Physio-Morphological and Biochemical Changes in Mungbean Vigna radiata (L.) Wilczek due to Drought Stress
Автор: Shubankar Das, Supatra Sen
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.21, 2025 года.
Бесплатный доступ
Crop plants growing under natural conditions are exposed to several environmental stresses, all affecting plant growth and development, which consequently hampers the productivity of crops plants. Drought is considered the single most devastating environmental stress, which decreases crop productivity more than any other environmental stress. Water stress was given in the potted Vigna radiate plants (for control 1st set - 4 pots were given regular water, for the 2nd set -3 days kept without water in the pot, for the 3rd set -5 days kept without water and for the 4th set -7days kept without water). The plants were then uprooted and collected for experimental work on the 3rd, 5th and 7th day for the 1st, 2nd and 3rd sets respectively for further studies with drought stress. Total chlorophyll and carotenoid decreased under drought stress while protein, amino acid and proline contents increased under stress. Scavenging enzymes catalase and peroxidase were assayed and while catalase activity increased under stress peroxidase activity was found to decrease under stress.
Environmental stress, scavenging enzymes, ROS, biochemical constituents
Короткий адрес: https://sciup.org/143185128
IDR: 143185128
Текст научной статьи Physio-Morphological and Biochemical Changes in Mungbean Vigna radiata (L.) Wilczek due to Drought Stress
Drought stress in plants refers to the physiological strain a plant experiences due to a lack of available water, typically occurring when soil moisture is insufficient or when water loss through transpiration and evaporation exceeds water uptake. This stress can have significant impacts on plant growth, development, and overall yield. Crop plants growing under natural conditions are exposed to several environmental stresses, all affecting plant growth and development, which consequently hampers the productivity of crops plants. Drought is considered the single most devastating environmental stress, which decreases crop productivity more than any other environmental stress. Drought is one of the major constraints limiting crop production worldwide. Crop growth models predict that this issue will be more severe in future with rising climate change. Drought impairs normal growth, disturbs water relations, and reduces water use efficiency in plants. Plants, however, have a variety of physiological and biochemical responses at cellular and whole organism levels,making it a more complex phenomenon.
MATERIALS AND METHODS
Mungbean seeds were rinsed thoroughly in distilled water. The mungbean seeds were then spread in 16 plastic pots uniformly filled with fresh garden soil. Water stress was given in the potted plants (for control 1st set -4 pots were given regular water, for the 2nd set -3 days kept without water in the pot, for the 3rd set -5 days kept without water and for the 4th set -7days kept without water). The plants were uprooted and collected for experimental work on the 3rd, 5th and 7th day for the 1st, 2nd and 3rd sets respectively for further studies with drought stress.
fter uprooting from the pots, the length of shoot and root was individually measured by a scale in centimeter unit. Leaf area was determined by drawing the outline of each leaf of a plant on a graph paper and estimating the area in sq. cm. fter uprooting from the pots, fresh weight of shoot, root and leaves were measured by the help of a digital balance. Now those plant parts were placed in a Petri dish and oven dried at 60°C for a few hours. Then the dry weight of each plant part was recorded. The leaves were individually placed in a petri dish filled with distilled water covering the lids for 2 hours. fter the stipulated time, the leaves from each set were blotted with blotting paper and immediately weighed as saturated weight. From the data collected, RWC was calculated.
Chlorophyll a, chlorophyll b and total chlorophyll content were calculated by the method of rnon (1949); amount of Carotenoid (carotene and xanthophyll) was estimated by the method of Kirk and llen (1965); Protein content was estimated according to Lowry et al. (1951); total amino acid content in leaves was estimated according to the Ninhydrin method of Lee and Takahashi (1966) with some modifications. Estimation of proline was done according to Bates et al. (1973); catalase enzyme activity was assayed by the method of Gasper and Lacoppe (1968) with slight modifications and peroxidase enzyme activity was assayed spectrophotometrically according to Chance and Maehly (1955) with slight modifications.
The results obtained in triplicates were subjected to analysis of variance ( NOV ) and S.E. (Standard Error) and C.D. (Critical Difference) at 5% and 1% levels calculated.
