Phytochemical screening and antifungal activities of ethanolic flower extracts of medicinally important plants against Aspergillus niger from Bhagalpur region
Автор: Kumari P., Singh M., Rani A.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.21, 2025 года.
Бесплатный доступ
Phytochemical screening and antifungal activity of ethanolic extracts of flowers from medicinally important plants Hibiscus sabdariffa , Moringa oleifera , Cassia fistula and Clerodendrum Infortunatum were investigated against Aspergillus niger , a common fungal pathogen. The study aimed to evaluate the presence of bioactive compounds in selected plant flowers and assess their potential antifungal efficacy. Phytochemical analysis revealed the presence of key secondary metabolites, including alkaloids, flavonoids, tannins, and phenols, known for their antimicrobial properties. The antifungal activity was tested using the Poisoned Food technique, a technique that involves incorporating the test substance into a growth medium to observe its inhibitory effects on fungal growth. The Diameter of growth was measured to determine the efficacy of the extracts. Results indicated significant antifungal activity in the flower extracts of Moringa oleifera and Cassia fistula , suggesting their potential use in the development of natural antifungal agents. This study highlights the importance of exploring indigenous medicinal plants for alternative treatments against fungal infections.
Phytochemical screening, antifungal activity, ethanolic extracts, medicinal plants, aspergillus niger
Короткий адрес: https://sciup.org/143184704
IDR: 143184704
Текст научной статьи Phytochemical screening and antifungal activities of ethanolic flower extracts of medicinally important plants against Aspergillus niger from Bhagalpur region
he increasing prevalence of fungal infections, particularly those caused by Aspergillus niger, has become a significant concern in both agricultural and medical fields. Aspergillus niger, a saprophytic fungus, is known for its role in various diseases affecting humans, plants, and animals. It thrives in humid conditions and can cause a range of health issues, particularly in immunocompromised individuals (Schuster et al., 2002), as it is capable of producing toxins such as ochratoxin A and fumonisins B2 and B4 (Nielsen et al., 2009). he emergence of drug-resistant strains of fungi has heightened the need for alternative antifungal agents derived from natural sources, such as medicinal plants.
Medicinal plants have been used for centuries in traditional medicine systems for their diverse therapeutic properties (Jain, 2007). Among them, Hibiscus sabdariffa , Moringa oleifera , Cassia fistula , and Clerodendrum infortunatum are well-known for their medicinal value and are widely used in folk medicine for treating a variety of ailments (Singh, 2015). hese plants are rich in bioactive compounds, such as alkaloids, flavonoids, phenols, tannins, and saponins, which have been shown to possess antimicrobial, antifungal, and antioxidant properties (Nandagoapalan et al. , 2016) (Salem et al. , 2022).
his study focuses on the phytochemical screening and antifungal activities of ethanolic extracts of flowers from Hibiscus sabdariffa , Moringa oleifera , Cassia fistula , and Clerodendrum infortunatum against Aspergillus niger isolated from the Bhagalpur region. Bhagalpur, located in the eastern part of India, is home to diverse flora with potential medicinal significance, yet many of these plants remain unexplored for their antifungal properties. he ethanolic extracts of these flowers are analyzed for the presence of key phytochemicals, and their antifungal efficacy is evaluated to explore their potential use as natural alternatives to synthetic antifungal agents.
his research aims to contribute to the growing body of knowledge on plant-based antifungal agents and to promote the use of indigenous plants in the development of eco-friendly and sustainable antifungal
therapies.
MATERIALS AND METHODS
CHEMICALS AND REAGENTS
All the chemicals used in the present study were of analytical grade and bought from Loba Chemicals. Aluminium chloride was bought from Merck and deionized X-tra pure water from Sigma chemicals. PDA (Potato Dextrose Agar RDM-PDA 01) was procured from Ready Med.
PREPARATION OF EXTRACT OF PLANT SAMPLES he flowers of four different medicinally important plants were collected from local regions of Bhagalpur, Bihar, India. he flowers were thoroughly washed with normal water followed by rinsing with distilled water. After complete draining of water, the plant samples were refrigerated separately for complete drying for about 20 days. he dried flowers were powdered in Usha Colt Mixer Jar not letting the jar heat. he ethanolic extract of dried flowers was prepared using Maceration technique (Ali-Shtayeh et al., 1999). About 4g of powder was soaked in 40 ml of absolute alcohol. After 48 hours, the solution was filtered through Whatman’s filter Paper No 41. he filtrate was evaporated on a water bath to obtain semi-solid dry ethanolic extract. he obtained extract was stored in air tight bottles at 4°C for further studies.
