Phytohormones and morphogenesis of root nodules and lateral roots of a legume plant
Автор: Glyanko A.K.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.14, 2018 года.
Бесплатный доступ
Data on the physiological role of phytohormones (mainly cytokinin and auxin) in the initiation of cortical cell division of the root, in the formation of nodule primordium and in its further organogenesis are summarized. The necessity of high level of cytokinin and low level of auxin for this process is proved. The mechanism leading to an increase in the cytokinin / auxin ratio is associated with the inhibition of auxin transport from the aerial organs to the root with the involvement of cytokinin signaling. Reducing cytokinin / auxin ratio against the background of inhibition of cytokinin signaling initiates the formation of lateral roots. The role of other phytohormones as well as flavonoids which have a positive (gibberellins, brassinosteroids) or negative influence (ethylene, abscisic, jasmonic and salicylic acids) on the formation of the root nodule is discussed. The key role of the rhizobial Nod factor signaling in the organogenesis of the nodule is emphasized. The schemes of reactions and signaling processes involved in the initiation of nodule primordium and lateral roots formation are given.
Rhizobia, legume-rhizobial symbiosis, nodule formation, phytohormones, auxin, cytokinin, nod factor, flavonoids, root nodule organogenesis, lateral roots
Короткий адрес: https://sciup.org/143166689
IDR: 143166689
Текст обзорной статьи Phytohormones and morphogenesis of root nodules and lateral roots of a legume plant
Phytohormones (auxins, cytokinins, gibberellins, brassinosteroids, ethylene, abscisic, jasmonic and salicylic acids) play a decisive role in plant life processes exerting a multifaceted influence on the growth and development of plants (Kulaeva, 1973; Polevoy, 1982; Woodward, Bartel, 2005; Zhao, 2010; Kiseleva et al. , 2012; Chandler et al ., 2015). Of these, the most attention in the scientific and practical aspects is given to auxins and cytokinins. Compounds possessing auxin activity stimulate cell elongation. Cytokinins affect the division and differentiation of cells.
Physiological functions of auxins and cytokinins
-
6 groups of compounds with auxin activity are known nowadays. Natural auxin – indolyl-3-acetic acid (IAA) and synthetic auxins – indolebutyric, naphthylacetic and 2,4-dichlorophenoxyacetic (2,4-D) acids are the most studied among them. Auxins synthesized in the apex tissues of the aerial organs are transported along the phloem actively and polarly into the root apex. Their action is associated with the elongation and proliferation of meristematic cells as well as with the phenomenon of apical dominance as a result of which the apical bud dominates in growth and the growth of the lateral buds is inhibited. Auxin basipetal transport is observed in the aerial organs (Lomax et al ., 2001) and the accumulation of auxin in apex can be due to its transport from young leaves and leaf primordia (Ljung et al ., 2001).
Cytokinins are found in the cells of various organisms. These natural and artificially synthesized compounds are called cytokinins for the ability to induce cell division of plants (cytokinesis). At present, about 200 such compounds of both natural and synthetic origin are known. The most famous of them are: 6-furfurylaminopurine (kinetin), zeatin, 6-benzylaminopurine (BAP). Cytokinins are synthesized in the root apex and transported upward along the xylem nonpolarly and passively. They remove auxin apical dominance causing stem buds development and shoot branching.
The combination of cytokinins with auxin leads to the switch of cell division to differentiation. The antagonistic effect of the action of auxin and cytokinin on the growth and organogenesis in plant tissue culture was revealed (Skoog, Miller, 1957). This antagonism reveals itself in the roots with apical dominance. During the initiation and maintenance of the root apical meristem, cytokinins and auxins function as antagonists acting in different zones of the meristem (Moubayidin et al., 2009). Auxin accumulates at the tip of the root of the apical meristem and supports cell division while cytokinin acts between the proximal meristem and the stretch zone being associated with the switch of cells from division to differentiation (Moubayidin et al., 2009). Treatment of Arabidopsis plants with exogenous auxin inhibits cytokinin biosynthesis (Nordstrom et al., 2004). However, it is possible that in other cases (apical meristem of the aerial organs) these hormones can act synergistically enhancing the effect of each of them at a certain concentration and ratio (Chandler, Werr, 2015).