RESULTS AND DISCUSSION
Drought stress, a common abiotic stress, significantly impacts plant growth and productivity by reducing water availability thereby causing physiological and biochemical changes. This can lead to reduced biomass, altered metabolism, and even yield losses. Plants respond to drought through various mechanisms, including morphological changes, physiological adjustments, and molecular responses. The shoot lengths of drought imposed plants decreased as compared to control while the root lengths increased significantly from the control (Table 1). The root lengths of stressed plants possibly increased in length in search of water and nutrients as compared to control. The leaf area of stressed plants significantly decreases (Table 2). The fresh and dry weights of the plants (Tables 3 and 4) also decrease considerably from the control. R.W.C. is found to decrease considerably as compared to control (Fig. 1). Dahanayake et al. (2014)
observed all morphological parameters viz. plant height, wet and dry weight of shoots and roots, root length, number of pods, wet and dry weight of pods, wet and dry weight of seeds were significantly affected by the water stress condition.
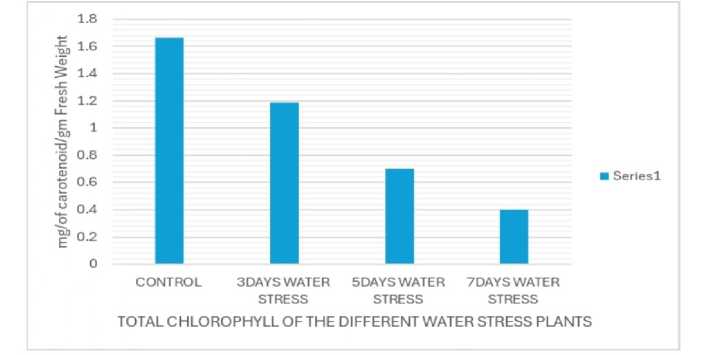
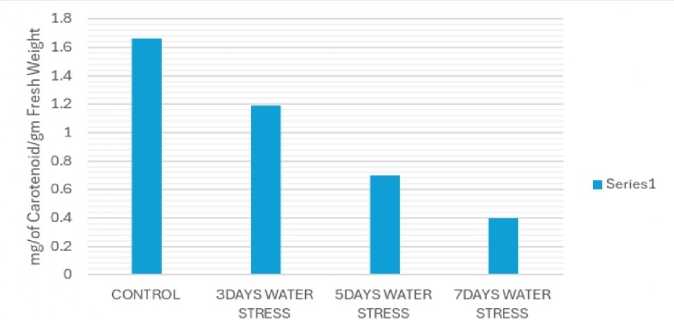
Drought stress, a major abiotic stress for plants, occurs when soil water availability is scarce, leading to reduced plant growth and yield (Sen and Mukherji 1998a). This stress impacts various physiological processes, including photosynthesis (Sen and Mukherji, 1998b, 1999) and nutrient uptake (Sen and Mukherji, 1997) and can even affect plant morphology and reproductive development. Plants respond to drought through various mechanisms. While in this study it is observed, decreased chlorophyll levels (Fig. 2) of the stressed plant compared to control plant but others have observed an increase, suggesting the response can vary depending on the plant species, environmental conditions and the intensity and duration of the stress. Of the two photosynthetic pigments classes, carotenoids show multifarious roles in drought tolerance including light harvesting and protection from oxidative damage caused by drought. In this experiment it is reported that the carotenoid level was decreased in the stressed plant campared to control plant (Fig. 3). Thus, specifically carotenoids are important for stress tolerance for plants experiencing water stress. Kanimozhi et al. (2021) observed Chlorophyll a and b , carotenoids (like carotenes and xanthophylls), and phycobilins are reduced in stressed plants of Vigna mungo.
From this work we could conclude that amount of protein was more in the stressed plant as compared to control plant (Fig. 4). Water stress induces the production of specific stress-responsive proteins, including Late Embryogenesis bundant (LE ) proteins and dehydrins, which help protect other proteins and cellular structures. These changes in protein expression are a key part of the plant’s response to drought and other water-related stresses. mino acids play a crucial role in plant responses to water stress, acting as both signaling molecules and building blocks for stress defense mechanisms. Our study shows that amino acid content was more in stressed plant (Fig. 5) as compared to control plant. Proline also showed the same trend (Fig. 6) as protein and amino acid content. Proline present in stressed plant can help plants maintain turgor pressure, protect cellular structures, and enhance antioxidant defense systems, ultimately improving stress tolerance. In plants under water stress, proline acts as an osmoprotectant, stabilizing cellular structures, and acting as an antioxidant to scavenge reactive oxygen species (Sen and Mukherji 1998c, Hayat et al. 2012) reported increased proline with increasing drought intensity and duration and there was a tremendous increase in amino acids content as well. Therefore, increasing osmolytes like proline with an increased drought period proves the role of proline in stress tolerance and helps in survival by maintaining osmotic potential, water influx, and detoxifying ROS.
