suggested that the relationship between reactive changes in the bronchial mucosa and NSCLC progression may be caused by the functional status of monocytic-macrophage cells as important participants in inflammation, which determines both the risk of premalignant changes in the epithelium and malignant progression. The purpose of the study was to investigate the phenotypic profile of peripheral blood monocytes and macrophages induced from monocytes in vitro depending on the state of respiratory epithelium in NSCLC patients. Material and Methods. The study included 39 patients with newly diagnosed NSCLC. Based on the morphological examination of small bronchi taken at the distance of 3-5 cm from the tumor, patients were divided into the following groups depending on the type of pretumor changes: no pretumor changes (n=6), isolated basal cell hyperplasia (BCH) (n=13), combination of BCH and squamous metaplasia (SM) (n=3), combination of unchanged epithelium and focal BCH (n=17). The phenotypic features of peripheral blood monocytes and in vitro-induced macrophages were assessed before treatment using flow cytometry. Results. The state of the respiratory epithelium in NSCLC patients prior to the start of anticancer treatment was associated with the phenotypic features of peripheral blood monocytes, but not with the profile of macrophages induced from them. Distortion of the response of induced macrophages to the polarizing stimuli was observed in NSCLC patients: the cultured cells responded to both M1 and M2 inducers (LPS and IL-4, respectively) with a phenotype shift to M2, while the CD206 marker expression varied depending on the presence and type of pretumor changes. Conclusion. The phenotypic profile of peripheral blood monocytes was associated with the state of the respiratory epithelium in NSCLC patients before anti-tumor treatment, but not with the phenotypic features of induced macrophages.
Non-small cell lung cancer, monocytes, macrophages, pretumor changes, progression, basal cell hyperplasia, squamous metaplasia, polarization
Короткий адрес: https://sciup.org/140302022
IDR: 140302022 | УДК: 616.24-006:612.112.95:576.3 | DOI: 10.21294/1814-4861-2023-22-4-55-64
Текст научной статьи Phenotypic profile of monocyte-macrophage lineage cells as a function of respiratory epithelium status
Non-small cell lung cancer (NSCLC) accounts for approximately 85 % of all lung cancers [1]. Despite high advances in the diagnosis and treatment of lung cancer, the overall 5-year survival rate for NSCLC remains low, reaching 68 % in patients with stage IB and ranging from 0 % to 10 % in patients with stages
IVA-IVB [2]. One of the main causes of death in lung cancer is the high risk of progression after antitumor treatment. The ability to predict the risk of NSCLC progression is an urgent goal of modern oncology.
The prognostic value of the respiratory bronchial epithelium at a distance from the primary tumor was described in earlier studies [3]. The presence of basal cell hyperplasia in the bronchial epithelium at a distance from the primary tumor is associated with a high risk of hematogenous metastasis, and its combination with squamous metaplasia increases the risk of local recurrence [3]. The mechanism underlying the identified phenomenon remains unclear. The relationship between reactive changes in the bronchial mucosa and NSCLC progression may be caused by the functional status of monocytic-macrophage cells as important participants in inflammation, determining both the risk of pretumor changes in the epithelium and malignant progression.
Currently, the role of monocyte-macrophage lineage cells in cancer pathogenesis has been well determined. Monocyte-macrophage lineage cells are known to be highly plastic, i.e., their phenotype can change under the influence of various factors of the internal environment and microenvironment. Thus, in response to LPS and IFN-γ, monocytes/macrophages undergo classical M1 activation, while alternative M2 activation is induced when exposed to IL-4/ IL-13. Classically activated M1 and alternatively activated M2 macrophages have anti-inflammatory and pro-inflammatory activity, correspondingly [4]. The role of tumor-associated macrophages (TAMs) in the pathogenesis of malignant growth is known to be determined by their functional polarization. High infiltration of M1-macrophages in tumor islets has been shown to be associated with increased overall survival in NSCLC. High infiltration of M2-macrophages in stroma and tumor islets was associated with decreased overall survival [5].
The surface molecules, such as TLR-2, TLR-4, CD80, CD86, MHC-II (HLA-DR) are known to be the markers of M1 polarization [6]. The M2 macrophages can be identified by the expression of surface markers, such as mannose receptor CD206, CD163, CD209, FIZZ1 and Ym1 [6].
