Physiological and biochemical responses of two maize cultivars (Corralejo and Tlaltizapon) under salt stress
Автор: Amdouni T., Mrah S., Msilini N., Zaghdoud M., Ouerghiabidi Z., Lachaal M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.10, 2014 года.
Бесплатный доступ
The aim of the present work was to study the effect of different concentrations of NaCl (0, 50 and 100mM) in two cultivars of maize (Corralejo and Tlaltizapan), on their nutritional and photosynthetic comportment. The measures focused on the physiological parameters (growth weight, hydration and nutritional status of plants) and biochemical (chlorophyll, PEPC activity, activity of some anti-oxidant enzymes and lipid peroxidation). Analysis of morphological parameters showed a yellowing of the extremity of leaves, at 100 mM of NaCl. These visual symptoms are associated with a decrease of chlorophyll. A decrease in potential growth was found in two cultivars, but less significant in Corralejo. The best salt tolerance of the latter was due to a better hydration of the leaves, to a lesser accumulation of Na + and Cl - in its leaves and a better selectivity K/Na. To identify the biochemical characteristics associated with the physiological behavior, we conducted measures activity of PEPC, the protein, catalase and peroxidase on the fourth leaf from the bottom. A negative correlation between the activity of PEPC and Na+ amount was found at 50 mM in the sensitive cultivar and at 100 mM of NaCl in tolerant cultivar Corralejo. Furthermore, the antioxidant response was marked by a greater accumulation of malondialdehyde, in Tlaltizapan at 100 mM of NaCl. At the same concentration, catalase peroxidase and SOD activities weren't decreased in this cultivar. This suggests that salt has created a stress oxidative state only in Tlaltizapan leaves. These results showed a better performance of Corralejo cultivar compared to Tlaltizapan cultivar, at 50 and 100 mM of NaCl.
Maize, growth weight, hydration, lipid peroxidation, proline, chlorophyll, salt stress
Короткий адрес: https://sciup.org/14323891
IDR: 14323891
Текст научной статьи Physiological and biochemical responses of two maize cultivars (Corralejo and Tlaltizapon) under salt stress
Salt stress in soil is one of the major stresses especially in arid and semi-arid regions and affects plant production in many parts of the world, particularly on irrigated land (Koca et al., 2007). It is estimated that 20% of the irrigated land in the world is presently affected by salinity (Yeo, 1999). This reduction in plant growth in saline environments could be due to either adverse water relations or the toxic effects of Na+ and Cl- ions accumulation on metabolism (Yeo and Flowers, 1983).
Many crop species are sensitive to high concentrations of salt with negative impacts on agricultural production. Maize ( Zea mays L .) is considered a moderately salt-sensitive plant, (Mass and Hoffman, 1977). Salt resistance of plants is a complex phenomenon that involves biochemical and physiological processes as well as morphological and developmental changes (Munns, 2002; Pitann et al., 2009). Plant salt tolerance has generally been studied in relation to regulatory mechanisms of ionic and osmotic homeostasis (Ashraf and Harris, 2004). The decrease in uptake of K+, Mg2+, Ca2+ and thereby as well as in growth at higher sodium concentration has also been reported (Koca et al., 2007). In addition, salt stress, like other abiotic stresses, also leads to oxidative stress through an increase in reactive oxygen species (ROS), such as superoxide (O 2 •-), hydrogen peroxide (H 2 O 2 ) and hydroxyl radicals (OH•) (Neill et al., 2002).
These oxygen species are highly cytotoxic and can seriously react with vital biomolecules such as lipids and proteins (Imlay, 2003) causing lipid peroxidation, protein denaturing and DNA mutation
(Breusegem et al., 2001; Quiles and Lopez. 2004). Evidence suggests that membranes are the primary sites of salinity injury to cells and organelles (Candan and Tarhan, 2003) because ROS can react with unsaturated fatty acids to cause peroxidation of essential membrane lipids in plasmalemma or intracellular organelles (Karabal et al., 2003). Fortunately, plants have developed various protective mechanisms to eliminate or reduce ROS, which are effective at different levels of stress-induced deterioration (Beak and Skinner, 2003). The enzymatic antioxidant system is one of the protective mechanisms including CAT (Kono and Fridovich, 1983) and POX (Gara et al., 2003).
Despite the importance of this cereal in Tunisia, until now, we do not have a veritable maize culture. This leads us to think of integrating foreign varieties that could ameliorate productivity and adapted to the conditions of drought and salinity. Therefore, the aim of this study was to evaluate the effects of salt stress on growth and on anti-oxidative enzymes activity in leaves of tow maize cultivars, in order to better understand the physiological and biochemical mechanisms of salt tolerance.