PHYTOCHEMICAL SCREENING he qualitative chemical test was performed for the identification of various phytochemicals (Alkaloids, flavonoids, terpenoids, glycosides, anthocyanins, proteins, steroids, coumarins, saponins, anthraquinones and phlobatannins) present in the ethanolic flower extracts of medicinally important plants from Bhagalpur region of Bihar, India (Egwaikhide et al., 2007).
TOTAL PHENOLIC CONTENT otal phenolic content was estimated by using the Folin-Cio-Calteau Reagent (McDonald, 2001) (Nagalakshmi et al., 2023). Briefly, to an aliquot of 500µl of extract in ethanol, 500µl of Folin-Ciocâlteu reagent was added. he solution was diluted by adding 6 ml of deionized distilled water followed by the addition of 1.5 ml of 20% sodium carbonate solution and then 1.9 ml of deionized distilled water was added. After incubation for 2 hours, the absorbance was recorded at 760 nm using Systronics117 UV–VIS Spectrophotometer. Gallic Acid was used as a reference phenolic compound for preparing standard curve and generating linear regression equation y = mx + c for expressing the results as milligram Gallic Acid Equivalents (GAE) per gram of Extract. he experiments were conducted in duplicates.
TOTAL FLAVONOID CONTENT otal flavonoid content was estimated by the aluminum chloride method (Chang et al., 2002). Briefly, to an aliquot of 500µl of extract, 4ml of ethanol and 1ml of 10% aluminum chloride were added followed by the addition of 1ml of 1M sodium acetate solution. he absorbance was noted after incubation for 45 minutes in the dark at 420 nm using a Systronics117 UV-VIS Spectrophotometer. Quercetin was used as standard for preparing standard curve and generating linear regression equation y = mx + c for expressing the results as milligram Quercetin equivalent (QE) per gram of extract. he experiments were conducted in duplicates.
ANTIFUNGAL ACTIVITY he antifungal activity of ethanolic extract of flowers of medicinally important plants is determined by Poisoned Food technique, a technique that involves incorporating the test substance into a growth medium to observe its inhibitory effects on fungal growth (Debbarmal et al., 2021). he PDA (Potato Dextrose Agar) and ethanolic dry extract were mixed in measured amount of distilled water to have the concentration of 1mg/ml. he solution was autoclaved at 121°C and 15psi. he PDA is poured in sterilized Petri plates and allowed to solidify. he fungal mycelium from 5-day old fungal culture is inoculated to the Petri plates aseptically using flame sterilized inoculation needle. he Petri plates are sealed with parafilm to avoid contamination and incubated at 37°C for six days. he Petri plate having PDA without ethanolic extract is considered as Negative control. he diameter of growth in mm is measured every 24 hrs. at the same time. he experiment is conducted in triplicates. he antifungal activity is calculated as follows:
Antifungal Activity (%) = (D C – D S / D C ) Χ 100
Where,
D C = Diameter of Growth in Negative control Petri plate.
D S = Diameter of growth in Petri plate containing plant extract.
STATISTICAL ANALYSIS
he results are expressed as mean ± standard deviation(SD) and calculated on Microsoft Excel. he correlation factor was calculated using Microsoft Excel function CORREL. he statistical significance of the results was examined using one-way ANOVA test (p<0.05) in Microsoft Excel.
RESULTS he flowers of four medicinally important plants namely Hibiscus sabdariffa, Moringa oleifera, Cassia fistula, and Clerodendrum Infortunatum were subjected for the preparation of ethanolic extract using maceration technique. hus, four different ethanolic extracts of flowers of medicinally important plants were prepared namely: ethanolic extract of flowers of Hibiscus sabdariffa (KF), Moringa oleifera (MOF), Cassia fistula (CFF), and Clerodendrum Infortunatum(CF).