The role of monocytes in the pathogenesis of malignant growth is primarily related to the replenishment of various cell populations of tumor microenvironment, including TAM [7]. It has been suggested that the status of monocytes in peripheral blood can determine the properties which tissue macrophages will exhibit in tumors [8]. The pool of peripheral blood monocytes is heterogeneous: 3 major populations of monocytes are identified: classical (CD14+CD16-,), non-classical (CD14dimCD16+,) and intermediate (CD14+CD16+) monocytes. As a model for studying the properties of macrophages in molecular biology, the method of their induction from peripheral blood monocytes by in vitro cultivation in the presence of various soluble factors is widely used [9–15].
The purpose of this study was to investigate the phenotypic differences of peripheral blood monocytes and in vitro induced macrophages in NSCLC patients depending on the pretumor changes of the bronchial respiratory epithelium.
Material and Methods
The study included 39 patients with first-time diagnosed non-small-cell lung cancer, stage I–III, (T1–4N0–3M0), who were admitted for treatment to the Cancer Research Institute of Tomsk NRMC of the RAS in the period from 2019 to 2022. The patients included 31 men and 8 women. The mean age of the group was 59.3 ± 17.3 years (42 to 81 years). Squamous cell cancer was verified in 18 patients, adenocarcinoma in 20 patients, and mixed type in 1 patient. All patients signed an informed consent to participate in the study. Venous heparinized blood and lung tissue samples 3–5 cm away from the primary tumor locus served as the study material. Patients were divided into groups depending on pretumor changes in the respiratory bronchial epithelium. The study was performed in accordance with the Declaration of Helsinki, 1964, and with the permission of the local ethical committee of the institute.
Morphological verification of pretumor changes of bronchial epithelium in small bronchi in patients with NSCLC
Evaluation of morphological changes in bronchial epithelium in NMSL patients was performed on small bronchial tissue samples taken during the operation at the distance of 3–5 cm from the primary tumor. Tissue samples were fixed in 10 % formalin for 18–24 hours and then were embedded in paraffin. Sections of 5 µm thickness were prepared from the fixed samples. Micropreparations were stained with hematoxylin and eosin solutions according to the standard protocol. Morphological examination was performed using an Axio Scope A1 light microscope (Carl Zeiss, Germany). Based on the cytological evaluation, the patients were divided into the following study groups:
-
1) NSCLC patients without pretumor changes in the respiratory epithelium (N), (n=6);
-
2) NSCLC patients with isolated basal cell hyperplasia (BCH+SM-) associated with a high risk of hematogenous metastasis (n=13);
-
3) NSCLC patients with the combined presence of BCH and squamous metaplasia (BCH+SM+), associated with the risk of local recurrence, (n=3);
-
4) NSCLC patients with a combination of unchanged epithelium and focal basal cell hyperplasia (N+BCH) in the respiratory epithelium (n=17).