MATERIALS AND METHODS
Seeds of maize (cv. Corralejo and Tlaltizapon ) were imbibed for one hour in deionized water at 4◦C, and then sown in Petri dishes with wet filter paper for germination in the dark at 25°C. Five days after germination; seedlings were transferred into pots containing nutrient solution of Hoagland and Arnon (1940) in a growth room. Photoperiod was 16 h with
150 µmol m-2 s-1 PAR at the plant level. Day and night temperature and relative humidity regimes were 22/18°C and 60/80%, respectively. Seventeen days after germination, an initial harvest was performed. Then, three treatments were started. In the first, seedlings were cultivated in the same nutrient solution and considered as control. In the other treatment, 50 and 100 mM NaCl was added to the medium. The final harvest was performed after 15 days of treatment. For each treatment, 8 plants were taken and the fresh weights of leaves, leave order 4, stems and roots were weighed. The samples were then oven-dried at 70°C for 72 h for the determination of DW. Besides, fresh samples from each plant were immediately frozen in liquid nitrogen and stored at -80°C until performing biochemical analysis.
Aliquots (0.2 g) of oven-dried ground material of all organs were digested with 25 ml nitric acid (HNO 3 ) 0.5%. Sodium (Na+) and potassium (K+) content were measured in the digests with a flame emission photometer (Jenway PFP7). Chloride (Cl-) content of the digested extracts was determined using a chloride analyser.
The level of lipid peroxidation was determined by a procedure based on the method of Heath and Packer (1968). 0.5 g fresh samples were ground in 5 ml of ice-cold phosphate buffer solution (0.05 mM, pH 7.8) containing 1% polyvinylpyrrolidone (w/v) (PVP). The homogenate was centrifuged at 10,000 g for 30 min. 2 ml of supernatant was mixed with 2 ml of thiobarbituric acid (TBA) (0.5% TBA, 20% trichloroacetate TCA). The mixture was heated at 100°C for 30 min, chilled on ice, and then centrifuged at 1000 g for 10 min. Absorbance of the supernatant was measured at 532 nm and adjusted for nonspecific absorbance at 510 nm and 560 nm.
The electrolyte leakage (EL) was determined as described by Dionisio-Sese and Tobita (1998). Leaves 4 discs of fresh seedlings were cut into 2– 3mm pieces and placed in test tubes containing 10 ml distilled water. The tubes were incubated in a water bath at 32°C for 2 hours and the initial electrical conductivity of the medium (EC1) was measured. The samples were autoclaved at 121°C for 20 min to release all electrolytes; cooled to 25°C and the final electrical conductivity (EC2) was measured. The EL was calculated from EL = (EC1/ EC2)100.
Fresh material of the leaves 4 obtained at 15 days after salt treatment were excised and immediately placed in liquid nitrogen. They were later ground in a 50 mM potassium phosphate buffer (pH 7.5) containing 1 mM EDTA, 1 mM DTT, 5% glycerol and 5% polyvinylpolypyrrolidone, and centrifuged for 20 min at 15000×g. The supernatant was used for determination of soluble proteins and enzymatic activities. The concentration of proteins was determined according to the method of Bradford (1976).
CAT activity was measured according to the modified method of Aebi (1984). The reaction mixture consisted of 25 mM potassium phosphate buffer (pH 7.0), 30 mM H2O2 and enzyme extract. The decomposition of H2O2 was followed by measuring the decrease in absorbance at 290 nm. Total peroxidase activity was assayed using guaiacol as an electron donor, with a reaction mixture containing 50 mM potassium phosphate (pH 7.0), 0.1 mM EDTA, 5 mM H2O2 and 10mM guaiacol, a method derived from Fielding and Hall (1978). The increase of absorbance, due to tetraguaiacol formation, was recorded at 470 nm through 3 min. All enzyme activities were expressed per mg of total proteins.
In our experiences, the native frosts used, don't contain any denaturing agents and permit to make migrate the native proteins. It will allow surveying the raw state activity and the total molecular weight of the total protein. The isoforms of superoxide dismutase (SOD) were separated by gel electrophoresis of the supernatant (stacking gel: 5% acrylamide, pH 6.8, 0.5M Tris-HCl buffer; resolving gel: 12%, pH 8.8, 1.5M Tris-HCl buffer).
