Physiological responses of Salvinia natans L. to aluminium stress and its interaction with putrescine
Автор: Mandal C., Ghosh N., Dey N., Adak M.K.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.9, 2013 года.
Бесплатный доступ
Salvinia natans L. a water fern is displayed with some of its physiological attributes in response to aluminium (Al) stress in aqua culture as well as its interaction with externally applied putrescine (put). At the tissue level the Al deposition is prominent and mostly distributed in the intracellular spaces as well as cellular interfaces. The accumulation of Al and its induced oxidative damages are also revealed through Evan’s blue staining. In both the cases dose dependent responses of Al induced oxidative damages and its mitigation with Put was the resultant. Under non enzymatic antioxidation pathways, anthocyanin and flavonoids were the two phenolics over expressed as a function of Al and ameliorated with Put application. The property of root membranes was changed with an up regulation of H +/ATPase activity which was moderated by Put.The peroxidase activity particularly those were restricted to the wall bound also showed variability according to Al doses as revealed through in gel staining. From these studies of Al accumulation and its concomitant changes in physiological attributes in Salvinia plants, the species could be selected as a potential hyper accumulator of Al. The role of Put in Al accumulation as well as its moderation has been discussed with reference to physiological activities.
Aluminium toxicity, antioxidative enzymes, putrescine, reactive oxygen species, salvinia sp
Короткий адрес: https://sciup.org/14323798
IDR: 14323798
Текст научной статьи Physiological responses of Salvinia natans L. to aluminium stress and its interaction with putrescine
Abbreviations: Put: Putrescine; ROS: Reactive Oxygen Species; EDTA: Ethylene diamine tetra acetic acid; DTT: Dithiotreitol; HEPES: 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; MES: 2- N- morpholino)ethanesulfonic acid; NBT: Nitroblue tetrazolium; SEM: Scanning Electron Microscope
Most of the cases plant suffers from metal toxicity out of heavy metals. Still some metal lower in molecular weight are also equally harmful for plant in detoriation of growth and development when absorb in excess by the plants aluminium (Al) is one of those and being the abundant metallic elements cover around 8 % of total earth’s crust (Achary et al., 2007). Of the abundant of Al salt like aluminium silicate, the metal becomes more toxic in soil with acidic condition because those are readily transformed into soluble and absorbable forms (e.g. aluminium hydroxide). On the other hand, chemically Al is though non-transitional metal still can induce in a number of ways for redox reaction, that’s why Al also behaves as pro-oxidant (Exley, 2004). The toxicity of Al is more pronounced in some specific compounds or moieties (e.g. Al-superoxides, semi-reduced radicals) by producing a number of reactive oxygen species (ROS) in plants (Roychoudhury and Pradhan, 2011). The most common effects are Al induced cell death in plants which is reported as ROS/ROI activated programmed cell death. In general the phytotoxic symptoms are displayed in a number of forms, like anamols in root structure, excessive proliference of lateral branches, thickening of cortical tissues and physiological wilting of the whole plants. This is more found with the sensitivity of Al toxicity which is markedly position at meristematic and apical zone of the roots. Injuries out of Al accumulation are more prone at the cellular level on cell wall apoplastic place, interior site of plasma membrane, cellular organelle and nucleus (Achary et al., 2007) At the biochemical level a number of biomolecules are reported to bind by nucleophilic interactions mostly by oxygen or/and nitrogen ligands like carboxylic acid, proteins, phospholipids and nucleic acid etc. Like other redox metal, Al is potential for cellular disintegration through a number of ways: lipid peroxidation, protein carbamylation, DNA damage etc. Moreover, Al is well marked in respect to redox reaction, particularly for dismutation super oxide in hydrogen peroxide at the acidic pH in the cellular environments. This metal is also sufficient to carryout phenton type of reaction for generation of peroxide (H2O2) and other free radicals, however, unlike as those of transition metals (Fe, Cu etc.) (Damanik et al., 2010). Therefore, the oxidative stress as induced by Al and its adjoining effects is undoubtedly established at toxic metal. Plant can since signalling to abiotic stress and its evocation and modulation by various elicitors like compound.
Polyamine (PAs) though not regarded as typical growth substances, still has been effective as an biomolecule to interact growth and physiological processes under normal and abiotic stresses as well (Tang and Newton, 2005). PAs are some special kind of amine containing aliphatic straight chain moieties, which is protonated at the physiological pH. With this property PAs are more offered to interact with negatively charged backbones of biomolecules with its cationic residues (i.e. R-NH4+) This offers a protective barrier on the cellular membrane to counter the invading ROS (Liu et al., 2008). In reference to the frequent PAs to interact stress response tri-amine spermidine (Spd) and tetra-amine spermine (Spm) are more documented. Moreover, putrescine (Put) a characteristically diamine are less explored in plant system under oxidative stress. However, efficacy of polyamines in crops, encountering or moderating physiological responses are not constant in result (Liu et al., 2008). In an instance a significant over accumulation of putrescine was surprisingly accompanied by no changes in spermidine and spermine in rice genotypes treated with excess salinity (Ghosh et al., 2011). Interestingly, this situation was accompanied with an opposite trend in susceptible rice varieties. On the contrary, wheat genotype behaves differently and irrespective of condition inductive to oxidative stress spermidine and spermine was evident as reliever (Annalisa et al., 2002). However, most of the cases, the reversal of oxidative damages like electrolyte leakages by membrane full in osmotic turgidity, protein carbamylation etc are predominantly done by spermidine like other higher polyamines. This had been documented in plants response to a number of stresses like chilling, salinity, water deficit etc (Roy et al., 2005; Mariale et al., 2004). On the protectively role of free radical scavenger spermine have been essential for different crop species and its cellular interaction is more deciphered in Arabidopsis mutants. On the other hand, Put may easily be catabolized yielding H2O2 as a byproduct (Farooq et al., 2009). In signalling to abiotic stress the later is granted for an effective elicitor to evoke tolerance in many stresses. Therefore, Put would be primarily choice for studying interact of metal induced oxidative stress in plants, contextually the present experiment Al is chosen to bind it out its interaction with Put.