Extraction of mononuclear cell fraction from peripheral blood
Venous blood was collected into heparin-treated vacutainer tubes in the morning on an empty stomach in an amount of 20 ml from each patient. Heparinized blood in a 1:1 ratio was mixed with a wash medium consisting of RPMI-1640 medium (PanEco, Russia) to which L-glutamine, penicillin, and streptomycin were previously added (PanEco, Russia). Mononuclear cells obtained on a density gradient (ρ=1.077g/L) were diluted in complete culture medium to a concentration
Table 1/Таблица 1
Clinico-morphological parameters of patients with non-small cell lung cancer Клинико-морфологические параметры пациентов с немелкоклеточным раком легкого
|
Clinico-morphological parameters/ Клинико-морфологические параметры |
N/ Неизмененный эпителий |
BCH+SM-/ Изолированная БКГ |
BCH+SM+/ Сочетание БКГ и ПМ |
N+BCH/ Очаговая БКГ |
|
Age, years/Возраст, лет |
63,7 ± 9,9 |
61,8 ± 8,5 |
75,0 ± 5,2 |
56,5 ± 17,1 |
|
Sex/Пол |
||||
|
Male/Муж |
5 (83,3 %) |
11 (84,6 %) |
2 (66,7 %) |
13 (76,6 %) |
|
Female/Жен |
1 (16,7 %) |
2 (15,4 %) |
1 (33,3 %) |
4 (23,5 %) |
|
Stage/Стадия |
||||
|
I |
3 (50,0 %) |
7 (53,8 %) |
– |
5 (31,3 %) |
|
II |
1 (16,7 %) |
2 (15,4 %) |
1 (33,3 %) |
5 (31,3 %) |
|
III |
2 (33,3 %) |
4 (30,8 %) |
2 (66,7 %) |
5 (31,3 %) |
|
Tumor size/Размер опухоли |
||||
|
Т1–2 |
6 (100,0 %) |
9 (69,2 %) |
2 (66,7 %) |
7 (41,2 %) |
|
Т3–4 |
– |
4 (30,8 %) |
1 (33,3 %) |
10 (58,8 %) |
Table 2/Таблица 2
Molecular markers of the main populations and types of macrophage polarization used in the study Молекулярные маркеры основных популяций и типа поляризации макрофагов, использованные в работе
Induction of macrophages from peripheral blood monocytes
The isolated mononuclear cells in the amount of 2×106 in 1 ml of complete culture medium were placed in a well of a 24-well flat-bottomed plate for cell cultivation (Greetmed). Three samples were placed for each assay: 1 – a control sample, 2 – an M1-macrophage induction sample, and 3 – an M2-macrophage induction sample. Macrophage colony-stimulating factor of macrophages (M-CSF) was added to all wells in the dose of 20 ng/ml. After 5 days of cell incubation in a 5 % CO2 atmosphere, 100 ng/ml lipopolysaccharide (LPS, Sigma) was added to the second sample, as an inducer of M1-macrophages and 10 ng/ml interleukin-4 (IL-4, Sigma) was added to the third sample, as an inducer of M2-macrophages. After 24 hours of incubation, the fraction of cells that did not adhere to the plate was removed by washing. The adherent cells, macrophages, were separated from the bottom of the well with a scraper, collected in tubes with complete culture medium, fixed, and then stained to study the induced macrophage phenotype.
Evaluation of the phenotype of monocytes and in vitro induced macrophages
Phenotypic features of populations of macrophages induced from monocytes were studied by state-of-the-art method flow cytofluorometry. The monocyte and macrophage markers studied in the work are presented in Table 2. All antibodies were obtained from BD (USA).
To 1×106 induced macrophages, 5 μL of antibodies to the antigens indicated in Table 1 were added according to the manufacturer’s recommendations. Analysis without staining (negative control) and analysis with isotypic antibodies (positive control) were performed in parallel. Phenotypic features of induced macrophage
CD14+16-HLA-DR+ monocytes
CD14+16-HLA-DR+ моноциты
Pretumor changes Предопухолевые изменения
Fig. 1. Phenotypic features of monocytes in NSCLC patients depending on the variant of pretumor changes: а) proportion of cells expressing CD206+; b) MFI of HLA-DR molecule expressed by CD14+16 monocytes (p – significance level of differences; MFI – mean fluorescence intensity measured in standard units; «N» – unchanged respiratory epithelium; «BSH+SM-» – isolated basal cell hyperplasia; «BCH+SM+» – combination of basal cell hyperplasia and squamous metaplasia basal cell hyperplasia; «N+BCH» – combination of unchanged epithelium and focal basal cell hyperplasia)
Рис. 1. Фенотипические особенности моноцитов у больных НМРЛ в зависимости от варианта предопухолевых изменений:
а) доля клеток, экспрессирующих CD206+; б) MFI молекулы HLA-DR, экспрессируемой CD14+16-моноцитами (p – уровень значимости различий; MFI – медиана интенсивности флюоресценции, измеряемая в условных единицах; «N» – неизмененный респираторный эпителий; «BSH+SM-» – изолированная базальноклеточная гиперплазия; «BCH+SM+» – сочетание базальноклеточной гиперплазии и плоскоклеточной метаплазии; «N+BCH» – сочетание неизмененного эпителия с очаговой базальноклеточной гиперплазией)
CD206+ monocytes
CD206+ моноциты
populations were analyzed on a BDFACSCantoII flow cytofluorimeter.