After the migration, the native gels 12% is incubated in the dark for 20 min in 2.45mM Nitro Blue Tetrazolium pH 7.8, 36mM phosphate buffer containing 28mM TEMED and 28 mM riboflavin. Thereafter, the gels were exposed to intense white light for 20 min in Phosphate of potassium (36 Mm, pH 7.8), TEMED (28 Mm) and Riboflavine (28 µM). SOD activity appears as bleached bands on the gels. Cu/Zn-SOD are inhibited by KCN and H 2 O 2 and Fe-SOD are inactivated by H 2 O 2 , whereas Mn-SOD are resistant to both inhibitors (Fridovich, 1989).
Statistical analysis performed with Statistica ™ software, using two-way ANOVA and Newman-Keuls test for post-hoc mean comparison. The ANOVA was performed over the whole set of data.
RESULTS
The growth responses to salt stress of two cultivars of maize were represented in Table I. At 0 mM NaCl, all cultivars produced the same total dry weight (1.9 g and 1.7 g respectively for Corralejos and Tlaltizapan). At 100 mM, the whole plant biomass decreased by 31% and 45%, respectively, in Corralejo and Tlaltizapon in comparison to the control. Considering plant organs, Table I shows that leave growth is the most affected by salinity followed by roots and stems. In addition, the applied NaCl inhibit the growth of maize plant and caused the decrease in the length of the aerial part with leaf yellowing especially in Tlaltizapon.
Particular interest is granted to the leaf of the order 4, this is the first leaf which appears just after salt treatment. Dry weight of leaves 4 was drastically reduced by 54% and 30% under 100 mM NaCl, respectively in Tlaltizapon and Corralejo. The 50 mM NaCl had non-significant effects on dry weight of these leaves.
Water content of three organs of Corralejo and Tlaltizapon (Table I) depended poorly on the treatments. At 100 mM, salt treatment induced no significant effect on leaf tissue hydration in Corralejo, but affected it (-57 %) in Tlaltizapon. In Corralejo as well as in Tlaltizapon, the hydration of the roots seems insensible to salt stress. On the other hand, the water content in the leaves order 4 decreased only in Tlaltizapan (-20%).
In this study, Na+ accumulation in Corralejo as well as Tlaltizapon showed a positive relationship with NaCl concentrations added in the culture medium. Under NaCl treatment, a significant variation in the areal part (leaves, leave 4 and stem) and roots Na+ content was observed (Table II). In the two cultivars, the roots Na+ content was greater than that of the leaves. Moreover, at 100 mM, the Na+ content was more important in Corralejo roots than in Tlaltizapon. In leaves, salt treatment increased Na+ content, especially in Tlaltizapon.
Chloride uptake showed important variations according to the NaCl concentration. Cl- is accumulated in the same way as Na+ in the different organs of maize plants, according to a decreasing pressure gradient of the roots toward the leaves. At 100 mM of NaCl, Corralejo plants accumulate less Clin their leaves and more in their roots. In addition, both cultivars accumulated more Na+ and Cl- in their leaves of order 4.
Salt treatment significantly reduced K+ content in all organs, but differently according to the varieties: the main reduction concerned leaves and stems in Tlaltizapon.
Membrane lipid peroxidation in the leaves 4, first leaf appear just after salt treatment, of two maize cultivars was assessed by the content of MDA and the EL (Figure 1). Under non-saline conditions, MDA concentration was similar in leaves of both cultivars. Salt treatment increased MDA concentration in Tlaltizapon, reaching 4,80 µmol.g-1 FW at 100 mM NaCl. However no significant effect was observed in leaves of Corralejo. These results are confirmed by electrolyte leakage. Results showed that salt stress impaired membrane permeability by increasing ion leakage in the leaves of both cultivars, but highly in Tlaltizapon.
Protein contents in the leaves 4 of two cultivars after 12 day of NaCl treatment are given in Figure 2. The results show that protein content was higher in the leaves of Corralejo, under control as well as salt conditions. NaCl treatment did not significantly affect protein content in both cultivars.
Activities of CAT and POX, (Figure 2) showed that, CAT activity in Corralejo leaves was significantly enhanced by 100 mM NaCl, however in seems it remain unchanged at 50 mM. in Tlaltizapon, CAT activity was not affected by salt treatment. As for CAT activity, only 100 mM NaCl significantly increased the activity of POX in leaves of Corralejo cultivar, however it was not significantly affected in Tlaltizapon.