Cytokinins are signaling molecules that initiate the expression of the corresponding genes. In Arabidopsis , cytokinin signaling is represented by two components – a histidine kinase receptor (eg, CRE1) and an ARR response regulator (cytokinin-responsive Arabidopsis response regulator) (Ferreira, Kieber, 2005). Cytokinins inhibit the formation of lateral roots but induce the morphogenesis of the root nodule and the formation of cecidia under the action of nematodes (Lohar et al ., 2004). It was found that the size of the root apical meristem in the triple Arabidopsis mutant increases in the enzymes of the cytokinins synthesis (isopentenyl transferases) (Ioio et al., 2007). These plants (mutants) also show a decrease in the rate of differentiation of meristematic cells. That indicates the regulation of this process with cytokinin by limiting the zone of the root meristem.
Thus, cytokinins regulate growth and ensure the formation of new structures and their shape. The appearance of primordia which are organs rudiments occurs under the influence of cytokinins as in the case of the morphogenesis of the root nodule. Transgenic plants with the superexpression of the cytokinin oxidase / dehydrogenase gene were characterized by the lowest cytokinin content and formed various phenotypes: with increased root meristem, growth of lateral roots at the border of proximal and apical meristem, increased root branching, and with the formation of accessory roots (Werner et al., 2003). The cell cycle is influenced by both hormones; the relative importance of each of them and the degree of their interaction are topical issues for researchers.
Literature data indicate the involvement of auxins and cytokinins in the processes of formation of legume-rhizobial symbiosis (LRS) (Oldroyd et al ., 2011; Liu et al ., 2015). This fact was first lined in 1936 (Thimann, 1936). Extensive research in this area was carried out in 1990s (Hirsch et al ., 1989; Hirsch, 1992; Hirsch, Fang, 1994; Hirsch et al ., 1997; Cooper, Long, 1994) that showed the involvement of these hormones in the initiation of nodule formation in experiments with the change in the concentration of exogenous cytokinin and the use of inhibitors of auxin transport. Later, the need for activation of cytokinin signaling for inducing nodule morphogenesis was proved in experiments with Medicago truncatula and Lotus japonicum mutants which are defective with the cytokinin receptor gene ( CRE1 and LHK1 ) (Murray et al ., 2007; Plet et al ., 2011). The functional significance of phytohormones in this process is different (Oldroyd, Downie, 2008). Auxins, cytokinins, gibberellins and brassinosteroids are positive LRS regulators, the others (abscisic, jasmonic, salicylic acids and ethylene) have a negative effect on the symbiosis.
Thus, auxins, cytokinins, gibberellins (GB), and brassinosteroids (BS) are the main endogenous compounds that initiate organogenesis of root nodules; their level and ratio are key factors in the formation of these structures (Ferguson et al ., 2005; Oldroyd, Downie , 2008).
Influence of phytohormones on the morphogenesis of root nodules
The physiological aspects of the effect of cytokinins and auxins on nodulating processes during LRS formation are the most studied area of the issue of mutualistic symbiosis (Oldroyd et al ., 2011, Murray, 2011). The question of the morphogenesis of lateral roots is also of interest. It should be noted that the result of phytohormones action depends on their combinations and the specific role of each of them and that their interaction is related to the effect on a certain process at the signaling level (Ferreira, Kieber, 2005).