To stain monocytes, antibodies were added to 100 µl of whole blood and incubated for 20 min in the dark at room temperature. The erythrocytes were then lysed with Lysing Solution (BD). Samples were incubated for 15 min in the dark and washed 2 times with Cell Wash. Phenotypic features were then analyzed.
The data were visualized and analyzed using FACSDiva Version 6.1.3 software. In whole blood samples a CD45+ monocyte was gated, from which CD14+CD16+classic subpopulation, CD14+CD16+intermediate subpopulation and CD14-CD16+nonclassic monocyte subpopulation were identified based on expression of CD14 and CD16 molecules. In each of these subpopulations, the proportion of CD68+, CD163+, CD206, and HLA-DR+ cells, as well as their subpopulations, twice positive for these markers were determined. For induced in vitro macrophages, the entire pool of CD45+ cells obtained after collection of the adherent fraction was examined, assuming that this pool contained only monocytic-macrophage cells after the manipulations performed (isolation on a density gradient and removal of the nonadherent fraction). The gating strategy was then performed according to the algorithm described for monocytes. The mean fluorescence intensity (MFI) in the population of interest, which reflects the density of the expressed marker, was also assessed.
Statistical analysis
Statistical analysis was performed using Statistica version 12 for Windows (StatSoft Inc). Normal distribution was confirmed using the Kolmogorov-Smirnov and Shapiro-Wilk tests, depending on the sample size. The studied parameters were not distributed according to the normal law, so descriptive statistics were presented in the form of medians (Me) and interquartile range (Q1:Q3). Mann-Whitney test for independent samples was used to identify significant differences between monocyte and in vitro induced macrophage parameters. Differences in comparison groups were considered significant when the level of significance (p<0.05) was reached. Data at the level of statistical trend were also discussed (p<0.1).
Results
Association between the phenotypic profile of peripheral blood monocytes in NSCLC patients and the variant of pretumor changes in the respiratory epithelium
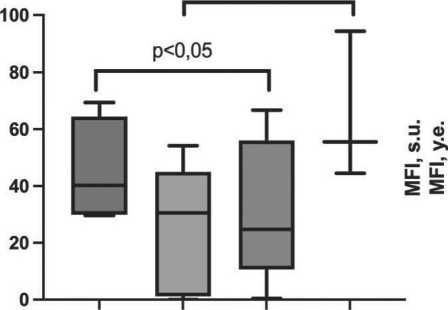
Peripheral blood monocytes from NSCLC patients were significantly more likely to have the M2-polar-ized CD206+ phenotype in the group of patients with unchanged epithelium 40.3 [30.3–59.5] % compared to the group of patients who had isolated BCH in the respiratory bronchial epithelium 24.8 [13.7–53.2] %, (p=0.018) (Fig. 1A). There was also an increase in the CD206+ monocyte population in the group of patients with a combination of BCH and SM of 55.5
CD206+ macrophages with LPS CD206+ макрофаги с добавлением LPS p<0,05
20000-1 । ।
N N+BCH BCH+SM- BCH+SM+
Pretumor changes Предопухолевые изменения
Fig. 2. Phenotypic features of in vitro induced macrophages without the addition of polarization inducers in NSCLC patients depending on the variant of pretumor changes (p – significance level of differences; «N» – unchanged respiratory epithelium; «BSH+SM-» – isolated basal cell hyperplasia; «BCH+SM+» – combination of basal cell hyperplasia and squamous metaplasia basal cell hyperplasia; «N+BCH» – combination of unchanged epithelium and focal basal cell hyperplasia)
Рис. 2. Фенотипические особенности индуцированных in vitro макрофагов без добавления индукторов поляризации у больных НМРЛ в зависимости от варианта предопухолевых изменений (p – уровень значимости различий; «N» – неизмененный респираторный эпителий; «BSH+SM-» – изолированная базальноклеточная гиперплазия; «BCH+SM+» – сочетание базальноклеточной гиперплазии и плоскоклеточной метаплазии; «N+BCH» – сочетание неизмененного эпителия с очаговой базальноклеточной гиперплазией)
Fig. 3. Phenotypic features of in vitro induced macrophages with LPS addition in NSCLC patients depending on the variant of pretumor changes (p – significance level of differences; MFI – mean fluorescence intensity measured in standard units; «N» – unchanged respiratory epithelium; «BSH+SM-» – isolated basal cell hyperplasia; «BCH+SM+» – combination of basal cell hyperplasia and squamous metaplasia basal cell hyperplasia; «N+BCH» – combination of unchanged epithelium and focal basal cell hyperplasia)
Рис. 3. Фенотипические особенности индуцированных in vitro макрофагов при добавлении LPS у больных НМРЛ в зависимости от варианта предопухолевых изменений (p – уровень значимости различий; MFI – медиана интенсивности флюоресценции, измеряемая в условных единицах; «N» – неизмененный респираторный эпителий; «BSH+SM-» – изолированная базальноклеточная гиперплазия; «BCH+SM+» – сочетание базальноклеточной гиперплазии и плоскоклеточной метаплазии; «N+BCH» – сочетание неизмененного эпителия с очаговой базальноклеточной гиперплазией)
[44.5–94.3] % compared with the group of patients with focal BCH of 27.5 [1.50–44.5] % (p=0.028).