PEPC activity was also measured in leaves 4 extracts from the two cultivars (Figure 3). In control medium, the two cultivars of plants present an equivalent PEPC activity. The presence of salt in the nutrition solution reduced activity of this enzyme, in the both maize cultivar. This reduction increased with NaCl concentration and reached 71% and 79% at 100 mM NaCl, respectively in Corralejo and Tlaltizapan.
There were striking differences in antioxidant enzyme activities between the two cultivars with increasing NaCl concentration (Figure 3). In Corralejo, we showed any remarkable change of SOD activity, whereas it decreases strongly to 50 mM and 100 mM of NaCl in Tlaltizapan. The tests of inhibition, by cyanide and H2O2 show that the band of the SOD detected is a Cu/Zn-SOD. The analysis of the Electrophoregrams to Basic guaiacol peroxidase was represented as two bands, in the both of cultivars. The activity of the isoforme 1 rest insensible to salt in Corralejo, it increased in this cultivar but decreased with increasing of NaCl concentration in Tlaltizapan. As for the isoforme 2, salt stress affects the activity of this enzyme only at Tlaltizapan.
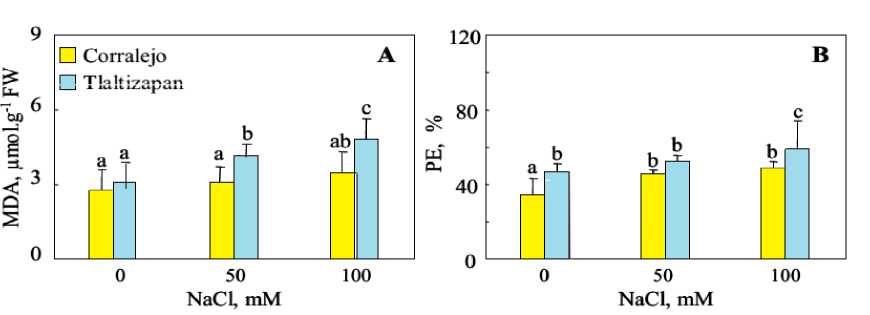
Figure 1. Concentration of malondialdehyde (MDA) (A) and electrolyte leakage levels (EL) (B) in leaves, stems and
roots of tow cultivar of maize plants, after 15 days of growth under NaCl (0, 50mM and 100mM NaCl). Data are means of 6 replicates. Bars labelled by the same letter are not statistically different according to the ANOVA test at P ≤ 0.05.
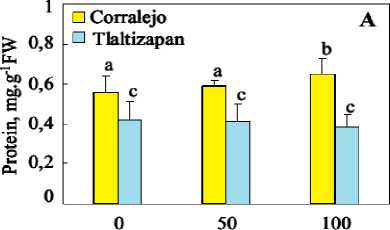
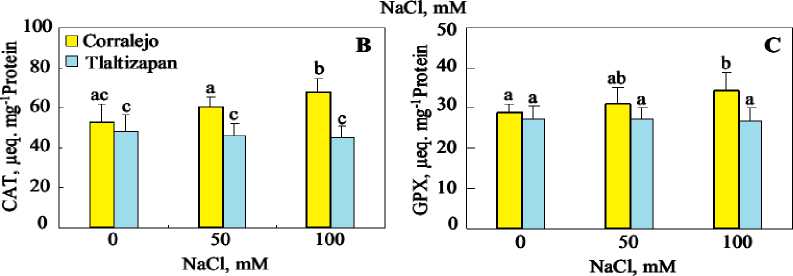
Figure 2. Total protein contents(A), catalase (B) and Guaiacol peroxidase (C) activities in leave in leave, stem and roots of tow cultivar of maize plants grown for 12 days in presence of different NaCl concentrations (0, 50mM and 100 mM NaCl). Data are means of 4 replicates. Bars labelled by the same letter are not statistically different according to the ANOVA test at P ≤ 0.05.
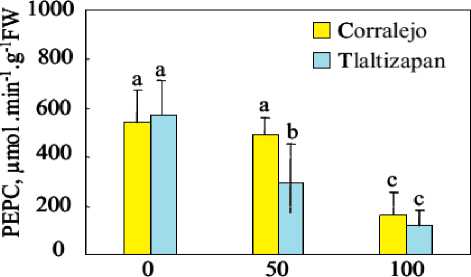
NaCl, mM
Figure 3. Effect of different levels of salinity (0, 50 and 100 mM NaCl) on the rate of phosphoenolpyruvate carboxylase kinase activity in leaves 4 of tow cultivar of maize (Corralejo and Tlaltizapon), after 15 days of growth under NaCl (0, 50mM and 100mM NaCl)). Data are means of 4 replicates. Bars labelled by the same letter are not statistically different according to the ANOVA test at P ≤ 0.05

Figure 4. Superoxide dismutase (SOD) native polyacrylamide gel in tow cultivar of maize (Corralejo and Tlaltizapon) plants grown for 12 days under different NaCl concentrations (0, 50mM and 100 mM NaCl).