Now, plants species are highly variable to display their activity withstanding or tolerating against toxic metals. This is facilitated by their super accumulation ability of metals by defolding of genetic plasticity of antioxidation pathways However, Al also is included to be targeted by some specific plants, particularly terrestrial species under acidic soil (Skinner et al., 2007). In most of the cases, angiosperm species are showed with wider variability and sustenance to Al contaminated zones and categorized as Al hyper accumulator (Boscolo et al., 2003). In contrast to angiospermic species, some non angiosperm though few in number have scored their ability to to hyper accumulates toxic metals. This is readily referred by illustration of Chinese brake fern Pteris vitatta Linn.) when thrives well under Arsenic contaminated soil (Kramer, 2010). Moreso, other pteridophytic fern species like Salvinia, Azolla, Marsillea are proven to be good quencher of heavy or toxic metals by their improved anti oxidation pathways (Xu et al., 2009) Salvinia natans (L.) could also be cited as aquatic fern species, free floating with wider adaptability in water bodies contaminated with industrial effluents. In addition, an appreciable over growth is also featured under such metal contaminated industrial effluents.
So, Salvinia could be thought as a plant material due to ability the absorb metal in excess and thrive well under such condition. With the inbuilt potential for metal tolerance, the cellular adjustment in the path ways of antioxidation is ought to be analyzed. In addition how exogenously applied elicitors like polyamine can modulate the antioxidation path way would also be justified specially in a non angiospermic plant like Salvinia Few reports have been contextually published with this aspect of metal tolerance in Salvinia , particularly for those of heavier in molecular weights (Dhir et al ., 2009). Still, a metal like Al which is also toxic to its maximum extends is yet to be understood in such plant species like Salvinia . In the present experiment, we described the Al absorption by the tissues and its induced ROS generation (O 2 - and H 2 O 2 ), histochemical detection of ROS by in vivo staining, changes in activities of membrane permeability protein (H+/ATPase), variation in phenolics (anthocyanin and flavonoids), activities of phenolic induced wall bound peroxidase were studied. The exhibited variation of those cellular responses was also discussed in relation to polyamine interaction under Al salt in Salvinia natans Linn.
MATERIALS AND METHODS
Plant material and Treatments:
Salvinia natans (L.), an aquatic fern was selected as the material for the present experiment.
Although Salvinia plants don’t have true roots but rhizoids like structure developed from the third leaf of each whorl and function like roots (Gifford and Foster, 1989). Plants were grown in unpolluted pond, then collected and washed with de-ionized water and used for experimental purposes. Plant sample was transferred into ¼ strength of Murashige and Skoog media (Murashige and Skoog, 1962) for seven days for acclimatization, followed by plants were treated with varying concentrations (0, 240, 360, 480 µM) of aluminium salt as potassium aluminium sulphate [KAl(SO 4 ) 2 .12 H 2 O] and 480 µM of Al-salt supplemented with 1 mM Put and maintained the acidic condition so that Al could be more soluble in Al3+ form and readily absorbed by plant (Giannakoula and Ries, 2010). All the sets were kept in a greenhouse under the condition: 37±1ºC of temperature, 75-85 % of relative humidity (RH) and photoperiod of 13 -11 hr light and dark for 7 days. There after, plants were harvested and stored in -70ºC for further use. Five number of replication (n=5) was done for each concentration of Al treatment.
Histochemical detection of tissue lyses by Al:
Root tips (10 mm) were excised, washed thoroughly with de ionized water and incubated in 0.25 % (w/v) solution of Evan’s blue for 25 min. On completion, the root tips were washed again and again to remove excess adhered stain on it and observed under microscope (Olympus, CKX41, Tokyo, Japan) to detect the damages of tissue by Al in the root (Baker and Mock, 1994). Similarly, roots of control and treated plants were excised and incubated in a saturated solution of N, N -dimethylformamide for ½ hr at room temperature followed by measured the tissue damage by taking the absorbance at 600nm using a UV-Vis spectrophotometer (CECIL, CE7200). On the other hand, for detection of the Al deposition in the tissue, the samples were preserved in fixative solution for 4 hr and then microtome sections were done and coated with carbon particles. SEM photographs were taken using SEM model Philips XL 30 attached with energy dispersed X-ray (EDX) unit.
Assay of wall bound peroxidase activity:
For the activity of wall bound peroxidase (EC 1.11.1.7), cell wall was isolated according to Asthir et al . (2009). 10 g of fresh sample was thoroughly washed under double distilled deionized water to remove all adhering debris. Then the samples were homogenized in 20 mM sodium phosphate buffer (pH 6.4) under cold condition. The homogenate was filtered through 4-5 layers of muslin cloth followed by centrifuged at 15,000 x g for 20 min at 4ºC. The supernatant was collected and mixed with acetone and kept at 4ºC to precipitate soluble peroxidase. An aliquot of acetone precipitate protein was re-dissolved in 20 mM phosphate buffer (pH 6.4) for soluble peroxidase activity. Concomitantly, the pellet was saved and suspended in 50 mM sodium phosphate buffer (pH 6.5) containing 1 M sodium chloride to release the ionically bound enzyme during an hr of incubation at 4ºC. The released enzyme was centrifuged at 10,000 x g for 15 min. Finally, the supernatant containing the ionically bound enzyme was used for spectrophotometric assay as suggested by Mika and Luthje (2003). The assay mixture for peroxidase activity was made with 50 mM sodium phosphate buffer (pH 6.5), 10 mM DTT, 15 mM H 2 O 2 and 4 mM ferulic acid. Each sample was incubated in 3.5 ml of reaction mixture. The changes in absorbance on every equal interval for a total of 3 min were recorded using the molar extinction coefficient of ferulic acid (Saroop et al .,
2002; Hu et al ., 2009).
For the isozymic expression of wall bound peroxidase, the protein sample was desalted by 0.05 mM CaCl 2 and separated through dialysis bag against a buffer of 50 mM Tris-HCl (pH 7.5), 0.1 mM EDTA, 1 mM PMSF, 1 mM β-ME, 20 % glycerol. The recovered supernatant was concentrated in vacuum lyophilizer at -40ºC. About 50 µg of protein was loaded in a native 12 % (w/v) PAGE against a buffer of 375 mM Tris-HCl (pH 8.8). The gel was stained for peroxidase activity in an assay mixture containing 100 mM sodium acetate (pH 6.0), 0.06 % H 2 O 2 and 6 mg ml-1 of chloronapthol (dissolved in methanol). The bands were made more resolved by washing with 0.01 % of glacial acetic acid.