Increased expression of HLA-DR molecule responsible for antigen presentation and induction of immune response in a subpopulation of classical CD14+16- monocytes was a distinctive feature of NSCLC patients with unchanged epithelium 14590 [7937–17322] s.u. compared with the group of patients with focal BCH in the respiratory bronchial epithelium of 5127 [3377–7315] s.u. (p=0.053, Fig. 1B).
Study of the phenotypic profile of model macrophages differentiated in vitro from monocytes of NSCLC patients with different variants of pretumor changes of respiratory epithelium
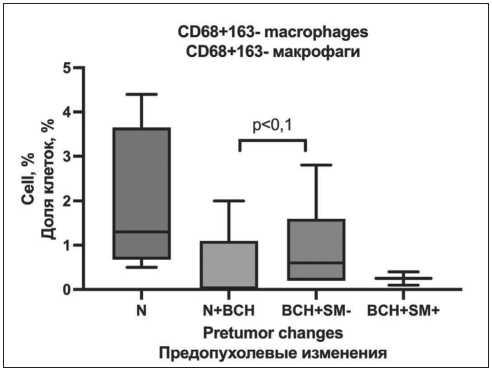
Macrophages cultured without addition of polarization inductors had no statistically significant differences in comparison groups. However, at the level of statistical trend, it was noted that in patients with the presence of isolated BCH in the bronchial epithelium, macrophages had the CD68+163- phenotype more frequently – 0.6 [0.2–1.6] % compared to NSCLC patients who had the combination of unchanged epithelium with focal BCH 0 [0.0–0.2] % (p=0.092) (Fig. 2).
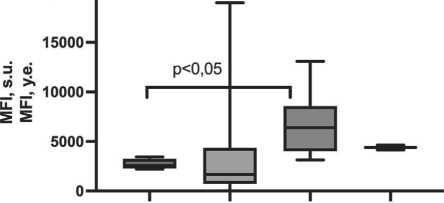
Phenotypic features of macrophages depending on the variant of pretumor changes of respiratory epithelium during incubation with LPS
Cell cultivation in the presence of LPS – inducer of M1-polarization was followed by expressive differences in the expression of M2-polarization marker CD206 on the surface of induced macrophages depending on the state of the respiratory epithelium. Thus, the maximum level of expression of this molecule was noted in patients with the presence of isolated BCH (6389 [4296–7075] s.u.); when BCH and SM were combined, this index was 4387 [4142–4631] s.u.; with unchanged epithelium – 2570 [2350–3048] s.u.; and reached the minimum values in focal BCH on the background of normal epithelium – 1654 [1441–4282] s.u., (p=0.018) (Fig. 3).