The auxin / cytokinin ratio is important for the regulation of many growth and development processes including organ regeneration from tissue culture (Skoog, Miller, 1957). In contrast to other hormones, a distinctive feature of cytokinins is their expression of the synthesis of the so-called early proteins – nodulines, characteristic for the early stage of LRS: ENOD2, ENOD11, ENOD12, ENOD40 and others (Bauer et al ., 1996). Moreover, the expression of noduline genes under the influence of cytokinins occurs earlier than the initiation of root nodule primordium formation (Hirsch, Fang, 1994). The question whether early noduline proteins participate in the organogenesis of the nodule remains in abeyance. Early nodulines are believed to be indicators of the status of endogenous cytokinin in symbiotic tissues (Hirsch et al., 1997). They are also believed to participate in the regulation of cortex cells (ENOD40) (Mathesius et al. , 2000). The fact is lined that under the influence of rhizobia, the content of cytokinins increases and expression of ENOD-protein genes takes place. This suggests the effect of rhizobia on the synthesis of cytokinins and the subsequent expression of ENOD genes under the influence of cytokinins.
Auxin transport inhibitors are known as inducers of pseudonodules formation and expression of ENOD40 – a gene associated with the initiation of nodule primordium formation (Fang, Hirsch, 1998; Mathesius et al., 2000). Trans-zeatin (cytokinin) is able to activate nodulation in legumes in experiments with rhizobia defective in the synthesis of Nod factors (Cooper, Long, 1994). Thus, modulation of auxin and cytokinin levels is an important key step in the root nodule formation. The important role of cytokinin in nodulation is confirmed by genetic studies. Thus, in spontaneous nodulation Lotus japonicus mutants carry a mutation in histidine kinase (LHK1), a cytokinin receptor (Tirichine et al., 2007). In addition, loss of CRE1 (orthologue of LHK1) function in alfalfa mutant reduces the formation of nodules to a large extent (Gonzalez-Rizzo et al., 2006). Lhk1 mutants lose the ability to initiate the formation of nodule primordia but do not affect the infection of the root with rhizobia (Murray et al., 2007). However, the effect of the mutation on the growth of infection threads which create complex infectious structures is shown. Hence, the formation of nodule primordium is not associated with the penetration (infection) of rhizobia into the root tissue through the epidermis. At the same time, exogenous cytokinin induces the expression of genes that are normally associated with nodule primordium formation (Fang, Hirsh, 1998; Mathesius et al., 2003). Mutations in the LHK1 gene indicate the central role of cytokinine in the organogenesis of the nodule in the cortex and simultaneously indicate their lack of influence on the rhizobial infection in the epidermis. Nevertheless, there is evidence (Gage, Margolin, 2000) about the involvement of cytokinin in the formation of pre-infection threads in the outer cortex cells. These threads develop in infection threads. Therefore, one can speak of the positive role of cytokinin in root hair infection as an alternative (Murray, 2011; Liu et al., 2015).
Positive influence on nodule formation is exerted by gibberellins (GB) and brassinosteroids (BS). The work of Ferguson et al . (2005) where low-growth pea mutants deficient in these hormones were used to prove the need for GB and BS to form nodules is noteworthy in this respect (Nomura et al ., 1997). It was found that all the mutants studied had a low ability to form nodules compared to the wild-type pea (Torstag variety). Adding exogenous GB to the plant growing environment restored the pea ability to form an optimal number of nodules comparable to the wild type. A similar pattern was also revealed for lateral roots. The authors conclude that GB and BS are involved in the processes of nodule formation in pea. It is assumed that the effect of GB in the roots on nodulation is direct and the influence of BS is mediated through a mechanism of nodulating process regulation in the roots localized in the aerial organs. However, the mechanisms of their action are not sufficiently studied and understood. There is evidence that auxin enhances GB production and interacts with BS (Ross et al ., 2000; Kim et al ., 2000).