Determination of anthocyanin and flavonoids:
Two major antioxidants, anthocyanin and flavonoids were assayed according to Eryilmaz (2006). For anthocyanin, 1 g of fresh sample was thoroughly homogenized in 80 % methanol–HCl (v/v) and incubated for 48 hr at 4ºC. The filtrate was centrifuged at 1,000 x g for 15 min at 4ºC. The absorbance was recorded at two different wavelengths. The subtracted values of absorption (i.e. A 530 - A 600 nm) were taken for quantification of anthocyanin. For flavonoid determination, the fresh sample was extracted in 80 % aqueous ethanol following centrifugation at 10,000 x g at room temperature for 20 min. The supernatant was added with a reaction mixture containing 5 % sodium nitrite (NaNO 2 ) and 10 % aluminium chloride (AlCl 3 ). The reaction mixture was diluted with 1 M NaOH and absorption was measured at 510 nm. The total flavonoid was determined with a standard of quercetein (Sigma) and expressed as μmol g-1 (Mohamed and Aly, 2008).
Isolation and assay of plasma membrane bound H+/ATPase activity:
5 g of the fresh root sample was excised and freezed in liquid nitrogen to isolate the plasma membrane. Extraction buffer contained 250 mM sucrose, 125 mM KCl, 5 mM EDTA, 2 mM PMSF, 10 μg ml-1 leupeptine, 20 μg ml-1 pepstatin, 1.5 % PVP, 0.1 % BSA, 1 mM DTT and 25 mM HEPES-KOH (pH 7.8). The roots were thoroughly homogenized and the supernatant was collected after centrifugation at 13,000 x g for 15 min at 4 ºC. The supernatant was ultra centrifuged at 80,000 x g for 60 min. After recovering the pellet, it was resuspended in a minimum volume of buffer containing 300 mM sucrose, 1 mM KCl, 1 mM EDTA, 2 mM DTT, 50 mM HEPES-KOH (pH 7.8). The microsomal pellet so recovered was used for assay of H+/ATPase EC 3.6.3.6) activity according to Janicka-Russak et al . (2008). The assay mixture was prepared with 25 mM Tris-MES (pH 6.5), 50 mM KCl, 3 mM ATP, 1 mM (NH 4 ) 2 MO 4 and 3 mM MgSO 4 . This assay mixture was replicated twice to titer the effect of promoter (1 mM KCl) and inhibitor (1 mM Na 3 VO 4 ) respectively. The reaction was initiated with 25 μg equivalent of microsomal pellet and incubated at 30 ºC for 30 min. Finally, a solution containing concentrated 2 % H 2 SO 4 , 5 % SDS, 0.7 % NaMO 4 and 10 % ascorbic acid was added to stop the reaction. The activity of H+/ATPase was determined by reading the absorbance at 820 nm as suggested by Roy et al . (2005). The protein was estimated with Bradford reagent (Bradford et al ., 1976).
The detection of the membrane bound proteins in the microsomal pellet was purified preliminarily by 80 % (NH4)2SO4 cut and then subjected to DEAE-Sephadex G100 column (Sigma), eluted with Tris-HCl buffer (pH 7.5). The eluted protein was measured with Bradford reagent. Eluted membrane protein was run in a 10 % native PAGE then stained with Coomasic Brilliant Blue (R-250, BIO RAD) to resolve the membrane bound proteins.
Statistical analysis:
All the observations were recorded with five replications (n=5) and data were expressed as mean ± SE. The statistical analysis was performed by oneway ANOVA analysis, taking P≤0.05 or 0.01 levels of significance.
RESULTS
Detection of metal in the tissues:
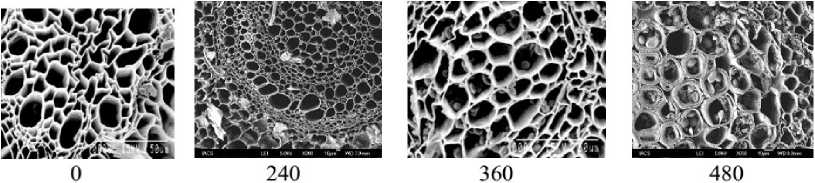
The accumulation of Al as a function of increasing concentration of KAl(SO 4 ) 2 12 H 2 O was detected through SEM study. The metal was identified (data not shown) with EDX spectra for its confirmation. The deposition of Al through the tissues was discriminately abundant through the sections of modified root. The metal was more profused on the epidermal cell and its inner wall (Fig. 1). This is followed by some diffuse metal in the cellular lumens of cortical tissue. However, the endodermis reaching the vascular bundle was least found with metal deposition. It is also noted that the distribution of Al was mostly profuse on the inner wall of the epidermal cells and that became significantly lesser when the plants were treated with Put. However, the cortical tissues recorded some scanty deposition of Al. The deposition of Al in the root tissue had significantly induced the oxidative damages as monitored through the Evan’s blue staining in-vivo. On critical observation, the sub-epical regions of the roots supposed to be more damage extending peripheries as it took dense staining.
A significant variation in staining intensities as a function of Al concentration as well as supplementation with Put was observed (Fig. 2A)
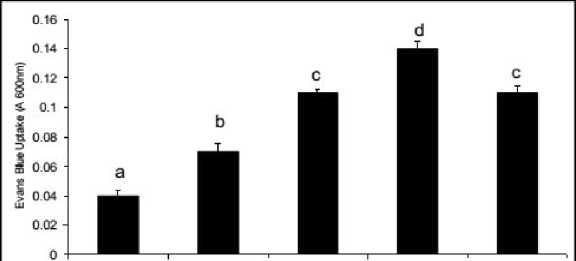
Form the staining with Evan’s blue the tissue lysis out of oxidative exposure was more clear when spectrophotometric assays was done at 600nm. The increase in intensities of the staining was significant (P≤0.05 or 0.01) at all doses of Al compare to control. The maximum tissue lysis was recorded at 480 μM of Al and it was 3.5 fold over the control Interestingly with the Put application the plants moderated tissue disintegration and it was recovered by 21.42 % (Fig. 2B).
