Photocatalytic properties of fluorinated tetraarylantimony carboxylates
Автор: Artemeva E.V.
Журнал: Вестник Южно-Уральского государственного университета. Серия: Химия @vestnik-susu-chemistry
Рубрика: Физическая химия
Статья в выпуске: 2 т.13, 2021 года.
Бесплатный доступ
Organic dyes are widely used in different kinds of manufacturing. As a result, they become common organic contaminants, and lead to water pollution. Therefore, the search of effective methods for such compound destruction is of interest. One of such methods is the photocatalytic destruction by antimony organic compounds. It has been found out that the incorporation of tetraarylantimony monocarboxylates (Ar4SbOC(O)R, Ar = Ph, R = C6F5 (1), CF2CF2CF3 (2), CF2Br (3); Ar = p-Tol, R = CF2CF3 (4), CF2CF2CF3 (5)) and further irradiation of solutions with UV radiation causes photocatalytic degradation of organic dyes methylene blue (MB) and methyl violet (MV) in their aqueous solutions. The change of concentration of organic dyes could be seen due to change in peak intensity at 554 nm and 665 nm in the UV spectra of MB and MV. Tetraphenylantimony carboxylates 1-3 showed higher photocatalytic activity than tetra(p-tolyl) antimony carboxylates 4, 5. Thus, MB decomposition was 90.5-98.8 %, MV: 97.7-100 % after 60 min irradiation of aqueous solutions containing compounds 1-3. While in the presence of 4, 5, decomposition of MB and MV was 55.1-53.2 % and 71.2-78.7 %, respectively. In both cases, methyl violet was susceptible to more total destruction than methylene blue. The experiments showed the probability of repeatable use of the photocatalysts. After full decay of the pigment, tetraarylantimony carboxylate precipitated by centrifugation, the precipitate was decanted and used for the next cycle. As a result of two photodegradation processes, the mass of tetraarylantimony carboxylates decreased on average by 50 % after washing and drying, but the carboxylates were still effective, which proved the possibility of the photocatalyst repeatable use and their stability.
Tetraarylantimony carboxylates, organic dyes, methyl violet, methylene blue, photocatalysis
Короткий адрес: https://sciup.org/147234258
IDR: 147234258 | УДК: 544.526.5+546.86+547.53.024 | DOI: 10.14529/chem210207
Текст научной статьи Photocatalytic properties of fluorinated tetraarylantimony carboxylates
A large range of organic contaminants introduced into natural water supplies or waste water treatment systems are known to be residual dyes from various sources. Organic dyes are widely used in the textile, cosmetic, pharmaceutical, tannery, paper and mining industries [1, 2] . At present, the scientific community is actively discussing the problem of extracting dyes from wastewater in order to reduce their impact on the environment [3–5]. The main problem when dyes get into water is their stability because of their complex aromatic composition and synthetic origin [6 –9] .
Another problem is their accumulation of dyes in water body resulting in the loss of dissolved oxygen, which creates anoxic environments that are toxic to marine organisms [10 –12] . According to the literature, some organic dyes are very toxic, they may cause carcinogenic, allergic and dermatic effects [13] .
Nowadays, different methods for water purification and the organic dyes decomposition are used. For example, use of biological [14 –16] , physical [17] or chemical treatment. The last includes the dyes degradation caused by polyurethane [18] , nanoparticles [19 –23] . Dye degradation generally means dye decolorization and mineralization in textile waste water. Decolorization is the destruction of the dye molecule chromophore group; organic compounds are also degraded into CO2 and H2O [24] .
Earlier, the photocatalytic activity of triarylantimony carboxylates has been studied [25].
In this work, we studied the possibility of photocatalytic destruction of organic dyes methylene blue and methyl violet in their aqueous solutions by adding tetraarylantimony monocarboxylates and further irradiation of the solutions with UV radiation.