The role of phenolic compounds (flavonoids) in the organogenesis of the nodule
It is known that legume flavonoids cause the expression of rhizobial nod -genes which lead to the synthesis of bacterial chitooligosaccharide – Nod factor, that initiate responses to the introduction of rhizobia in the host plant (Mulligan, Long, 1989). But the functions of flavonoids at the nodule formation do not end at that:
there is a sufficient amount of literature data indicating, in particular, regulation (modulation) of the local level of auxin in root tissues associated with the inhibition of auxin carrier proteins (PINs and PGPs) by flavonoids (Zhang et al ., 2009) and with the participation of these compounds and peroxidase in the fission of auxin (IAA) (Mathesius et al ., 2001). PINs (PIN-formed auxin) are auxin carrier proteins that belong to the family TC 2.A.69. Another family of auxin carrier proteins PGPs (phosphoglycoproteins) – TC 3.A.1 – is integrated into the plasma membrane. Flavonoids are suggested to modulate auxin transport by altering the phosphorylation of protein kinases and phospholipids (De Long et al ., 2002).
The localization of the synthesis of flavonoids which can inhibit auxin transport and thus alter the auxin flux in the root is one of the possible mechanisms for rhizobial infection for inducing hormonal changes in the cortex (Mathesius, 2001). Since 1988 it has been known (Jacobs, Rubery, 1988) that flavonoid compounds (quercetine,apigenin,kaempferol, etc.) can block the polar auxin transport in plants replacing the function of a natural inhibitor of the auxin transport NPA (1-naphthylphthalamic acid). This allows us to consider endogenous plant flavonoids as natural regulators of auxin flux in cells and polar auxin transport (Peer, Murphy, 2007; Adamowski, Friml, 2015).
Rhizobia can also have an effect on the balance of cytokinins / auxins in the root cortex through the induction of the synthesis of flavonoids. The main source of cytokinin is the meristematic root apex while the auxin source is the meristematic apex of the stem; their fluxes at the root are opposite in direction (Sakakibara, 2005; Leyser, 2006). Mutation in the enzyme of the biosynthesis of flavonoids –chalcone synthase – leads to the inhibition of the initiation of nodule primordium formation. That is associated with the regulation of the auxin flux in the root by flavonoids (Wasson et al ., 2006). In the opinion of (Peer, Murphy, 2007), flavonoids are rather modulators of auxin transport processes than regulators.
At the same time, it seems that other endogenous factors can also influence the acropetal (polar) transport of auxin (except phenols). Thus, according to
Fernandez-Marcos et al . (2011), nitrogen oxide (NO) disrupts the auxin transport in the roots of Arabidopsis by reducing the amount of protein PIN1 – an auxin transporter. On the other hand, the content of NO in Arabidopsis decreases under the influence of cytokinin (Liu et al ., 2013). According to other data, S-nitrosylation of the protein TIR1 (Transport Inhibitor Response1) – an auxin receptor – with NO activates auxin signaling in Arabidopsis (Terrile et al ., 2012). Thus, NO (as well as phenolic compounds) affects the growth of roots and, apparently, the initiation of the nodule primordium formation in legumes modifying the content of phytohormones.
Mechanisms of influence of phytohormones on the organogenesis of the nodule and lateral roots
The scheme of the formation of lateral roots and the morphogenesis of the nodule based on the concepts of the regulation of these processes by cytokinin and auxin are shown in Fig.1 and 2. The initiation of lateral roots occurs in the area of cell differentiation against the background of high auxin content in the stem cell center. Cytokinin signaling associated with the inhibition of auxin transporters (PINs, PGPs) is blocked; that creates conditions for auxin transport to the meristematic root apex and auxin accumulation at the sites of lateral roots formation.