Variation in anthocyanin and flavonoid content under Al toxicity:
Al, though not a heavy metals, however, can induced a systematic resistant’s against oxidative exposure so established. Out of antioxidation strategies anthocyanin and flavonoids are the two predominant moieties and were showed a significant variation in the plants of the present experiment also. For both anthocyanin and flavonoids a dose dependent responses were observed as function of Al being up regulated and down regulated respectively. For anthocyanin the effect was diminishing and it fall by 63.63 % at 480 µM of Al compared to control. On the contrary, the flavonoid content, maximum accumulation was recorded with 1.49 fold at highest concentration of Al (i.e.480 µM) over control though significant at all the concentration (P≤0.05 or 0.01). Interestingly Put was active in modulation of both the phenolics significantly. For anthocyanin it was retrieved by 1.62 fold. Whereas, for flavonoid the activity reduced by 19.79 %. (Fig. 3A and Fig. 3B).
H+/ATPase activity was significantly varied under Al stress:
In the present study the membrane functioning under condition of Al variation was monitored with invitro assay of vanadate insensitive H+/ATPase
Initially, the plasma membrane was isolated and purified for its bound protein with ammonium sulphate cut. The partial purified protein of plasma membrane is allowed to denature and separated on SDS PAGE. The resolve bands of the membrane bound protein were significantly variable according to Al doses, both in number and intensities (Fig 4A). In addition, it clearly showed the retrieval of bands with lower molecular weights when protein was extracted from Put supplemented plants. Thus it showed that the damages of membrane could be recovered by application of Put. On in-vitro assay of purified protein taking substrate as ATP the activity was determined by release of inorganic phosphate (pi) and it measured by spectrophotometrically. The activity of H+/ATPase showed a consistant increase along with Al concentrations and thereafter minimized with Put application. Thus it recorded a maximum of 2.81 fold increases in activity at highest concentration of Al over control, whereas Put reduced the activity by 15.1 % as compared to highest Al concentration (480 µM) (Fig. 4B). As usual the sensitivity of H+/ATPase was tested with both promoter (KCl) and inhibitor (vanadate) and showed a proportionate functioning of the activity according to the concentration of those. Interesting to note that the inhibition of activity by vanadate was significant at each concentration but Put can moderate the inhibition maximally. Again, the promotive effect of KCL for activity was similarly sustained when assayed with Put supplementation This showed that Put could be equally effective for both minimization and retrieval of enzyme activation regardless the case of inhibition and induction respectively (Table 1).
Variation in activities of wall bound peroxidase:
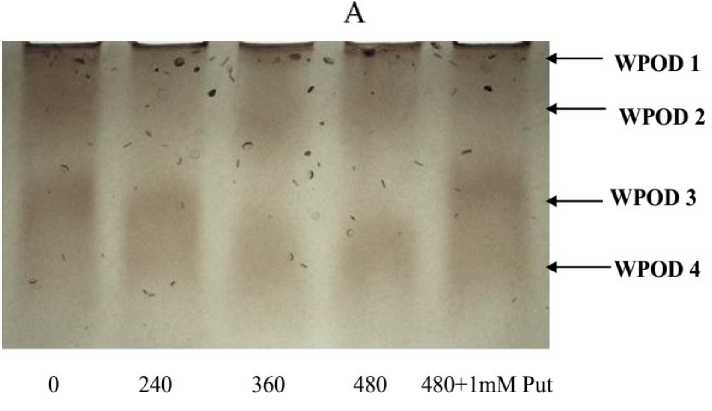
Ionic bound peroxidase on the cell wall and apoplastic spaces were isolated from the tissue with 1M NaCl. This enzyme is typified by its sensitivity with ferulic acid as electron donor. The variation in activity clearly suggests a stepper up regulation as a function of concentration of enzyme though a linear increase of activity was the feature with Al treatment but it peat 480 µM with 2.18 fold over control. As already stated the down regulation of activity by Put was 26.83 % as compared to highest concentration of Al (Fig. 5A). A separation followed by purification of isozymic forms of the wall bound peroxidase on native PAGE recorded clear variations among the treatments (Fig. 5B). Though the variation was not found in number of bands but the intensities of those were in increasing order so revealed. However the treatments of polyamine had also minimized band intensities as compared to Al doses.
Table 1. The kinetics of H+/ATPase activity (μM Pi mg-1 protein h-1) for treatment of induction and inhibition with promoter (KCl) and inhibitor (vanadate) respectively under various concentrations of Al salt (0, 240, 360, 480 µM) and 480 µM of Al salt supplemented with 1 mM Putrescine (480 µM + 1 mM Put)
|
Treatments |
0 µM |
240 µM |
360 µM |
480 µM |
480µM+1mM Put |
|
KCl (1.0 mM) |
11.4 |
15.8 |
21.6 |
28.3 |
23.5 |
|
Normal (0 mM) |
8.7 |
12.3 |
19.2 |
24.5 |
20.8 |
|
Vanadate (1.0 mM) |
3.2 |
4.1 |
4.9 |
5.2 |
4.7 |

480+ ImM Put
Figure 1. SEM study in Salvinia grown under varying concentration (0, 240, 360, 480 µM) and 480 µM of Al salt supplemented with 1 mM Putrescine (480 µM + 1 mM Put).