PHYTOCHEMICAL SCREENING he qualitative examination of ethanolic extracts of flowers from medicinally important plants (Hibiscus sabdariffa, Moringa oleifera, Cassia fistula, and Clerodendrum infortunatum) from Bhagalpur, Bihar, India, shows the presence of various phytochemicals, as shown in able 1. he ethanolic extracts were analyzed for the presence of alkaloids, carbohydrates, glycosides, flavonoids, terpenoids, coumarins, anthocyanins, proteins, steroids, phlobatannins, and saponins. All four extracts showed the presence of flavonoids, phenolics, carbohydrates, coumarins, steroids, saponins, glycosides, and terpenoids. Phlobatannins were not identified in the qualitative chemical test of the ethanolic extracts of the flowers of Hibiscus sabdariffa, Moringa oleifera, Cassia fistula, and Clerodendrum infortunatum. Proteins and anthraquinones were not identified in the extract of Moringa oleifera flowers. Alkaloids were not observed in the qualitative chemical tests of the ethanolic extracts of the flowers of Hibiscus sabdariffa,
Moringa oleifera , and Cassia fistula . Anthocyanins were also absent in the qualitative chemical test of the ethanolic extract of Cassia fistula .
TOTAL PHENOLIC CONTENT otal phenolic content was determined using the linear regression equation y=0.0007x+0.0559, with coefficient of determination, R2=0.9676, obtained from the standard curve of Gallic Acid. he larger value of R2 0.9676 suggests the significance of the curve and its reliability for the calculation of otal phenolic content.
he total phenolic content of the ethanolic extracts of flowers of different plants under study are in the order Clerodendrum infortunatum > Cassia fistula > Moringa oleifera > Hibiscus sabdariffa . he values of total phenolic content are shown in able 2. he highest total phenolic content is 242 ± 4.04 mg GAE/g of dry extract for ethanolic flower extract of Clerodendrum infortunatum and the lowest is 66 ± 8.08 mg GAE/g of dry extract for ethanolic flower extract of Hibiscus sabdariffa .
TOTAL FLAVONOID CONTENT otal Flavonoid Content was determined using the linear regression equation y=0.0224x+0.1422, with coefficient of determination, R2=0.965, obtained from the standard curve of Quercetin.
he total flavonoid content of ethanolic extracts of flowers of different plants under study are in the order Cassia fistula > Moringa oleifera > Hibiscus sabdariffa > Clerodendrum infortunatum . he values of total flavonoid content are shown in able 2. he total flavonoid content is highest at 40.178 ± 1.32 mg QE/g of dry extract for ethanolic flower extract of Cassia fistula and is lowest at 3.822 ± 0.189 mg QE/g of dry extract for ethanolic flower extract of Clerodendrum infortunatum .
ANTIFUNGAL ACTIVITY he antifungal activity of ethanolic extract of flowers of Hibiscus sabdariffa (KF), Moringa oleifera (MOF), Cassia fistula (CFF), and Clerodendrum Infortunatum(CF) at concentration of 1mg/ml was tested by poisoned food technique, which involves incorporating the test substance into a growth medium to observe its inhibitory effects against the growth of Aspergillus niger. he percentage of antifungal activity was calculated with reference to the negative control.
he growth of Aspergillus niger was highest in negative control Petri plate compared to the Petri plates containing ethanolic flower extracts of the plants under study (Figure 1). On day 3, fungal growth was significantly lower in the Petri plates containing ethanolic flower extracts of MOF and CFF compared to those with CF and KF extracts.
he diameter of Aspergillus niger growth in millimeters in the control and test Petri plates is shown in Figure 2. he growth diameter of Aspergillus niger was largest in the negative control Petri plate and smallest in the Petri plate with CFF extract. he minimum diameter of growth indicates the inhibitory effect of CFF extract on the growth of Aspergillus niger , demonstrating its antifungal activity. he daily measurements of fungal growth of Aspergillus niger in test and control showed significant variations, with p<0.05 in the One-Way ANOVA test.