Table 1. Plant height : (including root length and shoot length)
|
SET NO |
SHOOT LENGTH (cm) |
ROOT LENGTH (cm) |
LE F RE (length × breadth) cm2 |
|
CONTROL |
20.5 |
3.0 |
11.55 |
|
3 D YS W TER STRESSED PL NT |
17.5 |
4.3 |
9.00 |
|
5 D YS W TER STRESSED PL NT |
14.6 |
5.2 |
3.50 |
|
7 D YS W TER STRESSED PL NT |
10.5 |
9.5 |
3.20 |
|
ST ND RD ERROR |
2.13 |
1.40 |
2.06 |
|
CRITIC L DIFFERENCE |
16.52(1%) 3.30(5%) |
6.39(1%) 1.27(5%) |
86.01(1%) 17.22(5%) |
Table 2. verage leaf area of a single plant
|
Control leaf area (cm²) |
3 days stressed plant leaf area (cm²) |
5 days stressed plant leaf area (cm²) |
7 days stressed plant leaf area (cm²) |
Standard error |
Critical difference |
|
7.2 |
5.4 |
4.8 |
2.5 |
0.969 |
20.53(1%) 4.10(5%) |
Table 3. verage fresh weight of a single plant (including root, shoot, leaves):
|
SET NO |
PLANT WEIGHT (gm) |
ROOT WEIGHT (gm) |
SHOOT WEIGHT (gm) |
LEAF WEIGHT (gm) |
|
CONTROL |
1.057 |
0.170 |
0.407 |
0.160 |
|
3DAYS WATER STRESSED PLANT |
0.983 |
0.057 |
0.318 |
0.152 |
|
5 DAYS WATER STRESSED PLANT |
0.660 |
0.048 |
0.239 |
0.122 |
|
7 DAYS WATER STRESSED PLANT |
0.648 |
0.035 |
0.273 |
0.120 |
|
STANDARD ERROR |
0.107 |
0.031 |
0.036 |
0.010 |
|
CRITICAL DIFFERENCE |
9.10(1%) 1.82(5%) |
3.05(1%) 0.61(1%) |
65.37(1%) 13.07(1%) |
27.53(1%) 5.50(5%) |
Table 4. verage dry weight of a single plant
|
SET NO |
PLANT WEIGHT (gm) |
ROOT WEIGHT (gm) |
SHOOT WEIGHT (gm) |
LEAF WEIGHT (gm) |
|
CONTROL |
0.130 |
0.017 |
0.050 |
0.018 |
|
3 DAYS WATER STRESSED PLANT |
0.126 |
0.015 |
0.049 |
0.016 |
|
5 DAYS WATER STRESSED PLANT |
0.120 |
0.014 |
0.047 |
0.015 |
|
7 DAYS WATER STRESSED PLANT |
0.112 |
0.009 |
0.031 |
0.013 |
|
STANDARD ERROR |
0.0039 |
0.0017 |
0.0013 |
0.0012 |
|
CRITICAL DIFFERENCE |
96.15(1%) 19.23(2%) |
45.06(1%) 9.01(5%) |
58.79(1%) 11.79(5%) |
21.71(1%) 4.342(5%) |
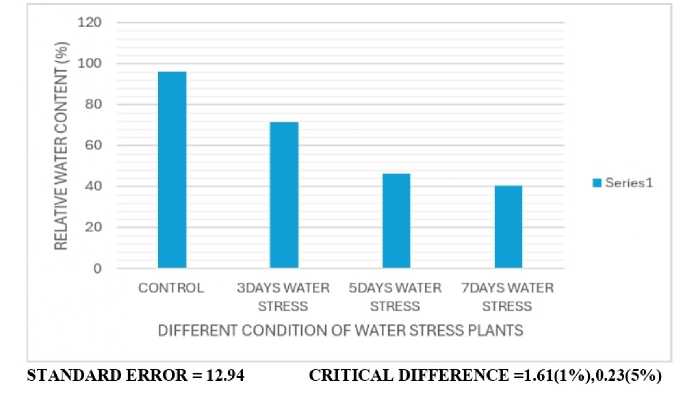
Figure 1: Estimation of relative water content (RWC)