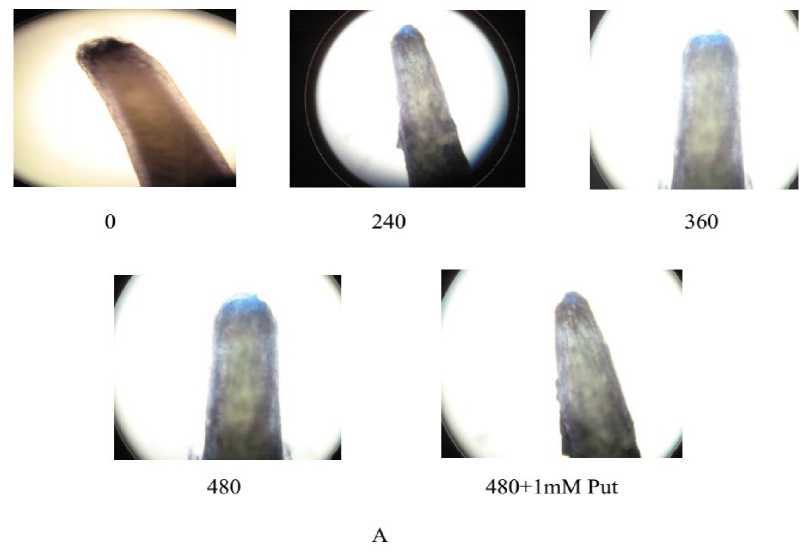
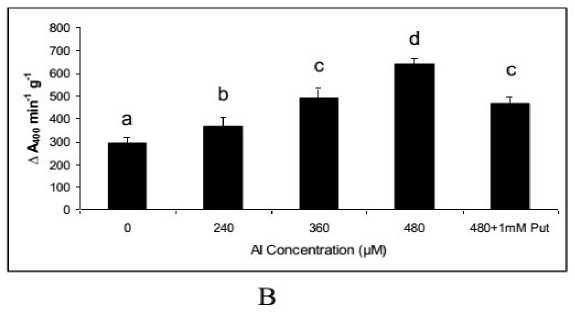
Figure 2. Evan’s blue absorbance (A), tissue disintegration detected by Evan’s blue staining (B) in the roots of Salvinia plants under varying concentrations of Al (0, 240, 360, 480 µM) and 480 µM of Al salt supplemented with 1 mM Putrescine (480 µM + 1 mM Put).
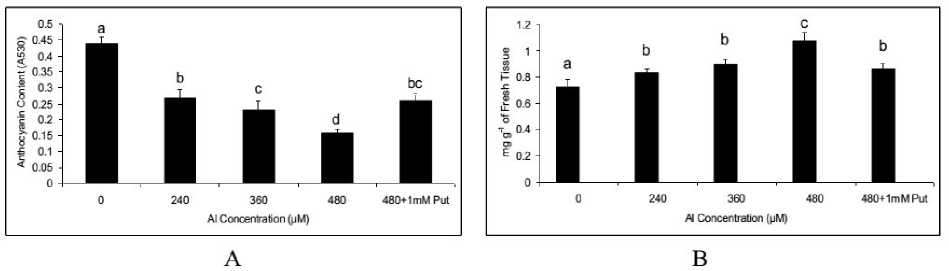
Figure 3. Anthocyanin (A) and Flavanoid (B) in Salvinia plants grown under varying concentration (0, 240, 360, 480 µM) and 480 µM of Al salt supplemented with 1 mM Putrescine (480 µM + 1 mM Put). The values are plotted with different letters above bars were significantly different from means (±SE ;) of replication (n=5), (P≤ 0.05) of each treatment.
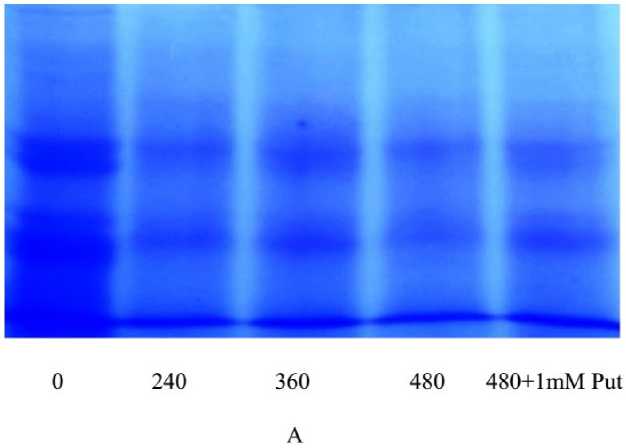
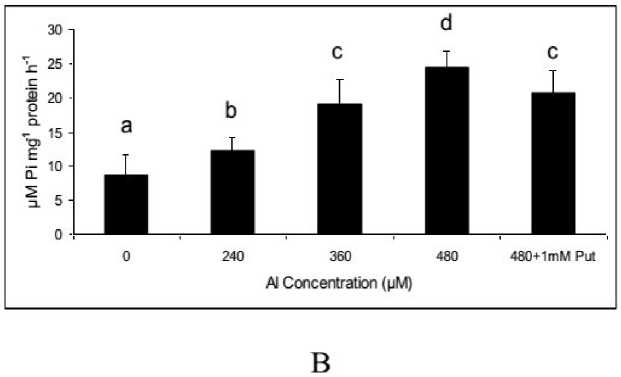
Figure 4. Separation of membrane bound protein of H+/ATPase on 10% polyacrylamide native gel (A) and H+/ATPase activity (B) in Salvinia plants grown under varying concentration (0, 240, 360, 480 µM) and 480 µM of Al salt supplemented with 1 mM Putrescine (480 µM + 1 mM Put). The values are plotted with different letters above bars were significantly different from means (±SE ;) of replication (n=5), (P≤ 0.05) of each treatment.
Ai Concentration (|M)
В
Figure 5. Wall bound peroxidase activity (A) and different isozymes: wall bound peroxidase (B) on 10% polyacrylamide native gel in Salvinia plants grown under varying concentration (0, 240, 360, 480 µM) and 480 µM of Al salt supplemented with 1 mM Putrescine (480 µM + 1 mM Put). The values are plotted with different letters above bars were significantly different from means (±SE ;) of replication (n=5), (P≤ 0.05) of each treatment.