Table 1. Dry weights (DW) and relative water contents (RWC) in leave, leave4, stem, and roots maize plants (Corralejo and Tlaltizapon) after 15 days of growth under NaCl (0, 50 and 100 mM NaCl) conditions.
|
NaCl, mM |
0 mM NaCl |
50 mM NaCl |
100 mM NaCl |
||||
|
Corralejo |
Tlaltizapon |
Corralejo |
Tlaltizapon |
Corralejo |
Tlaltizapon |
||
|
Leaves |
0.857±0.12a |
0.765±0.08ab |
0.735±0.11ab |
0.658±0.11ab |
0.591±0.17bc |
0.423±0.08c |
|
|
Dw, g |
Leaves 4 |
0.254±0.06ab |
0.287±0.05a |
0.290±0.08a |
0.207±0.04ab |
0.175±0.05bc |
0.133±0.02c |
|
Stems |
0.508±0.09ab |
0.538±0.15ab |
0.688±0.17a |
0.452±0.09b |
0.452±0.05b |
0.237±0.06c |
|
|
Roots |
0.493±0.10a |
0.383±0.03abc |
0.428±0.08ac |
0.367±0.06c |
0.275±0.05bcd |
0.248±0.05d |
|
|
Leaves |
6.01±0.43a |
7.80±1.00b |
6.0±0.11a |
6.301±1.12a |
5.8±2.70a |
3.41±0.65c |
|
|
RWC ml.g-1 DW |
Leaves 4 |
7.94±0.87abc |
8.66±0.77b |
6.99±0.72ac |
8.28±0.44ab |
7.33±0.96ac |
6.88±0.84c |
|
Stems |
12.71±2.73ab |
16.02±1.52c |
11.43±1.17ab |
14.02±3.02d |
8.45±0.79e |
11.63±2.41ab |
|
|
Roots |
14.33±1.98a |
13.41±1.44b |
13.11±1.42b |
13.30±1.71b |
12.60±2.07bc |
12.02±1.72c |
|
Table 2. Na+, K+ and Cl- content in leave, leave4, stem, and roots maize plants (Corralejo and Tlaltizapon) after 15 days of growth under NaCl (0, 50 and 100 mM NaCl) conditions.
|
NaCl, mM |
0 mM NaCl |
50 mM NaCl |
100 mM NaCl |
||||
|
Corralejo |
Tlaltizapon |
Corralejo |
Tlaltizapon |
Corralejo |
Tlaltizapon |
||
|
Leaves |
0.02±0.00a |
0.003±0.00a |
0.807±0.13b |
0.982±0.23c |
1.474±0.15d |
2.010±0.23e |
|
|
Na+, meq.g-1 MS |
Leaves 4 |
0.035±0.06a |
0.01±0.007a |
0.986±0.06b |
1.324±0.04c |
1.384±0.06c |
1.910±0.11d |
|
Stems |
0.017±0.00a |
0.02±0.005a |
1.046±0.09b |
1.938±0.15c |
1.747±0.25c |
2.259±0.16d |
|
|
Roots |
0.022±0.00a |
0.01±0.005a |
2.007±0.024b |
2.009±0.22b |
3. 005±0.09c |
2.342±0.12d |
|
|
Leaves |
1.115±0.06a |
1.305±0.13a |
0.853±0.073b |
0.748±0.18b |
0.209±0.03c |
0.243±0.07c |
|
|
Leaves 4 |
1.056±0.15a |
1.301±0.18d |
0.596±0.03b |
0.655±0.02b |
0.376±0.04c |
0.397±0.07c |
|
|
K+, meq.g-1 MS |
Stems |
1.076±0.22ac |
1.240±0.18a |
0.780±0.07b |
0.924±0.09c |
0.555±0.03d |
0.543±0.06d |
|
Roots |
0.722±0.04ab |
0.747±0.08b |
0.501±0.04c |
0.416±0.08c |
0.234±0.02d |
0.198±0.04d |
|
|
Leaves |
0.051±0.01a |
0.082±0.01a |
0.251±0.01b |
0.388±0.01c |
0.629±0.04d |
1.138±0.01e |
|
|
Cl-, meq.g-1 MS |
Leaves 4 |
0.015±0.04a |
0.025±0.01a |
0.514±0.02b |
0.641±0.02c |
1.114±0.02d |
1.010±0.03d |
|
Stems |
0.043±0.01a |
0.049±0.00a |
0.456±0.01b |
0.815±0.04c |
0.748±0.01d |
0.931±0.03e |
|
|
Roots |
0.034±0.01a |
0.022±0.01a |
0.594±0.02b |
0.501±0.03b |
1.623±0.09c |
1.113±0.06d |
|
Data are means of 8 replicates ± standard error. Bars labelled by the same letter are not statistically different according to the ANOVA test at P ≤ 0.05.