ExperimentalPhotocatalytic Activity Test
The photocatalytic activity of tetraarylantimony carboxylates was estimated by photodegradation of aqueous solutions of methylene blue (MB) and methyl violet (MV) dyes at room temperature (298 K). Experiments were performed according to the general procedure [25] : 20 mg of tetraarylantimony carboxylates of the general formula Ar 4 SbOC(O)R, Ar = Ph, R = C 6 F 5 ( 1 ), CF 2 CF 2 CF 3 ( 2 ), CF 2 Br ( 3 ); Ar = p-Tol, R = CF 2 CF 3 ( 4 ), CF 2 CF 2 CF 3 ( 5 ) (synthesized by the reaction of pentaarylantimony with a proper carboxylic acid 1:1) were added to 50 ml of an aqueous solution of MB or MV (concentration 4 mg/L). To establish adsorption-desorption equilibrium, the resulting suspension was stirred in the dark for 30 minutes. Then the suspension was irradiated under a UV lamp with constant stirring for one hour. The concentration of MS and MV was measured by UV spectroscopy every 15 minutes. The collected samples were centrifuged to separate tetraarylantimony carboxylates.
The visible UV diffuse reflectance spectrum (UV-Vis.) was obtained in the region of 200 to 800 nm using a Shimadzu UV2700 spectrometer. Barium sulfate with a reflectance of 100 % was used as a standard.
Results and Discussion
A change in the peak intensity at 554 nm and 665 nm for MV and MB, respectively, in their UV spectra, indicated a change in the concentration of dyes in the solution (Fig. 1 a, b). In a control experiment carried out under similar conditions in aqueous solutions without the addition of tetraarylantimony monocarboxylates, no decomposition of the MB and MV dyes occurred.
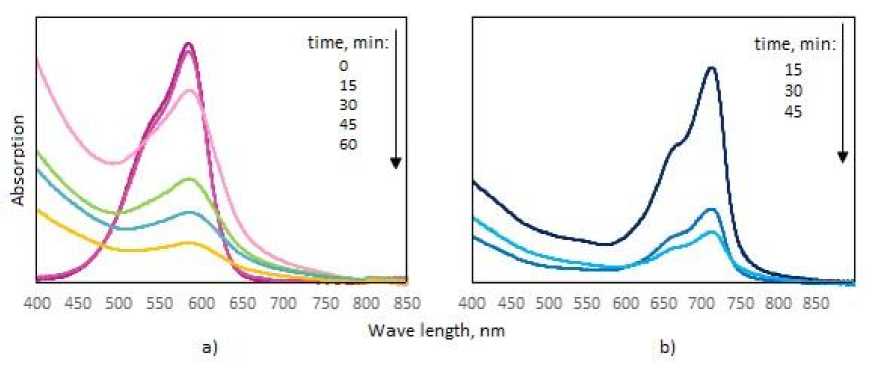
Fig. 1. (a) Absorption spectrum of MV solution in the presence of 4;
(b) absorption spectrum of MB solution in the presence of 1
It has been found that the photocatalytic activity of the compounds under study is different. Tetraphenylantimony carboxylates 1 – 3 exhibited higher photocatalytic activity than tetra(p-tolyl)antimony carboxylates 4 , 5 (Fig. 2).
Thus, upon 60 min irradiation of solutions containing compound 1 – 3 , the decomposition of MB was 90.5 %, 97.3 %, 98.8 %, MV: 97.7 %, 99.5 %, 100 %, respectively. Tetra(p-tolyl)antimony monocarboxylates 4 , 5 exhibit relatively low photocatalytic activity: in the presence of compound 4 , the decomposition of MB and MV occurred by 53.2 % and 78.7 %, respectively; in the presence of 5 – by 55.1 % and 71.2 %, respectively. The photocatalytic efficiency of all complexes is obviously higher for the decomposition of MV than MB. In general, the photocatalytic activity of 1 – 3 is comparable to those of triphenylantimony bis (trifluoromethylbenzoates), studied earlier. Thus, triphenylantimony bis (trifluoro-2-methylbenzoate) leads to the destruction of 90.5 % MB, and 90.6 % MV after 150 min and 90 min of irradiation, respectively [25] .