Phenotypic features of macrophages depending on the variant of pretumor changes of respiratory epithelium during incubation with IL-4
Addition of the M2 polarization inducer IL-4 to the macrophage culture medium was followed by almost the same expression rates of the CD206 molecule as when LPS was added, except for patients with a combination of BCH and SM: they showed an increase in the mean fluorescence of this marker
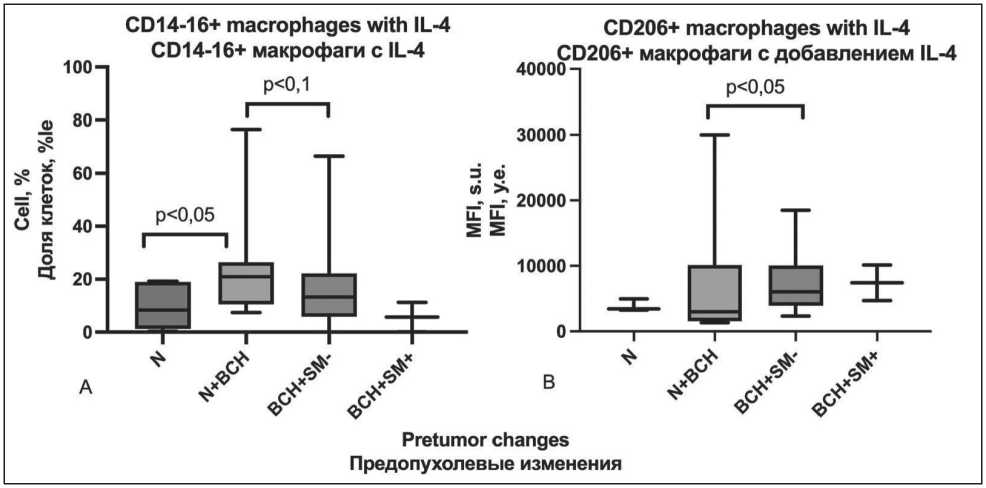
Fig. 4. Phenotypic features of in vitro induced macrophages with IL-4 addition in patients with NSCLC depending on the variant of pretumor changes: а) share of cells with CD14-16+ phenotype; b) MFI of CD206+ phenotype (p – significance level of differences;
MFI – mean fluorescence intensity measured in standard units; «N» – unchanged respiratory epithelium; «BSH+SM-» – isolated basal cell hyperplasia; «BCH+SM+» – combination of basal cell hyperplasia and squamous metaplasia basal cell hyperplasia; «N+BCH» – combination of unchanged epithelium and focal basal cell hyperplasia)
Рис. 4. Фенотипические особенности индуцированных in vitro макрофагов с добавлением IL-4 у больных НМРЛ в зависимости от варианта предопухолевых изменений: а) доля клеток, с фенотипом CD14-16+; б) MFI фенотипа CD206+ (p – уровень значимости различий; MFI – медиана интенсивности флюоресценции, измеряемая в условных единицах; «N» – неизмененный респираторный эпителий; «BSH+SM-» – изолированная базальноклеточная гиперплазия; «BCH+SM+» – сочетание базальноклеточной гиперплазии и плоскоклеточной метаплазии; «N+BCH» – сочетание неизмененного эпителия с очаговой базальноклеточной гиперплазией)
to 7427 [4711–10137] s.u. (Fig. 4). The presence of IL-4 in the cultivation medium caused an increase in the CD14-16+ subpopulation of cells exhibiting the phenotype of non-classical monocytes, in samples from patients with the presence of pretumor changes in the respiratory epithelium compared with those in patients with unchanged epithelium, reaching a level of statistical significance with the focal BCH group against a background of normal epithelium (5.3 [0.3–18.8] % and 20.9 [10.5–26.4] %, (p=0.022)) (Fig. 4).
Discussion
It is known that pretumor changes on the background of chronic inflammation in respiratory epithelium are an important stage of malignant transformation [16]. Pretumor changes in bronchial epithelium go through several stages: basal cell hyperplasia, squamous metaplasia, dysplasia (mild, medium and severe) and carcinoma in situ [16]. In fact, the presence of persistent or progressive high-grade dysplasia (moderate or severe) is a marker of increased risk for lung cancer. However, there are currently insufficient effective tools to detect these pretumor changes with the highest risk of progression to carcinoma in situ.