STANDARD ERROR = 0.013 CRITICAL DIFFERENCE = 21.51(1%), 4.30(5%)
Figure 2: Estimation of chlorophyll content
TOTAL CAROTENOID OF THE DIFFERENT WATER STRESS PLANTS
STANDARD ERROR = 0.278 CRITICAL DIFFERENCE 0.41(1%), 0.08(5%)
Figure 3: Estimation of carotenoid content
ф
1.2
и
Е
ф
□
СЛ Ф
Ф
О
■ Seriesl
BUFFER SOLUBLE PROTEIN CONTENT
STANDARD ERROR = 0.104
CRITICAL DIFFERENCE=13.33(1%), 2.66 (5%)
Figure 4: Estimation of buffer soluble protein content
E
£
E
STRESS
STRESS
STRESS
Seriesl
TOTAL AMINO ACID CONTENT IN THE DIFFERENT STRESS PLANTS
STANDARD ERROR =1.03
CRITICAL DIFFERENCE=2.42(1%), 0.48(5%)
-
Figure 5: Estimation of amino acid content
<л
tn
OJ
E
E
ф

CONTROL
3DAYS WATER STRESS


Seriesl
5DAYS WATER STRESS
7DAYS WATER STRESS
TOTAL PROLINE CONTENT IN THE DIFFERENT WATER STRESS PLANTS
STANDARD ERROR= 1.67
CRITICAL DIFFERENCE=47.37(1%), 9.47(5%)
-
Figure 6: Estimation of proline content
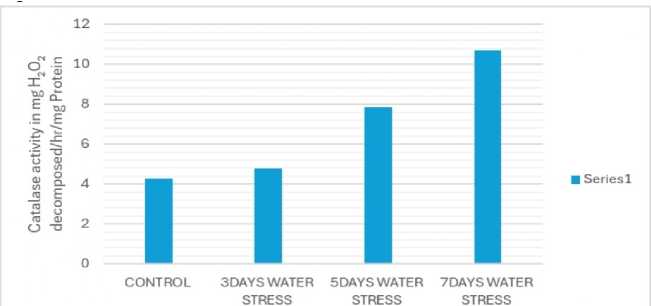
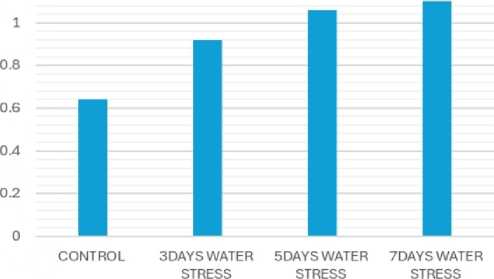
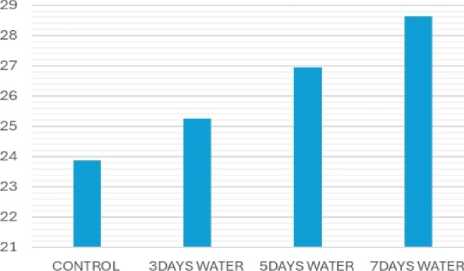
ACTIVITY OF CATALASE ENZYME IN THE DIFFERENT STRESS PLANTS
STANDARD ERROR= 1.49. CRITICAL DIFFERENCE= 9.16(1%), 1.83(5%)
Figure 7: ssay of catalase enzyme activity
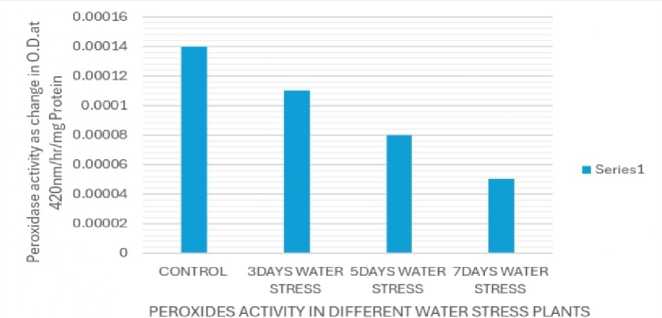
STANDARD ERROR=0.0001 CRITICAL DIFFERENCE=28.18(1%), 5.64(5%)
Figure 8: ssay of peroxidase enzyme activity
Due to water stress, catalase activity significantly increased (Fig. 7), while peroxidase activity decreased (Fig. 8). Catalase is a crucial enzyme in plants that helps to detoxify hydrogen peroxide (H2O2), a reactive oxygen species (ROS) that can damage cells under stress. Catalase and peroxidase are important antioxidant enzymes that play a crucial role in protecting plants from damage caused by abiotic stress like drought, salinity and extremes of temperatures. These enzymes work to scavenge harmful reactive oxygen species (ROS) generated as a result of abiotic stress (Sen, 2020, 2023).