To assess the possibility of the repeatable use of the photocatalysts, we studied the process of circulating photocatalysis of MB and MV under the treatment with 2, 4 and 5. After complete decay of the dyes, tetraarylantimony carboxylates were precipitated by centrifugation, the precipitates were decanted and used for the next cycle (Fig. 3). As a result of two cycles of photodegradation, the mass of tetraarylantimony carboxylates after washing and drying decreased on average by 50 %. However, as the graphs show, all the compounds were still effective towards dye decomposition and lead to the destruction of 49.5 % of MB, and 80.5 % – 90.0 % of MV after 1 hr irradiation.
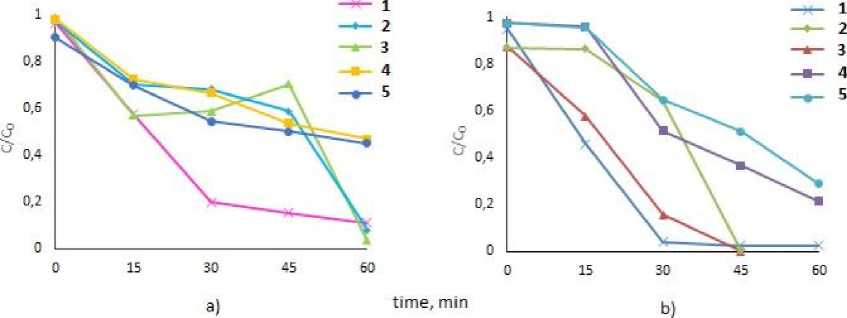
Fig. 2. Change in the reduced concentration (C / C0) under UV irradiation (t, min) of solutions of MV (a) and MB (b) dyes in the presence of tetraarylantimony carboxylates 1–5
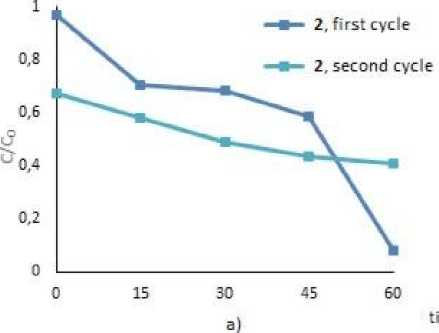
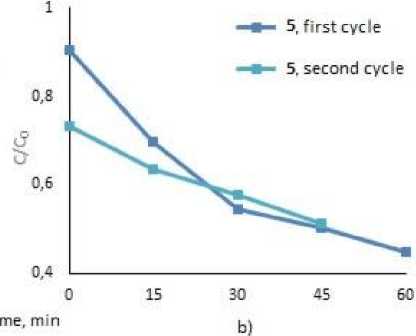
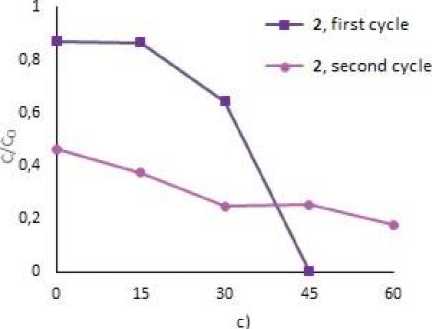
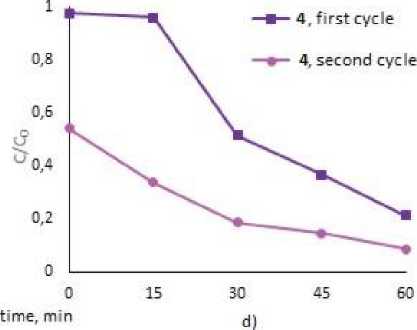
Fig. 3. Photocatalytic decomposition of MB with 2 (a), 5 (b), MV with 2 (c), 4 (d) (1 and 2 cycles)
Conclusion
Fluorinated tetraarylantimony carboxylates can be good candidate for the photocatalytic degradation of organic dyes: methylene blue and methyl violet. Meanwhile, tetraphenylantimony carboxylates are more effective towards both dyes, in comparison to tetra(p-tolyl)antimony compounds. However, methyl violet undergoes a fuller destruction than methylene blue.
