Physiological and Biochemical Responses of Aerva lanata (L.) Juss. ex Schult. under Heavy Metal Stress
Автор: Neema P., Jisha K.C.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.16, 2020 года.
Бесплатный доступ
Biotic and abiotic stresses exert a considerable influence on the growth and reproduction of plants. Temperature, pH, light, water and various chemical pollutants are the important abiotic stresses which directly affect the survival, growth, reproduction and geographical distribution of all plants. The present study aims to determine the stress related changes of Aerva lanata (L.) Juss. ex Schult. under different habitats. A. lanata is an important herb which is widely used for different therapeutic practices. It comes under the family Amaranthaceae. The physiological and biochemical analysis were conducted on the samples from polluted (road side and sides of polluted water body) and non- polluted habitats. The analysis showed remarkable variation among the samples of polluted and non-polluted plants. The results showed that A. lanata of non-polluted habitats were unstressed which was confirmed by the least amount of proline, malondialdehyde (MDA) and high amount of photosynthetic pigments. Heavy metal quantification also showed low amount of heavy metals (Zinc, Lead, Cadmium and Nickel) in samples from non- polluted areas when compared to polluted area, where the quantity of heavy metals is high. The present study revealed remarkable biochemical changes in the plants which were grown under polluted and non-polluted habitats. Moreover, this study also indicated the accumulation of heavy metals in various parts of A. lanata collected from polluted habitats when compared to samples collected from non-polluted habitats. Thus while collecting A. lanata for medicinal purposes, the polluted habitats should be avoided. This study is also very important for researchers who investigate the changes of plants under different stress factors.
Aerva lanata, heavy metal stress, photosynthesis, proline, protein
Короткий адрес: https://sciup.org/143173847
IDR: 143173847
Текст научной статьи Physiological and Biochemical Responses of Aerva lanata (L.) Juss. ex Schult. under Heavy Metal Stress
Medicinal plants have always been a resource for human health. There are many thousands of plants in use throughout the world, with tremendous range of action and degrees of potency. Most have specific action on particular body systems and are known to be suitable for treating certain type of diseases. Aerva lanata (L.) Juss. ex Schult. is one of the best medicinal plant used for different therapeutic practices. It belongs to the family Amaranthaceae. All plants may expose to different stresses and different plants show different types of responses towards various stresses. Some plants avoid stress by completing their life cycle as soon as possible and these stress avoiders have mechanisms that isolate their cells from the stress full conditions (Mooney et al., 1987).While other tolerates stress by altering their metabolism, which allows them to come to thermodynamic steady state with the stress but not suffer injury. In the case of medicinal plants, stresses may alter their medicinal properties as well. Moreover the phytoremediation potential of medicinal plants is good for environment, but in other terms they give bad effect, especially when they are used in drug designing.
The present research work deals with the comparison of various biochemical parameters and quantification of heavy metals (Zn, Pb, Cd and Ni) in the medicinal plant A. lanata growing under different habitats (polluted and non-polluted). For the study, plants were collected from three different areas, nonpolluted habitats, road side and sides of highly polluted water bodies. The samples collected from road side and canal considered as polluted plants. The different physiological and biochemical parameters such as fresh weight, dry weight, moisture content, total carbohydrate, photosynthetic pigments, total protein, proline, MDA and heavy metals were quantified in the samples.
MATERIALS AND METHODS
The research was carried out with Aerva lanata (L.) Juss. ex Schult. and the plants were collected from natural habitats, road side and canal side. The first one are considered relatively non polluted and later two are considered as polluted. For further reference, plants growing on the roadside of NH-17 are referred GOR, plants growing in the non-polluted area are referred as GON and those plants collected from polluted water side referred as GOW.
Plants were uprooted, kept in a polythene bag and brought to the laboratory. For the uniformity of experiments, 5th leaf from the tip was selected from each plant and from each habitat, three samples were collected and each of them mixed separately. Plants were washed thoroughly in running tap water and the water was blotted off with a blotting paper. The experiments were performed without any delay. All the experiments were repeated three times.