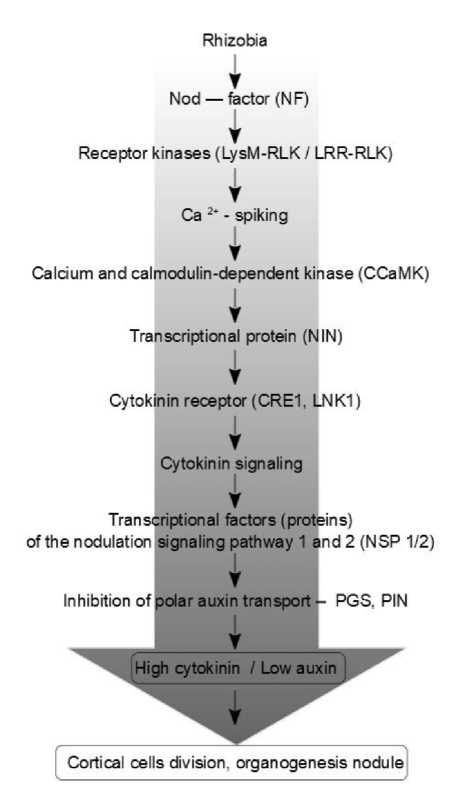
In the case of rhizobial infection, the main role is played by Nod factor activated by plant receptor-like kinases. It is established that the organogenesis initiation mechanisms of the root nodule are based on the regulation of cytokinin and auxin fluxes where rhizobial Nod factors (NFs) play a decisive role. They represent lipochitooligosaccharides that interact on the plasmalemma of epidermal cells with plant receptors (receptor-like kinases, LysM-RLK, LRR-RLK) and initiate the activity of the host plant signal systems (Glyan’ko, 2014). It is believed that NFs are the initial trigger of physiological, biochemical, genetic and other processes initiation that alter the direction of metabolism of the host plant and lead to the creation of a symbiotrophic organism (Ferguson et al ., 2010).
Nod factor localized on the surface of epidermal cells induces the transcription factor NIN (nodule inception)
and starts CRE1 receptor (histidine kinase) activation – cytokinin signaling. As a result, auxin transport is blocked due to the inhibition of transport proteins (PINs, PGPs, NPA) that leads to the accumulation of cytokinin which initiates the nodule morphogenesis. It can be represented schematically (Fig. 1).
The initiation of lateral and accessory roots formation is associated with the inhibition of cytokinin signaling by inducing the suppressors of this process RR7 and RR15 (Response Regulator genes) (Fig. 2). The suppressor of the auxin transporter PIN inhibits; that leads to a decrease in the cytokinin content and an increase in the auxin content localized at the sites of lateral roots (pericycle tissues). Loss of function of RR7 and RR15 leads to the disruption of the early embryogenesis in the root “stem-cells” system (Muller, Sheen, 2008). These events are preceded by the expression of “auxin” genes which is initiated by the interaction of auxin with the TIR1 / AFB receptor (Transport Inhibitor Response1 / Auxin Signaling F-Box) and the inactivation of the AUX / IAA repressor (Auxin / Indole-3-Acetic acid).
There arises a question on the origin of cytokinin in the cells of a symbiotrophic organism – plant or bacterial (rhizobial). It is believed that this is a plant cytokinin, although the ability of photosynthetic species Bradyrhizobium not producing the Nod factor to directly affect the hormonal level has been established. This bacterium is suggested to be possible to induce nodulation by supplying the plant with intermediates for the cytokinin synthesis. The legume Aeschynomene sensitive inoculated with Bradyrhizobium mutants which are incapable of synthesizing the Nod factor does not lose the ability to form root nodules (Giraud et al., 2007). These genetic models speak of two mechanisms for the initiation of the nodule organogenesis: with activation by the Nod factor and induction by cytokinin (Oldroyd, 2007). The penetration of Bradyrhizobium ssp into cortical cells of the root through cracks in the epidermis, bypassing root hairs, can be important evidence in using the cytokinin signal in initiating cortical cells division and nodule organogenesis instead of the Nod factor (Downie, 2007).