Acknowledgments
We are grateful to A.N. Efremov for the compound synthesis and A.V. Bulanova, O.K. Sharutina and V.V. Sharutin for the experimental supervising.
Список литературы Photocatalytic properties of fluorinated tetraarylantimony carboxylates
- Barnett J.C. Synthetic Organic Dyes, 1856-1901: An Introductory Literature Review of Their Use and Related Issues in Textile Conservation. Stud. Conserv., 2007, vol. 52, pp. 67-77. DOI: 10.1179/sic.2007.52.supplement-1.67.
- Guerra-Tapia A., Gonzalez-Guerra E. Hair Cosmetics: Dyes. Actas Dermo-Sifiliogr., 2014, vol. 105, no. 9, pp. 833-839. DOI: 10.1016/j.adengl.2014.02.003.
- Liang X., Zhao L. Room-Temperature Synthesis of Air-Stable Cobalt Nanoparticles and Their Highly Efficient Adsorption Ability for Congo Red. RSC Advances, 2012, vol. 2, no. 13, pp. 5485-5487. DOI: 10.1039/c2ra20240a.
- McMullan G., Meehan C., Conneely A., Kirby N., Robinson T., Nigam P., Banat I.M., Marchant R., Smyth W.F. Microbial Decolourisation and Degradation of Textile Dyes. Appl. Microbiol. Bio-technol., 2001, vol. 56, no. 1-2, pp. 81-87. DOI: 10.1007/s002530000587.
- Sharma P., Pant S., Poonia P., Kumari S., Dave V., Sharma S. Green Synthesis of Colloidal Copper Nanoparticles Capped with Tinospora Cordifolia and Its Application in Catalytic Degradation in Textile Dye: An Ecologically Sound Approach. J. Inorg. Organomet. Polym. Mater., 2018, vol. 28, no. 6, pp. 2463-2472. DOI: 10.1007/s10904-018-0933-5.
- Mohmood I., Lopes C.B., Lopes I., Ahmad I., Duarte A.C., Pereira E. Nanoscale Materials and Their Use in Water Contaminants Removal. Review. Environ. Sci. Pollut. Res., 2013, vol. 20, no. 3, pp. 1239-1260. DOI: 10.1007/s11356-012-1415-x.
- Lefebvre O., Moletta R. Treatment of Organic Pollution in Industrial Saline Wastewater: A Literature Review. Water Res., 2006, vol. 40, no. 20, pp. 3671-3682. DOI: 10.1016/j.watres.2006.08.027.
- Srinivasan A., Viraraghavan T. Decolorization of Dye Wastewaters by Biosorbents: A Review. J. Environ. Manage., 2010, vol. 91, no. 10, pp. 1915-1929. DOI: 10.1016/j.jenvman.2010.05.003.
- Karthikeyan S., Titus A., Gnanamani A., Mandal A.B., Sekaran G. Treatment of Textile Wastewater by Homogeneous and Heterogeneous Fenton Oxidation Processes. Desalination, 2011, vol. 281, no. 1, pp. 438-445. DOI: 10.1016/j.desal.2011.08.019.
- Ellouze E., Tahri N., Amar R. Ben Enhancement of Textile Wastewater Treatment Process Using Nanofiltration. Desalination, 2012, vol. 286, pp. 16-23. DOI: 10.1016/j.desal.2011.09.025.
- Turgay O., Ersoz G., Atalay S., Forss J., Welander U. The Treatment of Azo Dyes Found in Textile Industry Wastewater by Anaerobic Biological Method and Chemical Oxidation. Sep. Purif. Technol, 2011, vol. 79, no. 1, pp. 26-33. DOI: 10.1016/j.seppur.2011.03.007.
- Saratale R.G., Saratale G.D., Chang J.S., Govindwar S.P. Bacterial Decolorization and Degradation of Azo Dyes: A Review. J. Taiwan Inst. Chem. Eng., 2011, vol. 42, no. 1, pp. 138-157. DOI: 10.1016/j .jtice.2010.06.006.
- Tkaczyk A., Mitrowska K., Posyniak A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ., 2020, vol. 717, pp. 137222. DOI: 10.1016/j.scitotenv.2020.137222.