The physiological parameters like dry weight, dry weight percentage and moisture content percentage of samples were determined. Samples (leaves) were weighed using electronic balance. For fresh weight and dry weight measurements, the plants were blotted and wrapped separately in pre-weighed labelled aluminium foils. Fresh weight of the samples was determined by weighing them immediately after wrapping. For dry weight measurements the samples were kept in a hot air oven at 1000 C for one hour followed by at 600C for overnight. After 48 h, the samples were transferred to a desiccator, allowed to cool and then weighed. The samples were reweighed as described above at regular intervals (24h), until the weights became constant. The dry weight percentage was calculated by using the following formula:
Dry weight
Dry weight percentage = x 100
' Fresh weight
Moisture content percentage was calculated by using the following formula
Fresh weight - Dry weight Moisture content percentage = _ , . . x 1 OU
Fresh weight
Estimation of photosynthetic pigments (chlorophylls and carotenoids) was done according to the method of Arnon (1949). The total carbohydrate content was estimated according to Dubois et al . (1956) and total protein content of the plant material was estimated using Folin–Ciocatteau reagent as per the method of Lowry et al . (1951). Proline content was estimated according to the method of Bates et al. (1973) and the MDA content in the samples was estimated according to the method of Heath and Packer (1968).
For the heavy metal quantification, different plant parts root, stem and leaf tissues of these plants and soil around them were sampled and were dried at 60oC in a hot air oven. nown weight of the dried sample were digested by refluxing in 10:4 ratio of Nitric acid and perchloric acid until the solution become colourless using jeldahl’s flask heated in a sand bath. Then the digest was transferred to a standard flask and volume was made up to 50 ml and kept in screw capped containers. Atomic absorption spectrophotometer (ICPOES Optima 8000) available at College of Horticulture, AU, Mannuthy, Thrissur was used for the estimation of heavy metals present in the digested samples.
RESULTS
Dry Weight and Moisture Content Percentage
In the present study, dry weight of leaves was maximum in A. lanata plants grown in non-polluted habitats (GON) while compared to road side (GOR) and water side (GOW) samples. As far as the dry weight percentage and moisture content percentage is concerned, it was varied in polluted and non-polluted samples. In the collected samples from non-polluted habitats (GON), dry weight percentage was found to be 25% and moisture content percentage was 75% where as in the case of GOR and GOW, the dry weight percentage was found to be 20% and 16%, respectively. Moisture content percentage in GOR and GOW were 80% and 84% respectively (Table 1).
Photosynthetic Pigment Composition
In the case of photosynthetic pigment content, stress significantly reduced the quantity of photosynthetic pigments in the leaves. The leaves of A. lanata plants grown under non-stressed condition (GON) showed maximum quantity of total chlorophyll (1571.4 µg/gdw) when compared to GOR (935.5 µg/gdw) and GOW(1210.2 µg/gdw) plants (Fig. 1a).As far as the carotenoid content is concerned, similar changes were observed in the quantity of carotenoids in all the three samples. The leaves of A. lanata plants of GOR showed minimum quantity of carotenoid content (115.3 µg/gdw) when compared to GON (179.2 µg/gdw) and GOW (213.7 µg/gdw) (Fig. 1b).
Primary Metabolites
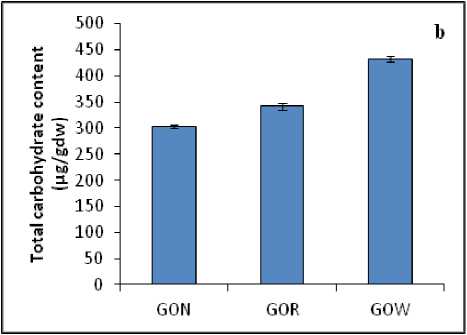
Primary metabolites like protein and carbohydrate showed variations in the plant samples collected from different habitats. The total protein content in the leaves of A. lananta under non-polluted habitat was less (180.2 µg/gdw) when compared to that of GOR (218.5 µg/gdw) and GOW (271.7µg/gdw) plants (Fig. 2a). Among the three samples studied, total carbohydrate content was found to be high in the samples which were grown under polluted (GOR-341.9 µg/gdw, GOW-432.7 µg/gdw) conditions when compared to plants grown in non-polluted(GON-302.8 µg/gdw) habitats (Fig. 2b).