On the opposite of that, monocyte-macrophage cells – tissue macrophages and monocytes as their plastic resource – are known to be important participants in both inflammation and tumor progression. It is possible that pretumor changes of respiratory epithelium can be determined by functional features of both tissue macrophages and circulating monocytes, which can transfer the properties acquired at earlier stages of life cycle to differentiating macrophages by epigenomic changes [17]. This phenomenon becomes particularly important under conditions of malignant growth, when monocytes become a resource for TAM [18].Thus, the properties of circulating monocytes can be associated not only with the state of bronchial epithelium at a distance from the tumor node, but also with its prognostic value, since the monocyte properties determine the functional profile of TAM in the tumor and, accordingly, its malignant potential.
This study revealed phenotypic differences between monocytes and in vitro induced macrophages in NSCLC patients in the groups with different variants of pretumor changes at a distance from the primary tumor node. In particular, when considering the prevalence of CD68+163- phenotype among in vitro induced macrophages without the addition of polarization inducers, we can note that in general the medians in the groups of patients with the presence of morphological changes in the respiratory epithelium are lower than in patients with unchanged epithelium.
For correct interpretation of the revealed phenotypic features of monocytic-macrophage cells it is necessary to have an understanding of the relationship between the phenotypic features and the functional orientation of this group of cells. Some groups of authors suggest using molecules such as CD80, CD86, HLA-DR, CD40, CD38, TLR4, etc. as markers of M1 polarization. Similarly, there are several markers used by different authors for M2 polarized cells. The most frequently mentioned are CD163, CD206,
CD204, less frequently MARCO-receptor, CD169, stabilin-1, CD36, CD9, etc. [19–21]. In this study, an increase in the population of macrophages expressing the HLA-DR marker as well as macrophages lacking the expression of M2 polarization markers – antigens CD163 and CD206 – should be considered a sign of M1 polarization.
This work demonstrated that the presence of pretumor changes is indeed associated with the features of the inflammatory infiltrate, particularly the functional and phenotypic characteristics of monocytemacrophage cells.
It should be noted that in NSCLC patients the response to polarizing stimuli is distorted: both to M1 and M2 inducers the cultured cells respond with a shift of the phenotype to the M2 side. Addition of the M1 polarization inducer LPS to the macrophage cultivation medium was followed by almost the same indicators of CD206 molecule expression as addition of IL-4, providing M2 polarization. The presence of isolated basal cell hyperplasia, including its combination with squamous metaplasia, is followed by inhibition of M1-polarization.
Conclusion
This study reveals the relationship of phenotypic profile of monocytes but not induced from them in vitro macrophages with the presence and variant of pretumor changes in the respiratory bronchial epithelium of NSCLC patients. Further work using new generation sequencing techniques is required to better understand the mechanisms of association between the functional profile of monocyte-macrophage cells and the state of the respiratory epithelium.
Список литературы Phenotypic profile of monocyte-macrophage lineage cells as a function of respiratory epithelium status
- Majem M., Juan O., Insa A., Reguart N., Trigo J.M., Carcereny E., García-Campelo R., García Y., Guirado M., Provencio M. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol. 2019; 21(1): 3–17. doi: 10.1007/s12094-018-1978-1.
- Duma N., Santana-Davila R., Molina J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc. 2019; 94(8): 1623–40. doi: 10.1016/j.mayocp.2019.01.013.
- Pankova O.V., Denisov E.V., Ponomaryova A.A., Gerashchenko T.S., Tuzikov S.A., Perelmuter V.M. Recurrence of squamous cell lung carcinoma is associated with the co-presence of reactive lesions in tumor-adjacent bronchial epithelium. Tumour Biol. 2016; 37(3): 3599–607. doi: 10.1007/s13277-015-4196-2.
- Yao Y., Xu X.H., Jin L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front Immunol. 2019; 10: 792. doi: 10.3389/fimmu.2019.00792.
- Jackute J., Zemaitis M., Pranys D., Sitkauskiene B., Miliauskas S., Vaitkiene S., Sakalauskas R. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018; 19(1): 3. doi: 10.1186/s12865-018-0241-4.
- Yao Y., Xu X.H., Jin L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front Immunol. 2019; 10: 792. doi: 10.3389/fimmu.2019.00792.
- Cassetta L., Pollard J.W. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018; 17(12): 887–904. doi: 10.1038/nrd.2018.169.