All extracts showed antifungal activity against Aspergillus niger at varying percentage as illustrated in Figure 3. he percentage of antifungal activity is highest for CFF extract and lowest for the CF and KF extracts, with the percentages being approximately same for CF and KF extracts. he percentage of antifungal activity of MOF extract was lower than that of CFF extract. here was a statistically significant difference in the antifungal activity percentages among the extracts, with p<0.05 in one-way ANOVA test.
here was a strong positive correlation between total flavonoid content and percentage antifungal activity, with a correlation coefficient of 0.908. his suggests that flavonoids may contribute significantly to the antifungal properties of the extracts. In contrast, the correlation between total phenolic content and percentage antifungal activity was low, with a coefficient of 0.157.
Table 1. Phytochemicals present in ethanolic extracts of flowers of medicinally important plants
|
Phytochemicals |
Hibiscus sabdariffa (KF) |
Moringa oleifera (MOF) |
Cassia fistula (CFF) |
Clerodendrum Infortunatum (CF) |
|
Alkaloids |
- |
- |
- |
+ |
|
Carbohydrates |
+ |
+ |
+ |
+ |
|
Glycosides |
+ |
+ |
+ |
+ |
|
Phenols |
+ |
+ |
+ |
+ |
|
Flavonoids |
+ |
+ |
+ |
+ |
|
erpenoids |
+ |
+ |
+ |
+ |
|
Protein &Amino Acids |
+ |
- |
- |
+ |
|
coumarins |
+ |
+ |
+ |
+ |
|
Saponins |
+ |
+ |
+ |
+ |
|
Steroids |
+ |
+ |
+ |
+ |
|
Anthraquinone |
+ |
- |
+ |
+ |
|
Anthocyanin |
+ |
+ |
- |
+ |
|
Phlobatanins |
- |
- |
- |
- |
|
Key: + means present and - means absent |
||||

Figure 1: ( A ) Growth of Aspergillus niger in negative control Petri plate on (i) Day 1, (ii) Day 2, (iii) Day 3, ( B ) Growth of Aspergillus niger in Petri plate containing ethanolic flower extract of Moringa oliefera (MOF), on (i) Day 1, (ii) Day 2, (iii) Day 3, ( C ) Growth of Aspergillus niger in Petri plate containing ethanolic flower extract of Clerodendrum infortunatum (CF), on (I) Day 1, (ii) Day 2, (iii) Day 3, ( D ) Growth of Aspergillus niger in Petri plate containing ethanolic flower extract of Hibiscus sabdariffa (KF), on (i) Day 1, (ii) Day 2, (iii) Day 3, ( E ) Growth of Aspergillus niger in Petri plate containing ethanolic flower extract of Cassia fistula (CFF), on (i) Day 1, (ii) Day 2, (iii) Day 3.
Table 2. otal flavonoid content and total phenolic content
|
Samples as ethanolic extract |
Milligram Quercetin equivalent per gram of dry extract |
Milligram Gallic Acid Equivalent per gram of dry extract |
|
Hibiscus sabdariffa (KF) |
19.594 ± 0.94 |
66 ± 8.08 |
|
Moringa oleifera (MOF) |
31.356 ± 0.94 |
107.429 ± 2.02 |
|
Cassia fistula (CFF) |
40.178 ± 1.32 |
201.714 ± 2.02 |
|
Clerodendrum Infortunatum (CF) |
3.822 ± 0.189 |
242 ± 4.04 |