Proline and MDA Content
Proline and MDA are regarded as the indicators of stress. In the present research work proline and MDA content showed significant variations among the different samples. The proline content was found to be higher in the samples which were grown under polluted water body GOW (95.7 µg/gdw). Whereas the proline content was 27.1 µg/gdw and 71.4 µg/gdw in the leaves of non-polluted (GON) and roadside (GOR) samples respectively (Fig. 3a). As far as the MDA content was concerned, it was noticed that extend of lipid peroxidation was more for plants collected from polluted water bodies and those from road side (GOW-30.3 µg/gdw and GOR-24.7µg/gdw) over the plants collected from non-polluted area (GON-18.9 µg/gdw) (Fig. 3b).
Heavy Metal Quantification
Among the four heavy metals studied, Zinc, Lead and Cadmium showed significant accumulation in various parts of A. lanata growing under polluted habitats when compared to A. lanata plants growing in non-polluted habitats. Zn content was high in all samples than Ni, Pd and Cd. The Nickel content was almost equal in all the three samples (GON, GOR and GOW) (Table 2).
|
Plants |
Dry weight% |
Moisture percentage content% |
|
GON |
25 |
75 |
|
GOR |
20 |
80 |
|
GOW |
16 |
4 |
Table 2. Zinc, Lead, Cadmium and Nickel accumulation in leaves, stem and roots of A. lanata grown under three different habitat (non-polluted-GON, Road side-GOR and canal side-GOW).
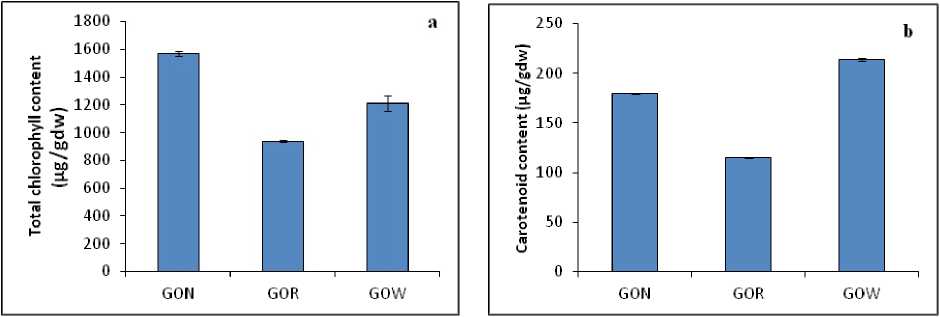
Figure 1: ( a ) Total chlorophyll content and ( b ) Carotenoid content in the leaves of A. lanata grown under three different habitats (non-polluted-GON, Road side-GOR and canal side-GOW). The vertical bars represent SE of the mean value of recordings from three independent experiments each with a minimum of three replicates.
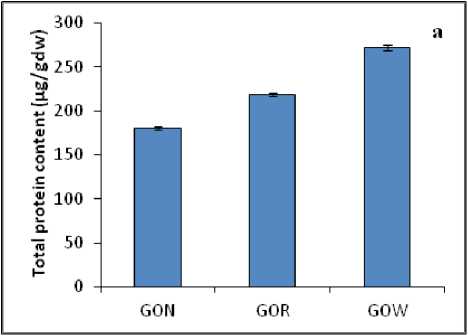
Figure 2 : ( a ) Total protein content and ( b ) Total carbohydrate content in the leaves of A. lanata grown under three different habitats (non-polluted-GON, Road side-GOR and canal side-GOW). The vertical bars represent SE of the mean value of recordings from three independent experiments each with a minimum of three replicates.