- Poschke I., Mao Y., Adamson L., Salazar-Onfray F., Masucci G., Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. 2012; 61(6): 827–38. doi: 10.1007/s00262-011-1143-y.
- Cao X., Yakala G.K., van den Hil F.E., Cochrane A., Mummery C.L., Orlova V.V. Differentiation and Functional Comparison of Monocytes and Macrophages from hiPSCs with Peripheral Blood Derivatives. Stem Cell Reports. 2019; 12(6): 1282–97. doi: 10.1016/j.stemcr.2019.05.003.
- Chen H.J., Li Yim A.Y.F., Griffith G.R., de Jonge W.J., Mannens M.M.A.M., Ferrero E., Henneman P., de Winther M.P.J. Meta-Analysis of in vitro-Differentiated Macrophages Identifies Transcriptomic Signatures That Classify Disease Macrophages in vivo. Front Immunol. 2019; 10: 2887. doi: 10.3389/fimmu.2019.02887.
- Foulds G.A., Vadakekolathu J., Abdel-Fatah T.M.A., Nagarajan D., Reeder S., Johnson C., Hood S., Moseley P.M., Chan S.Y.T., Pockley A.G., Rutella S., McArdle S.E.B. Immune-Phenotyping and Transcriptomic Profiling of Peripheral Blood Mononuclear Cells From Patients With Breast Cancer: Identification of a 3 Gene Signature Which Predicts Relapse of Triple Negative Breast Cancer. Front Immunol. 2018; 9: 2028. doi: 10.3389/fimmu.2018.02028.
- Gurvich O.L., Puttonen K.A., Bailey A., Kailaanmäki A., Skirdenko V., Sivonen M., Pietikäinen S., Parker N.R., Ylä-Herttuala S., Kekarainen T. Transcriptomics uncovers substantial variability associated with alterations in manufacturing processes of macrophage cell therapy products. Scientific reports. 2020; 10(1). doi: 10.1038/s41598-020-70967-2.
- Hamidzadeh K., Belew A.T., El-Sayed N.M., Mosser D.M. The transition of M-CSF-derived human macrophages to a growth-promoting phenotype. Blood Adv. 2020; 4(21): 5460–72. doi: 10.1182/bloodadvances.2020002683.
- Jin X., Kruth H.S. Culture of Macrophage Colony-stimulating Factor Differentiated Human Monocyte-derived Macrophages. J Vis Exp. 2016; (112). doi: 10.3791/54244.
- Lukic A., Larssen P., Fauland A., Samuelsson B., Wheelock C.E., Gabrielsson S., Radmark O. GM-CSF- and M-CSF-primed macrophages present similar resolving but distinct inflammatory lipid mediator signatures. FASEB J. 2017; 31(10): 4370–81. doi: 10.1096/fj.201700319R.
- Beane J.E., Mazzilli S.A., Campbell J.D., Duclos G., Krysan K., Moy C., Perdomo C., Schaffer M., Liu G., Zhang S., Liu H., Vick J., Dhillon S.S., Platero S.J., Dubinett S.M., Stevenson C., Reid M.E., Lenburg M.E., Spira A.E. Molecular subtyping reveals immune alterations associated with progression of bronchial premalignant lesions. Nat Commun. 2019; 10(1): 1856. doi: 10.1038/s41467-019-09834-2.
- Chen S., Yang J., Wei Y., Wei X. Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell Mol Immunol. 2020; 17(1): 36–49. doi: 10.1038/s41423-019-0315-0.
- Larionova I., Kazakova E., Patysheva M., Kzhyshkowska J. Transcriptional, Epigenetic and Metabolic Programming of Tumor-Associated Macrophages. Cancers (Basel). 2020; 12(6): 1411. doi: 10.3390/cancers12061411.
- Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12(4): 253–68. doi: 10.1038/nri3175.
- Larionova I., Tuguzbaeva G., Ponomaryova A., Stakheyeva M., Cherdyntseva N., Pavlov V., Choinzonov E., Kzhyshkowska J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front Oncol. 2020; 10. doi: 10.3389/fonc.2020.566511.
- Orecchioni M., Ghosheh Y., Pramod A.B., Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Frontiers in immunology. 2019; 10: 1084. doi: 10.3389/fimmu.2019.01084.