|
Note: the values are represented as mean ± SD |
||
Table 3. Correlation coefficient between otal Flavonoid Content, otal phenolic Content and % Antifungal activity
|
mean daily diameter of growth in mm |
% Antifungal Activity |
|
|
PC |
-0.26387 |
0.157747 |
|
FC |
-0.85869 |
0.908774 |
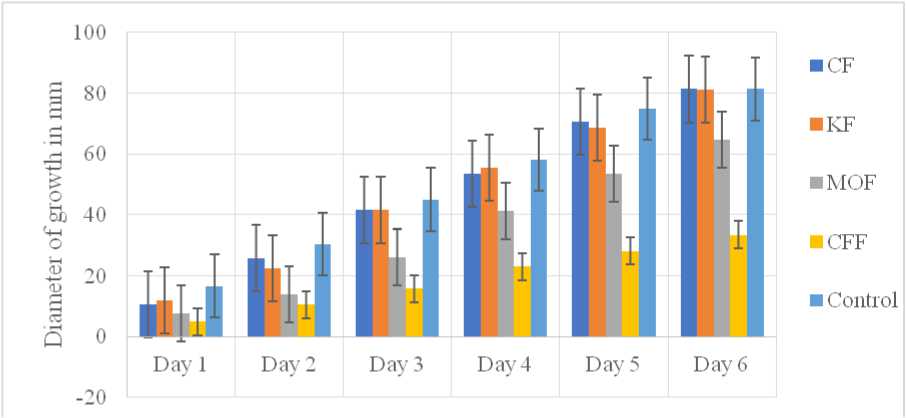
Figure 2: Diameter of Growth of Aspergillus niger
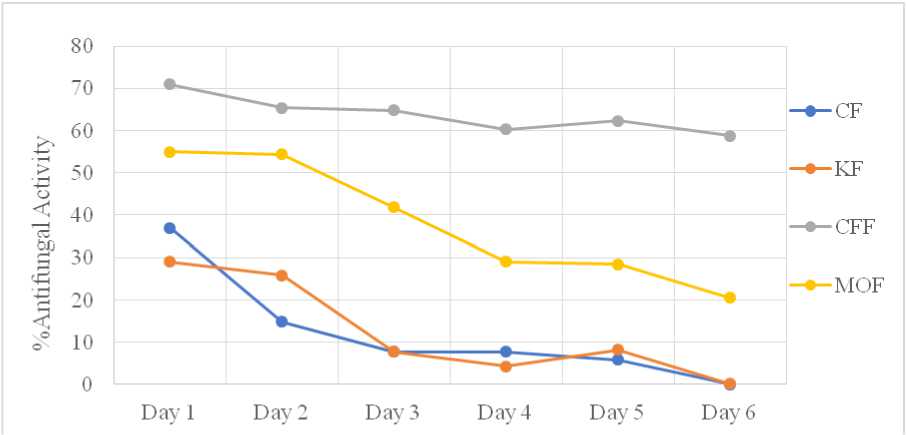
Figure 3: % of Antifungal Activity of Aspergillus niger
DISCUSSION
Chemical tests for phytochemicals revealed the presence of phytochemicals such as alkaloids, flavonoids, phenols, terpenoids, saponins, glycosides etc. in the ethanolic extracts. In the present study, flavonoids, phenolics, carbohydrates, coumarins, steroids, saponins, glycosides and terpenoids were detected in the ethanolic extract of flowers of Hibiscus sabdariffa (KF), Moringa oleifera (MOF), Cassia fistula (CFF), and Clerodendrum Infortunatum (CF). Similar phytochemicals have been reported by studies (Salem et al. , 2022, Don-lawson et al. , 2021, Chandra et al. 2024, Kekuda et al. , 2019, Dutta et al. , 2016).
Antifungal activity of ethanolic extracts of MOF, KF, CF and CFF was studied in the present work. he figure 1(A) to 1(E) illustrate the growth of Aspergillus niger in the presence of different plant extracts at concentration of 1 mg/ml in PDA. Figure 1 shows the fungal growth in the negative control Petri plate, where growth was highest. In contrast, the presence of plant extracts in other plates inhibited fungal growth to varying degrees. hese extracts showed the presence of secondary metabolites such as alkaloids, phenols, and flavonoids which are responsible for the antifungal activity.
Flavonoids, structurally diverse secondary metabolites in plants, are known to inhibit fungal growth by disrupting plasma membranes, inducing mitochondrial dysfunction, interfering with cell wall construction and cell division, and inhibiting RNA, protein synthesis, and efflux-mediated pumping systems (Al Aboody, 2020). Phenols, another group of secondary metabolites, function as antimicrobial agents by damaging membrane structural integrity in a nonspecific manner and inhibiting electron transport enzymes (Bhattacharya, 2010).
he present study reported significant amounts of total phenolic and flavonoid content in all the samples under investigation. he highest total flavonoid content was found in the CFF extract (40.178 ± 1.32 mg QE/g of dry extract), followed by the MOF extract (31.356 ± 0.94 mg QE/g of dry extract), and the lowest in the CF extract
(3.822 ± 0.189 mg QE/g of dry extract). he total phenolic content followed a similar pattern, except for CF, which had the highest phenolic content (242 ± 4.04 mg GAE/g of dry extract).