|
Samples |
Zn (µg/gdw) |
Pb (µg/gdw) |
Cd (µg/gdw) |
Ni (µg/gdw) |
|
GON leaf |
96 |
3 |
0.5 |
2 |
|
GON stem |
78.5 |
0.5 |
0.05 |
1.5 |
|
GON root |
73 |
2 |
1 |
1.5 |
|
GOR leaf |
158.5 |
3.5 |
4.5 |
2 |
|
GOR stem |
142 |
1.5 |
1.5 |
1.5 |
|
GOR root |
134 |
2 |
1.5 |
2 |
|
GOW leaf |
119.5 |
1 |
1 |
1.5 |
|
GOW stem |
39 |
2 |
2.5 |
1.5 |
|
GOW root |
30.5 |
2 |
1 |
1 |
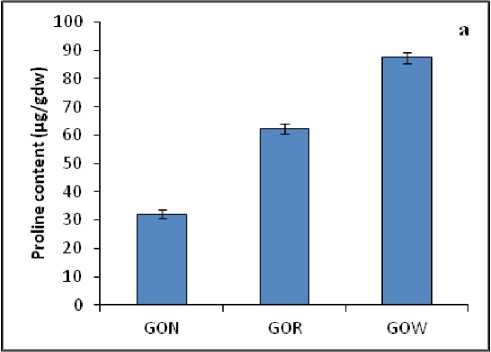
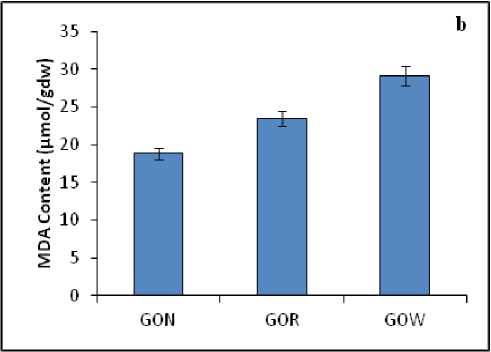
Figure : ( a ) Proline and ( b ) MDA content in the leaves of A. lanata grown under three different habitats (non-polluted-GON, Road side-GOR and canal side-GOW). The vertical bars represent SE of the mean value of recordings from three independent experiments each with a minimum of three replicates.
DISCUSSION
From the results it was clear that the plants which were grown under polluted and non-polluted habitats showed significant variations in the growth attributes as well as in the metabolism. The growth attributes like fresh weight and dry weight of samples were less under polluted habitats when compared to those of nonpolluted habitats. Under stressed conditions it was already reported that growth attributes like fresh weight and dry weight decreases in plants when compared with the plants which were grown under unstressed condition (Pessarakli 2001). It may be due to increased utilization of metabolites for competing with stress rather than for growth and reproduction of plants (Sagi et al., 1997).
As far as the photosynthetic pigment composition was concerned, it was found that the chlorophyll pigment content was less in the plants which were grown under polluted habitats. This reduction in the photosynthetic pigment content in the leaves of plants growing under polluted habitats may be due to the reduced primary metabolism of plants under stressed conditions. The decrease could be due to the breakdown of chlorophyll or due to the inhibition of fresh synthesis by the adverse growth conditions prevailing on roadsides and polluted water sides which includes various abiotic stress factors such as heavy metals and other pollutants. Decrease in chlorophyll content was already reported in Walnut under salt stress (Akca and Samsunlu 2012). Decrease in total chlorophyll content may be due to ion accumulation and functional disorder observed during stoma opening and closing under salinity stress (Seemann and Critchley 1985). The other possibility of reduction in chlorophyll content in A. lanata (GOR, GOW) could be due to the over accumulation of heavy metals in this species affecting the chlorophyll content. An indication that metals can really interfere with pigments and influence the chlorophyll content in plants is the heavy metal substituted chlorophylls that were found in some plants during heavy metal stress. The substitution of Mg2+ ion in chlorophyll molecules by metal ions such as Cu2+, Zn2+, Cd2+, Hg2+, Pb2+ or Ni2+ that was observed in water plants was a reason for the breakdown of chlorophyll thereby affecting photosynthesis negatively ( upper et al., 2000).