DISCUSSION
In a number of communications the oxidative damages have been described in its various facts and mostly in crop plants. It remains prudent for plant as irreversible changes of cellular redox preliminary. This is followed by initially loss of membrane lipid protein carbamylation etc and reaches to the level of DNA formation. Salvinia natans (Linn) as our plant material for the experiment in a fern species were floating in nature and basically devoid of true roots. Under submerged condition, the plant develops rhizoidal structures by modifying one of the leaves at each whorl which function as roots (Gifford and Foster, 1989). It is quite in agreement that Al is most effective in the root of plants particularly at the meristematic zone. More so distal transition and apical elongation zone are also characterized with higher sensitivity of Al toxicity (Sonia, 2012) Irrespective of the vulnerable sites of Al toxicity, at the cellular level it effects on primary cell wall cellular membrane, cytosolic protein moieties and ultimately the nucleus. The electrostatic attraction of Al with its position change to the negative backbones of nucleic acid and some specific domens of proteins leads to damages of those macromolecules. Therefore, identification of Al as a metal to specific sites of tissues are most important to assays the potential of the plants to sequester the metal. In the present experiment as a SEM studies couple with has revealed the major deposition of Al on the inner faces of cell membrane. It is interesting to know that the Al was most readily deposited on those sites, where chances of metal induced damages are less in comparison to be cytosolic in occurrence. This holds true with the other heavy metals where sequestering of ions in apolplastic spaces of tissues. Even, the scanty nature of Al distribution in the vascular lumens but profuse in cortical tissues also tends for less transportation towards leafy areal part. Undoubtedly this feature could support Salvinia as an effective hyper accumulator of Al through phyto sequestration. Thus, with the absorption of Al admitted well to disintegrate the tissue that had been recorded with Evan’s blue uptake. In result it is depicted a significant increase of the stain concentration as monitored with optical density according to Al doses. Moreover, this value had significantly full with application of putrescine Al absorption in excess followed by generation of ROS/ROI that induces the tissue lysis in predominantly attributed by lipid and protein oxidation. Therefore, the semialdehyde and carbamylated derivatives of protein and its formation typical coloration with Evan’s blue indexed the tissue damages by oxidative stress Thus metal induced ROS (eg. Al in the present case) initially turns over membrane lipid, loss of chlorophyll/ chlorophyll binding proteins, membrane spanning channel proteins and finally solubilization of enzyme protein also (Korenkov et al., 2007). Polyamine has established a dual role preventing the oxidative damages by down regulation of ROS generation and making a protective shield of those biomolecules from oxidative exposure with its polycataionic backbones. What ever the cases, Evan’s blue staining otherwise also indicates that protein carbamylation happens to be a sensitive trait for oxidative damages and even than lipid peroxidation. Evan’s blue thus evident as a reliable technique to monitor the in-vivo peroxidation and carbamylation even out of oxidative stress. So, tissue disintegration is otherwise detected according to degree of staining intensities under toxic metals like Al and others (Baker and Mock, 1994). Regardless the cases all these might cooperatively have reduced the overall growth Salvinia plants as an initial symptom of Al toxicity. However, the growth data as we have reported in earlier communication (Mandal et al., 2013), and henceforth not presenting herein.
As already reported that metal stress could render its effect by purterbance of osmotic balances in the tissues along specific ion effects. Al would be of no exception and its abundants in tissues can alter the water relation by modifying cellular membrane properties (Janicka-Russak et al., 2008) The membrane bound H+/ATPase activity happens to be one of the most important path ways for maintaing the cellular ion status, particularly for potassium. Thus a significant variation under Al doses as well as Put supplementation has modulated significantly in the Salvinia plant of the present experiment. Al being a non redox metal also can induce the potential of the root membrane and thus a full in ATP might pose a serious bottleneck for the cellular functions. Of those H+/ATPase might be the most predominant where availability of ATP is the most crucial as substrate requirement Kabała and Janicka-Russak, 2011). At cellular level, regardless of toxic metals, the H+/ATPase is evident to bind at its auto phosphorilating domain by the metal and is under the regulation of the enzyme. Put as found in the present experiment had over come the suppression of the enzyme activity under Al. It could be logistic that the affinity of metal to the binding domain of the H+/ATPase is reduced and thereby the activity is restored from the metal toxicity (Liu et al., 2006) The act of Put as a reliever of metal toxicity may also hypothesized to sustain the native structure of the membrane for proper location of the proteins (like H+/ATPase) and thus sustains the functioning in proper way (Koizumi et al., 2011).
In response to oxidative damages as already found in many studies out of Al contamination. Plants are also equipt with some special biochemical reaction to encounter those. Like other heavy metals the toxicity of Al is also moderated by plants through antioxidative pathway by adopting non enzymatic and enzymatic cascade (Achary et al., 2007). The integrate interaction of plants function with elevated oxidative status. Special classes of enzymes are induced to be over expressed and hyper activity under the condition. Depending upon their tissues and intensities of the oxidative exposure out of ROS enzymatic function are variable in different tissues spaces (Nakano and Asada, 1981). More commonly, peroxidase a special kind of oxidoreductase is required to lysis H2O2 under a diverse abiotic stresses, Al toxicity being one of them (Boscolo et al., 2003). Besides the lysis of H2O2 and allied pathways for ROS detoxification. Peroxidases are also offered for normal growth and development of tissue through cell wall formation. This is more exemplified in the cross linking reaction of phenolic residues for their polymerization and to be part of lignifications (Larue et al., 2010). This process is more effective in case of stiffening of cell wall for making less prone off oxidative damages and in fact helps a significant trait to metal induced damages in the plant (Kramer, 2010). A special class of membrane bound peroxidase that had been isolated from the Salvinia plant under present experiment is required to act with H2O2 to generate some anionic phenolic residues. The extraction of partial purification and its polypeptidic separation through native PAGE clearly reveals the expression potential of the plants under Al toxicity. As reveal in the present case the variation of isoenzymic patterns of those wall bound peroxidases more with their intesities advocates the physibility of tolerents to Al toxicity (Almagro et al., 2009). Moreover, the resumption of enzyme activity with Put supplementation, other wise mightbe indicative of the facts that the plants are relived from oxidative damages. In other communication polyamines have also down regulated the oxidative exposure in integrated ways so that plants remains less demand in over express the wall bound peroxidase activity as reported (Asthir et al., 2009). Thereby normal activity of peroxidase is sustained as usual in a non stresses condition.
In relation to some existing non enzymatic antioxidant compounds phenolic plays a predominant role in many plant systems, when exposed to oxidative stress. Cells function intrinsically under elevated oxidative states is the related to either down regulation of ROS/ROI generation and / or diminish the energy to regulate the oxidative damages. For both the cases, flavonoid and anthocyanin plays a vital role for interaction of metal induced oxidative exposure. In fact Al being a lighter metal could proportionately generate ROS, though not in similar intensities with other redox metal and known to enhance oxidation of phospholipids and proteins (Achary et al., 2007) In our earlier communication this had been confined for Salvinia with an elevated level of protein oxidation as an adhered trait for Al toxicity (Mandal et al., 2013). Flavanoids owing to their molecular configuration has an intrinsic property to chelate the metal along with its radical scavenging activity (Mohamed and Aly, 2008). The binding ability of Al to the flavonoids as furnished by electrostatic interaction is more circumvented by maintenance of acidic pH in the cells. The secretion of organic acid and its pre formation to flavonoid residues shares the charge imbalance and facilitate the legand formation with Al+3 (Peter, 2001). The over expression of flavonoid under Al stress as recorded in the present investigation could therefore be thought as a reliable trait to accommodate the oxidative stress in Salvinia.