here was a strong correlation between total flavonoid content and antifungal activity ( able 3), further supporting the role of flavonoids as fungicidal agents. In contrast, the correlation between total phenolic content and antifungal activity was lower, suggesting that phenolics have a lesser fungicidal effect. However, previous studies have highlighted phenolic compounds as potential antifungal agents (Bhattacharya et al. , 2010, Zabka et al. , 2013). he lower correlation coefficient for phenolic content and antifungal activity in this study may be due to the unexpectedly high phenolic content in CF. When the phenolic content of CF is excluded, the correlation coefficient becomes significantly higher (0.945), suggesting that both phenolic and flavonoid compounds may contribute to the antifungal activity of the ethanolic extracts.
Additionally, the correlation between the daily diameter of fungal growth and total phenolic and flavonoid content was similar to that of antifungal activity but with opposite signs, demonstrating the fungicidal effect of flavonoids. An increase in flavonoid content may be associated with a reduction in the diameter of fungal growth, and conversely, lower flavonoid content may correspond to larger fungal growth.
he ethanolic extracts of the plant samples in this study showed significant antifungal activity. All extracts inhibited the growth of Aspergillus niger , as indicated by the differences in fungal growth diameters in Petri plates with extracts compared to the negative control (i.e., the plate without any extract). he inhibitory effect on Day 3, was highest for the CFF extract (65%), followed by MOF (42%), and lowest for CF and KF (8%).
he antifungal activity of methanolic MOF extracts against Aspergillus niger has been previously reported (Asghar et al., 2022; Das et al., 2015), though limited data is available for ethanolic MOF extracts. he leaf and bark extracts of Moringa oleifera have also shown antifungal activity against Aspergillus niger (Aondo et al., 2018). he present study confirms the significant antifungal activity of MOF ethanolic extract against Aspergillus niger. Methanolic extracts of CFF have previously been reported to exhibit strong antifungal effects against Aspergillus niger (Archana et al., 2022), which aligns with the results of this study. Hydroethanolic KF extracts have been shown to have fungicidal effects on Aspergillus niger, though its infusion did not display such activity (Jabeura et al., 2017). In the present study, KF extract showed negligible antifungal activity, with its fungal growth diameter being almost similar to that of the negative control. Similarly, the CF extract exhibited a comparable outcome. Although limited data is available on the antifungal activity of CF extracts, the leaf extract of Clerodendrum infortunatum has been reported to have antifungal activity against Aspergillus niger (Waliullah et al., 2014).
he results of the present study do not fully align with those of other researchers. his discrepancy may be attributed to several factors, including differences in geographical regions and soil composition where the plants were grown, the seasons in which the plants were collected, and the stages of plant growth. Additionally, variations in the solvents (Dhakite and Gadpayale, 2024) and methods used for extraction could also contribute to these differences (Figueiredo et al. , 2008; Mohiuddin, 2019).
CONCLUSION he present study demonstrated significant antifungal activity in ethanolic extracts of Clerodendrum infortunatum (CF), Moringa oliefera (MOF), Cassia fistula (CFF), and Hibiscus sabdariffa (KF) against Aspergillus niger, correlating with the total flavonoid and phenolic contents present in the extracts. he highest total flavonoid content was observed in the CFF extract, followed by MOF, while CF exhibited the highest total phenolic content. he strong correlation between flavonoid content and antifungal activity highlights the potential of flavonoids as effective fungicidal agents.
Moreover, the observed discrepancies between this study and previous research can be attributed to various factors, including geographical variations, soil composition, plant collection seasons, growth stages, and differences in extraction methods and solvents.
hese findings underscore the importance of standardizing extraction protocols and considering environmental factors in future studies to enhance the comparability of results across different research efforts. Overall, this research contributes valuable insights into the antifungal potential of plant extracts and their bioactive compounds, paving the way for further exploration of natural antifungal agents.
ACKNOWLEDGEMENT
First author is grateful to the Department of Chemistry and Department of Botany, S. M. College, Bhagalpur for providing lab facilities to carry out the research work.
CONFLICTS OF INTEREST he authors declare that they have no potential conflicts of interest.