DISCUSSION
Soil salinity is a prevalent abiotic stress for plants that generally leads to growth arrest and even plant death (Munns and Tester, 2008). Growth inhibition is a common response to salinity; plant growth is one of the most important agricultural indices of salt stress tolerance as indicated by different studies (Parida and Das, 2005). Our results showed that the NaCl salinity reduced growth of the studied species, and the extent of reduction was difference among the cultivars. Tlaltizapan showed a higher growth reduction under salt conditions as compared to Corralejo.
Indeed, leaves growth of Tlaltizapan was more affected than that of Corralejo. Beatriz et al. (2001) showed that inhibition of leaves biomass is one of the primary effects of salt stress and is probably due to inhibition of leaves elongation induced by salt stress in maize (Zea mays). Variance analysis showed that relative growth rate values of cultivars were affected significantly by salt treatment. From our results, it may be expressed that Corralejo is more tolerant than Tlaltizapan. Similar results obtained from researches on maize were reported by other researchers (Ashrafuzzaman et al., 2003; Neto et al., 2004).
Increasing levels of NaCl induced a progressive absorption of Na and Cl in plant, agreeing with Turan et al. (2007a, b). Excessive Na concentration in the plant tissue hinders nutrient balance, and causes toxicity (Bernstein, 1963). Accumulation of Cl in the root tissue is disruptive of the membrane uptake mechanisms, and increased translocation of Cl to the shoots (Yousif et al., 1972). When NaCl was applied to the soil, the levels of K in plant were reduced in accordance with the antagonism between Na and K (Azevedo and Tabosa, 2000). Cramer et al. (1985) showed that excess NaCl leads to the loss of potassium due to membrane depolarization by sodium. In Tlaltizapan plants, leaves are more vulnerable than roots to Na+, simply because Na+ and Cl– accumulate to higher levels in leaves than in roots.
Contrary, Corralejo developed a physiological strategy of tolerance limiting the accumulation of Na and Cl in leaves. These results are consistent with that of Tester and Davenport (2003).
To date, very little is known about the toxic effects of Cl- ions in the plant cell (Teakle and Tyerman, 2010). It is also reported that sensitivity of some crops to salinity is due to the inability to keep Na+ and Cl- out of transpiration streams (Gorham et al., 1990). Starting from the physiological part, we can say that leaves 4 enables us to compare the two cultivars. That is why the biochemical part focuses on this leaf unjustly especially as it is the first leaf appear just after salt treatment.
Effect of salt stress on the plant tissues were determined by measuring of MDA content and the solute leakage from the cells. Our results showed that electrolyte leakage and lipid peroxidation increased as the stress level rose up, only in Tlaltizapan plants. The increase in MDA content was a clear indication for oxidative stress in these plants, which thus seemed to suffer from a more acute saline aggression than Corralejo cultivar. It has been reported that MDA content was correlated with leaf H 2 O 2 accumulation in two corn varieties (Hajlaoui et al., 2009).
Excess of ROS causes phytotoxic reactions such as lipid peroxidation, protein degradation and DNA mutation (Pitzschke and Hirt, 2006). To protect themselves against these toxic oxygen intermediates, a simultaneous increase antioxidant system such as catalase and peroxidases in salt treated plants. Our results show that salt treatment significantly increased CAT and GPX activities in Corralejo in the presence of
100 mM NaCl. However, this response was not observed in Tlaltizapon. A decline in CAT and GPX activities has been described in basilica plants cultivated at 50 mM NaCl levels (Attia et al., 2009). This can be attributed either to inhibition of catalase biosynthesis or to inactivation of this enzyme (Feierabend and Dehne, 1996). The decrease of catalase and other peroxidase activity, as observed in our work, is expected to lead to over accumulation of H 2 O 2 and damaging membrane permeability, as described previously. Such a situation has been described by several authors (Gupta and Datta, 2003), and discussed in relation to the signaling role of H 2 O 2 for morphogenesis. As a result, SOD (superoxide dismutase) increased in Corralejo with the increase of salt stress, Esfandiari et al. (2007) show that SOD (superoxide dismutase) increased in Sardari with the increase of salt stress, while in the case of Alvand, SOD showed constant activity at all salt stress levels. The superoxide dismutase (SOD) can be found in various cell compartments and it catalyses the disproportion of two O 2 ·- radicals to H 2 O 2 and O 2 (Scandalios, 1993).