Rhizobia
Nod — factor (NF)
Receptor kinases (LysM-RLK / LRR-RLK)
Calcium and calmodulin-dependent kinase (CCaMK)
Transcriptional protein (NIN)
Cytokinin receptor (CRE1, LNK1)
Cytokinin signaling
Transcriptional factors (proteins)
of the nodulation signaling pathway 1 and 2 (NSP 1/2)
Inhibition of polar auxin transport
PGS, PIN
| Cortical cells division, organogenesis nodule
Ca 2* - spiking
Figure 1. Morphogenesis of the nodule involving cytokine and auxin
Auxin
Auxin receptor (TIR1 /AFB)
Transcription Repressor (Aux/IAA)
Auxin signaling
Response regulator genes (RR7, RR15)
Cytokinin signaling -] PINs
(High auxin / Low cytokinin)
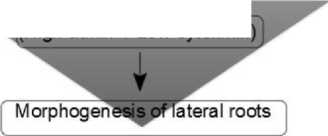
Figure 2. Morphogenesis of lateral roots involving cytokinin and auxin
Rhizobia
Nod-factor NF (rhizobiaI lipochitooligosaccharide)
Receptor-like kinases
Ca 21 - spiking
Ca :" and calmodulin-dependent kinase (CCaMK)
Epidermis
Transcriptional factors ofthe
Synthesis of early noduhne proteins nodulation signaling pathway —> (NSP 1/2) 1 .

Cortex
Epidermal signal ?
Cytokinin —► Transcriptionalprotein NIN (nodule inception)
Cytokinin receptor (CRE1, LNK1)
Transcriptional factors NSP 1/2
Inhibition of polar auxin transport -| Carrier protein (PGS, PIN)
< Auxin
Low auxin I high cytokinin
Cortical cell division, organogenesis nodule
Figure 3. Scheme of the signal transmission from the epidermis into the cortex during the initiation of the root nodule organogenesis
The role of hormones in the formation of nodules was initially based on the results of experiments with a change in the level of cytokinin and the use of inhibitors of auxin transport in the roots (Cooper, Long, 1994). Later, experiments with Lotus japonicum mutants on the cytokinin LHK1 receptor and alfalfa mutants on its CRE1 orthologue confirmed the need for cytokinin to induce the nodule morphogenesis (Tirichine et al ., 2007).
Cytokinin signaling is localized in the internal tissues of the root (cortex, pericycle) and is not associated with the epidermis. The immediate result of cytokinin signaling is the CRE1-dependent change in polar auxin transport which is inhibited by rhizobia and the purified Nod factor (Plet et al., 2011). The inhibition of the auxin polar transport leading to a decrease in the auxin level and the response of the auxin- responsive gene GH3 in the meristem of the nodule are the initial factors of the nodule organogenesis that are observed when root zones are treated with the purified Nod factor or rhizobia (Mathesius et al., 1998). Suppression of cytokinin signaling through the cytokinin receptor CRE1 reduces the number of nodules and increases the number of lateral roots (Gonzalez-Rizzo et al., 2006). While inhibiting auxin signaling, the opposite is true: the number of nodules increases and the formation of lateral roots decreases (Kuppusamy et al., 2009; Champion et al., 2015). Thus, according to Champion et al. (2015), inhibition of auxin signaling by the auxin signal regulator (I-3-AA7) in actinorhizal mutualistic symbiosis leads to an increase in nodulation and N2 fixation in the woody species Casuarina glauca inoculated with the soil nitrogen-fixing bacterium Frankia.