The protein largely determines the functional properties of all the living matter and serve as the organic bases of the life. The plants cannot avoid the exposure to stress factors but adapt morphologically and physiologically by some other mechanism. In the present study there was significant changes in the level of total protein was observed in the leaves of plants under non-polluted and polluted habitats. It may be due to the production of extra proteins in the leaves under stress in A. lanata plants. It was supported by Morimoto (1993) and Feder (2006) in their studies that, all most all stress induces the production of a group of protein called heat shock proteins (HSP). Heat stress as well as other stress can together induce gene expression that was not expressed under normal conditions.
The amount of carbohydrate indicates the photosynthetic efficiency of the plant. The increase in total carbohydrate under stressed conditions, in turn may be due to the high primary metabolic activity under stresses. It was already reported that carbohydrate changes are particularly related with physiological process such as photosynthesis, translocation and respiration. The rate and extent of increasing sugar contents depend on the environmental conditions, species and even on the genotype within the same species ( ameli and Losel 1993). The increase in sugar content in response to water stress is already reported by many researchers (Epron and Dreyer 1996).
Increase in the proline content in the samples GOW and GOR indicate that the plants were under stress. Proline being a common compactable solute in cells, its accumulation reduces the adverse effect of stress in plants. Proline is an imino acid and its accumulation in stressed plants has a protective function (Hare and Cress 1997). Numerous studies showed that the proline content increase under different environmental stresses. Proline accumulation has been reported during conditions of drought (Choudhary et al., 2005), high light and UV irradiation (Saradhi et al., 1995), heavy metals (Schat et al., 1997), and in response to biotic stresses (Fabro et al., 2004).
The lipid peroxidation is a well-established metabolism of cellular injury in both plants and animals, and it is used as an indicator of oxidative stress in cell and tissue. Lipid peroxidase derived from poly unsaturated fatty acid, is unstable and decomposes to form a complex series of compounds. These include reactive carbonyl compounds and among these Malondialdyhide (MDA) is the most abundant one. Measurement of MDA is widely used as an indicator of lipid peroxidation. In our study it was found that the plants which are growing under polluted habitats showed more MDA than the plants which were grown under non-polluted habitats. The increased amount of MDA shows that the plants were under stress. There were many reports on the increased amount of MDA in plants under stressed condition when compared to nonstressed conditions (Jisha and Puthur 2016).
Zinc accumulation was found to be higher in all samples especially in road side. From this data we can find that accumulation of heavy metals was more in leaves and stem. The metal concentrations in shoots are particularly greater indicating the special ability of the plant to absorb and transport metals and store them in their above-ground part. Heavy metal belongs to a group of non-biodegradable, persistent inorganic chemical constituents with the atomic mass over 20 and the density than 5 g cm-3 that have toxic, genotoxic and mutagenic effects on humans or animals and plants. There is a chance for oxidative stress and it supported by the reports of Mishra and Prakash (2010), excess Zn2+ in cells can produce ROS and adversely affects integration and permeability of membrane.
From the present study, it was understood that the A. lanata collected from polluted areas showed variations in various physiological and biochemical attributes when compared with the samples collected from non-polluted areas. The analysis showed clear difference in the chlorophyll, MDA, protein, proline and carbohydrate content among the samples of GON, GOR and GOW. Besides this, heavy metal quantification of samples showed comparatively high amount of heavy metals in A. lanata of polluted plants than non-polluted plants. Thus the present study revealed remarkable biochemical changes in the plants which were grown under polluted habitats and the study also suggests the collection of A. lanata plants from non-polluted habitats for medicinal purposes, because there will be a definite changes in the medicinal properties of the plants under stress.
Список литературы Physiological and Biochemical Responses of Aerva lanata (L.) Juss. ex Schult. under Heavy Metal Stress
- Akca, Y. and Samsunlu, E. (2012) The effect of salt stress on growth, chlorophyll content, proline and nutrient accumulation, and K/NA ratio in Walnut. Pak. J. Bot., 44: 1513--1520.
- Arnon, D.I. (1949) Copper enzymes in isolated chloroplast polyphenoloxidase in Beta vulgaris. Plant Physiol., 24: 1--5.