Список литературы Physiological and biochemical responses of two maize cultivars (Corralejo and Tlaltizapon) under salt stress
- Arulanantham, A.R., Rao, I.M. and Terry, N. (1990) Limiting factors in photosynthesis. IV. Regeneration of ribulose 1.5-bisphosphate limits photosynthesis at low photochemical capacity. Plant. Physiol., 93, 1465-1475
- Ashraf, M. and Harris, P.J.C. (2004) Potential biochemical indicators of salinity tolerance in plant. Plant. Sci., 166, 3-16
- Ashrafuzzaman, M., Halim Khan, M.A. and Shahidullah, S.M. (2003) Response of Vegetative Growth of Maize (Zea mays L.) to a Range of Salinity. OnLine J. Biol. Sci., 3(2), 253-258
- Attia, S., Beltrán, L., DE Herde, A. and Hensen, J. (2009) Architect friendly: a comparison of ten different building performance simulation tools. Proceedings of the 11th International IBPSA Conference. Glasgow, Scotland
- Azevedo neto, A.D and Tabosa, J.N. (2000) Estresse salino em plˆantulas de milho: Parte I An´alise do crescimento. Rev. Bras. Eng. Agr. Amb., 1, 159-164
- Beak, K.H. and Skinner, D.Z. (2003) Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant. Sci., 165, 1221-1227
- Beatriz, G., Piestun, N. and Bernstein, N. (2001) Salinity-induced inhibition of leaf Elongation in Maize is not mediated bay changes in cell wall acidification capacity. Plant. Physiol., 125, 1419-1423
- Bernstein, L. (1963) Osmotic adjustment of plants to saline media. II. Dynamic phase. Am. J. Bot., 48, 909-918
- Bradford, M.M. (1976) A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. An. Biochem., 72, 248-254
- Breusegem, F.V., Vranova, E.J.F., Dat, D. and Inze, (2001) The role of active oxygen species in plant signal transduction. Plant. Sci., 161, 405-414
- Candan, N.L. and Tarhan, L. (2003) The correlation between antioxidant enzyme activities and lipid peroxidation levels in Mentha pulegium organs grown in Ca2+, Mg2+, Cu2+, Zn2+ and Mn2+ stress conditions. Plant. Sci., 163, 769-779
- Cramer, G.R., Läuchli, A. and Polito, V.S. (1985) Displacement of Ca2+from the plasmalemma of root cells. A primary response to salt stress? Plant. Physiol., 79,297-211
- Dionisio-sese, M.L. and Tobita, S. (1998) Antioxidant responses of rice seedlings to salinity stress. Plant. Sci., 135, 1-9
- Esfandiari. E., Shekari1. F., Shekari. F., Esfandiari1. M. (2007) the effect of salt stress on antioxidant enzymes’ activity and lipid peroxidation on the wheat seedling. Not. Bot. Hort. Agrobot. Cluj., 1,1842-4309
- Feierabend, J. and Dehne, S. (1996) Fate of the porphyrin cofactors during the light dependent turnover of catalase and of the photosysem II reaction center protein DI in mature rye leaves. Planta, 198, 413-422
- Fridivich, I. (1989) Superoxide dismutases: An adaptation to a paramagnetic gas. Journal of Biological Chemistry, 264: 7761-7764
- Gara, L. D., Pinto, M.C. and Tommasi, F. (2003) The antioxidant systems vis-á-vis reactive oxygen species during plant-pathogen interaction. Plant. Physiol. Biochem., 41, 863-870
- Gorham, J., Wyn Jones, R.G. and Bristol, A. (1990) Partial characterization of the trait for enhanced K+/Na+ discrimination in the D-genome of wheat. Planta, 180, 590-597
- Gupta, S.D. and Datta, S. (2003) Antioxidant enzyme activities during in vitro morphogenesis of gladiolus and the effect of application of antioxidants on plant regeneration. Plant. Biol., 47, 179-183
- Hajlaoui, H., Denden, M. and El Ayeb, N. (2009) Differential responses of two maize (Zea mays L.) varieties to salt stress: changes on polyphenols composition of foliage and oxidative damages. Int. Crop. Product., 30,144-151