CONCLUSIONS
This study demonstrates the significant effects of drought stress on plant growth and productivity, highlighting that reduced water availability leads to physiological and biochemical changes that negatively impact biomass and yield. The decrease in shoot length and leaf area, along with an increase in root length, indicates that plants adapt by extending their roots in search of water, but this adaptation results in reduced fresh and dry weights.
dditionally, the decline in relative water content (RWC) and chlorophyll levels can impair photosynthesis, while decreased carotenoid levels highlight the complexity of responses to water stress. Increased levels of stress-responsive proteins, amino acids, and proline suggest these compounds are crucial for enhancing drought tolerance by maintaining cellular integrity. The rise in catalase activity, coupled with a decrease in peroxidase activity, indicates a shift in the antioxidant response to detoxify reactive oxygen species. Overall, understanding these mechanisms is essential for developing strategies to improve drought resistance in agriculture.
ACKNOWLEDGEMENT
The authors are thankful to the PG Department of Botany, sutosh College for providing necessary chemicals and equipment required for the study.
CONFLICTS OF INTEREST ll authors declare that they have no conflicts of interest.
REFERENCES rnon, D.I. (1949). Copper enzymes isolated chloroplasts, polyphenol oxidase in Beta vulgaris. Plant Physiol., 24, 1-15.
Bates, L. S., Waldren, R. P. ., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and soil , 39, 205-207.
Chance, B., and. Maehly, .C., (1955). ssay of catalases and peroxidases. In S.P. Colowick, N.O. Kaplan, eds., Methods in Enzymology, Volume 2, cademic Press, New York, 764-775.
Dahanayake Nilanthi, L Ranawake and DD Senadhipathy (2014) Effects Of Water Stress On The Growth nd Reproduction Of Black Gram (Vigna Mungo L.). Tropical Agricultural Research & Extension , 17(1), 45-48.
Gaspar, T., and Lacoppe, J. (1968). The effect of CCC and mo-1618 on growth, catalase, peroxidase and indoleacetic acid oxidase activity of young barley seedlings. Physiologia Plantarum , 21(5), 11041109.
Hayat S, Hayat Q, lyemeni MN, Wani S, Pichtel J, hmad . (2012) Role of proline under changing environments: a review. Plant Signal Behav. 7(11), 1456-66.
Kanimozhi, R., B nandharaj, P V. Murali (2021). Effect of Drought Stress on Growth, Photosynthetic Pigments and Biochemical Changes in Black Gram (Vigna mungo L) at Flowering Stage, International Journal of Scientific Research in Chemistry (IJSRCH , 6 (6), 07-15.
Kirk, J. T. O., & llen, R. L. (1965). Dependence of total carotenoids and chlorophyll “a” and “b” of leaf extracts in different solvents. Biochem. Soc. Trans, 603, 591.
Lee, Y. P., & Takahashi, T. (1966). n improved colorimetric determination of amino acids with the use of ninhydrin. Analytical biochemistry , 14(1), 7177.
Lowry, O. H., Rosebrough, N. J., Farr, . L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent, Journal of Biological Chemistry , 193, 265-275.
Sen, S. and S. Mukherji (1997). Changes in Mineral composition in belmoschus esculentus (L.) Moench (Okra) and Lycopersicon esculentum Mill. (Tomato) as affected by different seasons. Journal of National Botanical Society , 51, 47-51.
Sen, S. and S. Mukherji (1998a) Seasonal changes in growth characteristics in belmoschus esculentus (L.) Moench and Lycopersicon esculentum Mill. Indian Biologist , 30(2), 60-66
Sen, S. and S. Mukherji (1998b) Seasonal changes in chlorophyll content, chlorophyllase activity, photosynthetic non- cyclic electron transport and CO2 uptake in Lycopersicon esculentum Mill. Research Journal of Chemistry and Environment , 2(3), 57- 61.
Sen, S. and Mukherji, S. (1998c) Seasonal effects on nitrogenous compounds in two crop plants. Environment and Ecology , 16(4), 871-874
Sen, S. and S. Mukerji (1999) Changes in photosynthetic parameters in belmoschus esculentus (L.) Moench as affected by seasonal environmental conditions. Asian Journal of
Microbiology Biotechnology and Environmental
Science , 1(3-4), 157-161 (1999)
Sen, S. (2020) ntioxidative Defense In Plants In Response To Seasonal Environmental Stress, Asian Journal of Science and Technology, 11 (3), 10849-10862.
Sen, S. (2023). Impact of Seasonal Stress on Reactive Oxygen Species and Scavenging Enzymes of Two Crop Plants Growing Under Tropical Indian Conditions. J ournal of Stress Physiology & Biochemistry , 19(4), 43-55.
JOURNAL OF STRESS PHYSIOLOGY & BIOCHEMISTRY Vol. 21 No. 4 2025