- Bates, L.S., Waldren, R.P. and Teare, I.D. (1973) Rapid determination of free proline for water stress studies. Plant Soil, 39: 205--208.
- Choudhary, N.L., Sairam R.K. and Tyagi, A. (2005) Expression of deltal-pyrroline-5-carboxylase synthetase gene during drought in rice (Oryza sativa L.). Ind. J. Biochem. Biophy., 42: 366--370.
- Dubois, M., Gilles, K.A., Rebers, P.A., Hmilton, J.K. and Smith, F. (1956) Colorimetric method for determination of sugar and related substances. Anal. Chem., 26: 350--356.
- Epron, D. and Dreyer, E. (1996) Starch and soluble carbohydrates in leaves of water-stressed oak saplings. Annal. Sci. foresti. INRA/EDP Sci., 53: 263--268.
- Fabro, G., Kovács, I., Pavet, V., Szabados, L. and Alvarez, M.E. (2004) Proline accumulation and AtP5CS2 gene activation induced by plant pathogen incompatible interactions in Arabidopsis. Mol. Plant-Microbe Interact., 17: 343--350.
- Feder, M.E. (2006) Integrative biology of stress: molecular actors, the ecological theater, and the evolutionary play. International Symposium on Environmental Factors, Cellular Stress and Evolution, Varanasi, India, October 13–15, 21.
- Hare, P. and Cress, W. (1997) Metabolic implication of stress induced proline accumulation in plants. Plant Growth Regul., 21: 79--102.
- Heath, R. and Packer, L. (1968) Photoperoxidation in isolated chloropkast. I Kinetics and stoichiometry of fatty acid peroxidation. Pak. J. Bot ., 125: 189--198.
- Jisha, K.C. and Puthur, J.T. (2016) Seed Priming with Beta-Amino Butyric Acid Improves Abiotic Stress Tolerance in Rice Seedlings. Rice Sci., 23: 242--254.
- Kameli, A. and Losel, D.M. (1996) Growth and sugar accumulation in durum wheat plants under water stress. New Phytol., 132: 57--62.
- Kupper, H., Lombi, E., Zhao, F.J. and McGrath, S.P. (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyper accumulator Arabidopsis halleri. Planta, 212: 75--84.
- Lowry, O.H., Rosenbrough, N.J., Farr, A. L. and Randall, R.J. (1951) Protein measurement with Folinphenol reagent. J. Biol. Chem., 193: 265--275.
- Mishra, P.K. and Prakash, V. (2010) Response of nonenzymatic antioxidants to Zinc induced stress at different pH in Giycine max L. cv. Merril. Academic J. Plant Sci., 3: 1--10.
- Mooney, H.A., Percy, R.W. and Schlinger, J. (1987) Plant physiological ecology today. Biosci., 18—20.
- Morimoto, R.I. (1993) Cells in stress: The transcriptional activation of heat shock genes. Sci., 259: 1409--1410.
- Pessarakli, M. (2001) Physiological responses of Cotton (Gossypium hirsutum L.) to salt stress. In Pessarakli, M. (ed.), Handbook of plant and Crop Physiology. Marcel Dekker, New York, pp. 681-696.
- Sagi, M., Dovrat, A., Kipnis, T. and Lips, S.H. (1997) Ionic balance and the production of biomass and organic nitrogen as affected by salinity and N source in annual ryegrass (Lolium multiflorum Lam). J. Plant Nutr., 20: 1291--1316.
- Saradhi, P.P., Arora S.A. and Prasad, K. (1995) Proline accumulates in plants exposed to UV radiation and protects against UV induced peroxidation. Biochem. Biophy. Res. Comm., 209: 1--5.
- Schat, H., Sharma, S.S. and Vooijs, R. (1997) Heavy metal induced accumulation of free proline in a metal-tolerant and a non-tolerant ecotype of Silene vulgaris. Plant Physiol., 101: 477--482.
- Seemann, J.R. and Critchly, C. (1985) Effect of salt tress on growth, ion content, stomatal behavior and photosynthetic capacity of salt sensitive species Phaseolus vulgaris (L.). Planta, 164: 15--16.