- Heath, R.L. and Packer L. (1968) Photoperoxidation in Isolated Chloroplasts.2. Role of Electron Transfer. Arch. Biochem. Biophys.,125, 189-198
- Hoagland, D.R. and Arnon, D.I. (1940) Crop production in artificial culture solutions and in soils with special reference to factors influencing yield absorption of inorganic nutrients. Soil Sci. Soc. Am., 50, 463-483
- Imlay, J.A. (2003) Pathways of oxidative damage. Ann. Rev. Microbiol., 57, 395-418
- Karabal, E., Yücel, M. and Öktem, H.A. (2003) Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant. Sci., 164, 925-933
- Koca, M., Bor, M., Ozdemir, F. and Turkan, I. (2007) The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot., 60, 344-351
- Kono, Y. and Fridovich, I. (1983) Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J. Biol. Chem., 258, 6015-6019
- Mass, E.V. and Hoffman, G.J. (1977) Crop salt-current assessment. ASCE Irrigation and Drainage Division, 103, 115-137
- Munns, R. (2002) Comparative physiology of salt and water stress. Plant. Cell. Environ.,25, 239-250
- Munns, R. and Tester, M. (2008) Mechanisms of salinity tolerance. Ann. Rev. Plant. Physiol., 59, 651-681
- Neill, S.J., Desikan, R. and Hancock, J. (2002) Hydrogen peroxide signaling. Curr. Opin. Plant. Biol., 5, 388-395
- Neto, A.D., Prisco, J.T., Eneas-Filho Lacerda, C.D., Silva, J.V, Costa,P.H.A. and Gomes-Filho, E. (2004) Effects of Salt Stress on Plant Growth. Stomatal Response and Solute Accumulation of Different Maize Genotypes. Braz. J. Plant. Physiol., 16(1), 31-38
- Parida, A.K. and Das, A.B. (2005) Salt tolerance and salinity effects on plants. Rev. Ecotoxicol. Environ. Saf., 60, 324-349
- Pitann, B., Schubert, S. and Mühling, K.H. (2009) Decline in leaf growth under salt stress is due to an inhibition of H+-pumping activity and increase in apoplastic pH of maize leaves. J. Plant. Nutr. Soil. Sci., 172, 535-543
- Pitzschke, A. and Hirt, H. (2006) Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant. Physiol., 141, 351-356
- Quiles, M.J. and Lopez, N.I. (2004) Photoinhibition of photosystems I and II induced by exposure to high light intensity during oat plant grown effects on the chloroplastic NADH dehydrogenase complex. Plant. Sci., 166, 815-823
- Scandalios, J.G. (1993) Oxygen stress and superoxide dismutase, Plant. Physiol., 101, 712-726
- Teakle, N. L. and Tyerman, S. D. (2010) Mechanisms of Cl-transport contributing to salt tolerance. Plant. Cell. Environ., 33, 566-589
- Tester, M. and Davenport, R. (2003) Na+ tolerance and Na+ transport in higher plants. Ann. Bot., 91, 503-527
- Turan, M.A., Katkat, A.V. and Taban, S. (2007b) Variations in proline, chlorophyll and mineral elements contents of wheat plants grown under salinity stress. J. Agron., 6, 137-141
- Turan, M.A., Türkmen, N. and Taban, S. (2007a) Effect of NaCl on stomatal resistance and proline, chlorophyll, Na, Cl and K concentrations of lentil plants. J. Agron., 6, 378-381
- Yeo, A.R. (1999) Predicting the interaction between the effects of salinity and climate change on crop plants. Int. Soc Hort. Sci., 78, 159-174
- Yeo, A.R. and Flowers, T.J. (1983) Varietal differences in the toxicity of sodium ions in rice leaves. Plant. Physiol., 59, 189-195
- Yousfi, N., Slama, I., Ghnaya, T., Savouré, A. and Abdelly, C. (2010) Effects of water deficit stress on growth, water relations and osmolyte accumulation in Medicago truncatula and Medicago laciniata populations. C. R. Biol., 333, 205-213
- Yousif, H.Y., Bingham, F.T. and Yermason, D.M. (1972) Growth, mineral composition, and seed oil of sesame (Sesamum indicum L.) as affected by NaCl. Soil. Sci. Soc. Am.J., 36, 450-453