- Popli S., Patel U.D. Destruction of Azo Dyes by Anaerobic-Aerobic Sequential Biological Treatment: A Review. Int. J. Environ. Sci. Technol., 2015, vol. 12, no. 1, pp. 405-420. DOI: 10.1007/s13762-014-0499-x.
- Li D., Li Q., Mao D., Bai N., Dong H. A Versatile Bio-Based Material for Efficiently Removing Toxic Dyes, Heavy Metal Ions and Emulsified Oil Droplets from Water Simultaneously. Bioresour. Technol., 2017, vol. 245, pp. 649-655. DOI: 10.1016/j.biortech.2017.09.016.
- Mishra S., Mohanty P., Maiti A. Bacterial Mediated Bio-Decolourization of Wastewater Containing Mixed Reactive Dyes Using Jack-Fruit Seed as Co-Substrate: Process Optimization. J. Cleaner Prod, 2019, vol. 235, pp. 21-33. DOI: 10.1016/j.jclepro.2019.06.328.
- Magureanu M., Mandache N.B., Parvulescu V.I. Degradation of Organic Dyes in Water by Electrical Discharges. Plasma Chem. Plasma Process., 2007, vol. 27, no. 5, pp. 589-598. DOI: 10.1007/s11090-007-9087-x.
- Sultan M. Polyurethane for Removal of Organic Dyes from Textile Wastewater. Environmental Chem. Lett., 2017, vol. 15, no. 2, pp. 347-366. DOI: 10.1007/s10311-016-0597-8.
- Bogireddy N.K.R., Kiran Kumar H.A., Mandal B.K. Biofabricated Silver Nanoparticles as Green Catalyst in the Degradation of Different Textile Dyes. J. Environmental Chem. Eng., 2016, vol. 4, no. 1, pp. 56-64. DOI: 10.1016/j.jece.2015.11.004.
- Li D., Zheng H., Wang Q., Wang X., Jiang W., Zhang Z., Yang Y. A Novel Double-Cylindrical-Shell Photoreactor Immobilized with Monolayer TiO2-Coated Silica Gel Beads for Photoca-talytic Degradation of Rhodamine B and Methyl Orange in Aqueous Solution. Sep. Purif. Technol., 2014, vol. 123, pp. 130-138. DOI: 10.1016/j.seppur.2013.12.029.
- Siby J., Beena M. Microwave-Assisted Green Synthesis of Silver Nanoparticles and the Study on Catalytic Activity in the Degradation of Dyes. J. Mol. Liq., 2015, vol. 204, pp. 184-191. DOI: 10.1016/j.molliq.2015.01.027.
- Vidhu V.K., Philip D. Catalytic Degradation of Organic Dyes Using Biosynthesized Silver Nanoparticles. Micron, 2014, vol. 56, pp. 54-62. DOI: 10.1016/j.micron.2013.10.006.
- Naraginti S., Stephen F.B., Radhakrishnan A., Sivakumar A. Zirconium and Silver Co-Doped TiO2 Nanoparticles as Visible Light Catalyst for Reduction of 4-Nitrophenol, Degradation of Methyl Orange and Methylene Blue. Spectrochim. Acta - Part A: Molecular and Biomolecular Spectroscopy, 2015, vol. 135, pp. 814-819. DOI: 10.1016/j.saa.2014.07.070.
- Vujevic D., Papic S., Koprivanac N., Bozic A.L. Decolorization and Mineralization of Reactive Dye by UV/Fenton Process. Sep. Sci. Technol., 2010, vol. 45, no. 11, pp. 1637-1643. DOI: 10.1080/01496395.2010.487734.
- Zhang X.Y., Cui L., Zhang X., Jin F., Fan Y.H. Two Organoantimony (V) Coordination Complexes Modulated by Isomers of Trifluoromethylbenzoate Ligands: Syntheses, Crystal Structure, Photodegradation Properties. J. Mol. Struct., 2017, vol. 1134, pp. 742-750. DOI: 10.1016/j.molstruc.2017.01.039.