Out of number of antioxidative enzymes, peroxidase constitutes those which required phenolics residues as electron donor for lysis of H2O2 under sorts of abiotic stress. In addition, peroxidases are integral parts of plant growth and development even under normal conditions. As for example, cell wall lignification is accomplished by catalizing the oxidative polymerization of phenolic residues by special peroxiadase (Larue et al., 2010) The later are mostly located on cell wall and apoplastic places and is identified and characterized as a reliable stress for cell wall rigidity, particularly, under metal stress (Asthir et al., 2009). The wall bound peroxidase are required NAD(P)H oxidase mediated generation of H2O2 on the cell wall. H2O2 might be used as a substrate to be acted by wall bound peroxidases to generate free radicals. The free radicals are required for cross linking or polymerization of phenolics on the cell wall (Asthir et al., 2009). Salvinia as also found with distinct variation in wall bound peroxidases in the present experiment also that could be a trait for Al induced resistance. Moreover, when the purified protein was done to separate different expressed polypeptide the isozymic bands varying in molecular sizes and intensities were resolved. In another study, the over expressed bands for different isozymes of such peroxidases were characterized in plants under Al contamination (Almagro et al., 2009). The activity and expression potential of such enzymes are thought to be involved for the lignifications of cell wall with phenolic residues. In the present study the up regulation of flavonoids in the Salvinia plants has rendered it another protection for Al induced oxidative exposure Where as anthocyanin, another phenolics recorded to be down regulated under the same condition Flavonoids are a special class of poly phenolics where free hydroxyl group projected from the molecular configuration are actively participated to quench the excess energy of ROS (Mohamed and Aly, 2008). On vthe other hand anthocyanin are derivative of such phenolics where hydroxylation is replace by addition of sugar moieties in the molecule. Thus a characteristic features for its solubility in the cell sap is attended. The up regulation of flavonids with its diver’s configuration sets the most established non-enzymatic antioxidant path ways in plants. Flavonoids and anthocyanin are also offered as efficient metal chelators with its hydroxylation relation of free carbonyl groups. The binding ability of flavonoids with Al has also been established to be increased under some organic acids like citrate and malate (Mohamed and Aly, 2008). Therefore, Al tolerant genotype is well known to secret more organic acids and thus circumvents the chelation of Al with the plant exudates. On the other hand along with some other polyphenolics are also required to quench the H2O2 by glutathione peroxidase, particularly in the apoplast. In fact glutathione peroxidaes can favour the flavonoids as alternative electron donors instead of ascorbates. However, the later is granted the most available electron donor peroxidase reaction (Sakihama et al., 2007). With this understanding the up regulation of phenolics with reference to flavonoids could be taken as a reliable traits for tolerants against Al induced oxidative damages.
In conclusion, it seems sensible that for the resistance of Al toxicity, plants besides the exclusion of metal could also operate another mechanism through antioxidation system. Salvinia plants, in present case, seemed to evoke that mechanism under Al stress as well as modulated by application of Put. This improved antioxidation system required to minimize the oxidative damages of the plants as evident from the experiments. The induction of secondary metabolites that might act as non-enzymatic antioxidants like flavonoids, anthocyanin, etc. has been evident for Al tolerance and more sensitized with Put. In addition, Salvinia plants have also marked its efficiency for sustaining the activity of H+/ATPase and wall bound peroxidase under Al contamination. Wall bound peroxidase attained its importance in lignifications process of cell wall and thus may favor the stiffening of tissues against mechanical injury, if any. With all these features, conclusively, it is likely to justify that the Salvinia plants and its tolerance to Al is due to intrinsic detoxification capacity by improved antioxidation system. This had more been accomplished with Put application. Therefore, this work is validated with its relevance for expression and functioning of polyamine through the modulation of antioxidation pathways. In addition, Salvinia could be realized as a potential hyper accumulating fern species and could be useful in managing of Al contaminated fallows. Therefore, more expectedly it could be potential in phyto-remediation process.
ACKNOWLEDGEMENT
I would like to heartily acknowledge DST-PURSE programme for financial assistance. I would also like to acknowledge UGC and Department of Botany, University of Kalyani.
Список литературы Physiological responses of Salvinia natans L. to aluminium stress and its interaction with putrescine
- Achary, V.M.M., Jena, S., Panda, K. and Panda, B. (2007) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicolo. Environment. Safety., 70, 300-310
- Almagro, L., Ros, L.V.G., Belchi-Navarro, S., Bru, R., Barcelo, A.R. and Pedreno, M.A. (2009) Class III peroxidases in plant defence reactions. J. Experiment. Bot., 60, 377-390
- Annalisa, T., Richard, M., Napier, M., Franceschetti, M.A.V. and Nello, B. (2002) Spermidine-binding proteins. Purification and expression analysis in maize. Plant. Physiol., 128, 1303-1312
- Asthir, B., Koundal, A. and Bains N.S. (2009) Kinetic and thermodynamic behaviour of wall-bound peroxidase from wheat leaves infected with stripe rust. Plant. Growth. Regul., 59, 117-124
- Baker, C.J. and Mock, N.M. (1994) An improved method for monitoring cell deathin cell suspension and leaf disc assays using evan’s blue. Plant. Cell. Tissue. Organ. Cult., 39, 7-12
- Boscolo, P.R., Menossi, M. and Jorge, R.A. (2003) Aluminum-induced oxidative stress in maize. Phytochemistry., 62, 181-189
- Bradford, M.M. (1976) Rapid and sensitive method for quantitation of micro gram quantities of protein utilizing the principle of protein-binding dye. Ann. Biochem., 72, 248-254
- Damanik, R.I., Mahmood, M., Ismail, M.R., Ahmad, S. and Zain, A.M. (2010) Responses of antioxidative enzymes in Malaysian rice (Oryza sativa L.) cultivars under submergence condition. Acta. Physiol. Plant., 32, 739-747
- Dhir, B., Sharmila, P., PardhaSaradhi, P. and Nasim, S.A. (2009) Physiological and antioxidant responses of Salvinia natans exposed to chromium-rich waste water. Ecotoxicol. Environment. Safety., 72, 1790-1797
- Eryılmaz, F. (2006) The relationships between salt stress and anthocyanin content in higher plants. Biotechnol. Equip., 20, 47-52
- Exley, C. (2004) The pro-oxidant activity of aluminum. Free. Radic. Biol. Med., 36, 380-387
- Farooq, M., Wahid, A., Kobayashi, N., Fujita, D. and Basra, S.M.A. (2009) Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev., 29, 185-212
- Ghosh, N., Adak, M.K., Ghosh, P.D., Gupta, S., SenGupta, D.N. and Mandal, C. (2011) Differential responses of two rice varieties to salt stress. Plant. Biotechnol. Rep., 5, 89-103
- Giannakoula, C.N. and Ries, S.K. (2010) Superoxide dismutase. Plant. Physiol., 59, 309-314
- Gifford, E.M. and Foster, AS (1989) Morphology and evolution of vascular plants. W.H., Freeman and Company, New York
- Hu, Y., Ge, Y., Zang, C., Zu, T. and Cheng, W. (2009) Cd toxicity and translocation in rice seedlings are reduced by hydrogen peroxide treatments. Plant. Growth. Regul., 5, 51-61
- Janicka-Russak, M., Kaba1a, K., Burzynski, M. and K1obus, G. (2008) Response of plasma membrane H+/ATPase to heavy metal stress in Cucumis sativus roots. J. Experiment. Bot., 59, 3721-3728
- Kabała, K. and Janicka-Russak, M. (2011) Differential regulation of vacuolar H+/ATPase and H+/PPase in Cucumis sativus roots by zinc and nickel. Plant. Sci., 180, 531-9
- Koizumi, Y., Hara, Y., Yazaki, Y., Sakano, K. and Ishizawa, K. (2011) Involvement of plasma membrane H+-ATPase in anoxic elongation of stems in pondweed (Potamogeton distinctus) turions. New. Phytologist., 190, 421-430
- Korenkov, V., Hirschi, K., Crutchfield, J.D. and Wagner, G.J. (2007) Enhancing tonoplast Cd/H antiporter activity increases Cd, Zn and Mn tolerance, and impacts root/shoot Cd partitioning in Nicotiana tabacum L. Planta., 226, 1379-1387
- Kramer, U. (2010) Metal Hyperaccumulation in Plants. Annual. Review. Plant. Biology., 6, 517-534
- Larue, C., Korboulewsky, N., Wang, R. and Mevy, J.P. (2010) Depollution potential of three macrophytes: exudated, wall-bound and intracellular peroxidase activities plus intracellular phenol concentrations. Bioresou. Technol., 101, 7951-7957
- Liu, J., Yu, B.J. and Liu, Y.L. (2006) Effects of spermidine and spermine levels on salt tolerance associated with tonoplast H+/ATPase and H+/PPase activities in barley roots. Plant. Growth. Regul., 49, 119-126
- Liu, J.H., Inove, H., Moriguchi, T. (2008) Salt stress mediated changes in free polyamine titers and expression of genes responsible for polyamine biosynthesis of apple in vitro shoots. Environ. Exp. Bot. 62, 28-35
- Mandal, C., Ghosh, N., Maiti, S., Das, K., Gupta, S., Dey, N. and Adak, M.K. (2013) Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it’s modulation by polyamine. Physiol. Mol. Biol. Plants. 19, 91-103
- Mariale, S., Sanchez, D.H., Guirado, V.A. and Riiz, O.A. (2004) Spermine accumulation under salt stress. J. Plant. Physiol. 161, 35-4
- Mika, A. and Luthje, S. (2003) Properties of guaiacol peroxidase isolated from corn root plasma membranes. Plant. Physiol. 132, 1489-1498
- Mohamed, A.A. and Aly, A.A. (2008) Alteration of some secondary metabolites and enzymes activity by using exogenous antioxidant compound in onion plants grown under seawater salt stress. American. Eurasian. J. Sci. 3, 139-146
- Murashige, T. and Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473-497
- Nakano, Y. and Asada, K. (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22, 867-880
- Peter, W., Gloria, M.P., Alba, L.C., Jorge, E.M. and Idupulapati, M.R. (2001) The High Level of Aluminum Resistance in Signalgrass Is Not Associated with Known Mechanisms of External Aluminum Detoxification in Root Apices. Plant. Physiology. 125, 1473-1484
- Roy, P., Niyogi, K., SenGupta, D.N. and Ghosh, B. (2005) Spermidine treatment to rice seedlings recovers salinity stress-induced damage of plasma membrane and PM-bound H+/ATPase in salt-tolerant and salt-sensitive rice cultivars. Plant. Sci. 168, 583-591
- Roychoudhury, A. and Pradhan, S. (2011) Role of Potentially Important Microbes in Bioremediation. Science. Culture. 77, 324-330
- Sakihama, Y., Cohen, M.F., Grace, S.C. and Yamasaki, H. (2007) Plant phenolic antioxidant and prooxidant activities:phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 177, 67-80
- Saroop, S., Chanda, S.V. and Singh, Y.D. (2002) Changes in soluble and ionically bound peroxidase activities during brassica juncea seed development. Bulg. J. Plant. Physiol. 28, 26-34
- Skinner, K., Wright, N. and Porter-Goff, E. (2007) Mercury uptake and accumulation by four aquatic plants. Environmental. Pollution. 145, 234-237
- Sonia, S. (2012) Aluminium Toxicity Targets in Plants. J. Botany DOI: 10.1155/2012/219462
- Tang, W. and Newton, R.J. (2005) Polyamine reduces salt induced oxidative damage by increasing the activity of antioxidant enzyme and decreasing lipid peroxidation. Plant. Growth. Regul. 46, 31-43
- Xu, Q.S., Ji, W.D., Yang, H.Y., Wang, H.X., Xu, Y., Zhao, J. and Shi, G.X. (2009) Cadmium accumulation and phytotoxicity in an aquatic fern, Salvinia natans (Linn.). Acta. Ecologica. Sinica 29, 3019-3027