In other experiments, the effect of rhizobial infection on the level of IAA and cytokinins (zeatin + zeatin riboside) in the rhizobia responsive root zone (0-20 mm) of etiolated pea seedlings was studied (Glyan’ko et al., 2007, Akimova, Sokolova, 2012). It was shown that an increase in the content of both IAA and cytokinins was observed a 24-hour period after the inoculation in the studied root zone. Moreover, the activity of enzymes that decompose IAA – peroxidase and IAA oxidase – decreased.These data can probably be explained by the duration of the experiments (1 day) when the rudimentary nodule primordia cells entered the active phase of division and multiplication. Increased doses of auxin and changes in the ratio between hormones are apparently required for these processes. This is confirmed by data that indicate a strong activation of the auxin-responsive gene GH3.1 (Gretchen Hagen3.1) in the meristem of the embryonic nodule (Grunewald et al., 2009). Other data (Penmetsa, Cook, 1997; Olah et al., 2005) indicate the stimulation of the lateral roots formation in legumes with the purified Nod factor. Due to these data, an alternative view on the role of auxin in the initiation of nodule formation is possible. One can talk about the suppressive role of auxin at an early stage of nodule primordium development with the subsequent positive function at the later stages of nodule organogenesis. In connection with this, it is required to study the signaling role of auxin and cytokinin at various stages of nodule development relative to its initial stage – the initiation of nodule primordium formation (Timmers, 2008). On the other hand, there is evidence of the involvement of auxin signaling in the rhizobial infectious process, in particular in the formation of an infection thread in the root hairs (Breakspear et al., 2014; Laplaze et al., 2015). According to some data, auxin signaling can be a part of a mechanism that suppresses plant immunity in pathogenesis as well as in a symbiotic infection (Gourion et al., 2015).
Phytohormones with a negative effect on the processes of nodulation
Protective (stress) plant hormones (ethylene, abscisic, jasmonic and salicylic acids) play an important role in regulating the multiple responses of the epidermal root cells to bacterial invasion including rhizobia (Oldroyd, Downie, 2008). In this case, the invasion of plant pathogens and mutualistic bacteria into cells is carried out in a similar way with overcoming the plant protective systems in which these hormones are involved (Krugova, 2009). The main target of the action of these hormones is to prevent bacterial infection of plant cells and tissues. This also applies to the rhizobial infection which can be suppressed with the participation of these phytohormones. It should be noted that although the negative effect of these hormones on the rhizobial infection has been proven, the mechanisms by which the microsymbiont bypasses the protective systems of the host plant (macrosymbiont) are not fully understood (Gourion et al., 2015). On the other hand, these hormones undoubtedly interact with auxin and cytokinin in planta (Ding, Oldroyd, 2009).
The most studied in this regard is ethylene gas which has an inhibitory effect on the growth of the stem in length and which accelerates the ripening of the fruit. It was found that ethylene during the rhizobial infection inhibits in the epidermis of root hair cells such processes as root hair deformation, Ca2+- spiking formation, early nodulins synthesis expression, infection threads formation, and nitrogen-fixing ability of nodules in a legume (Grobbelaar et al., 1971; Heidstra et al., 1997; Oldroyd et al., 2001; Guinel, Geil, 2002). In experiments with ethylene-responsive alfalfa mutants, hyperinfection of the roots of Sinorhizobium meliloti with the formation of an excess of nodules was shown; it was 10 times greater than in the wild type of alfalfa (Permetsa, Cook, 1997). The inhibitory effect of ethylene on both rhizobial infection and nodulation is removed by inhibitors of ethylene synthesis (Peters, Crist-Estes, 1989). Ethylene has a negative effect on the Nod factor-induced Ca2+ - signaling in the root hairs of alfalfa and inhibits the induction of two genes of noduline proteins (Oldroyd et al., 2001) but does not affect the growth of root hairs in alfalfa seedlings which is also induced by NF-signaling (Heidstra et al., 1997). The conclusion that follows from these results is that ethylene suppresses the separate stages of signal transduction of the rhizobial Nod factor cascade in the epidermis and cortex and thus negatively affects the level of infection and nodulation (Oldroyd et al., 2001). But at the same time, experiments with the roots of the semi-aquatic plant Sesbania rastrata have shown that ethylene and ROS are involved in the NF signaling in the realization of the symbiotic pathway leading to the formation of pre-infectious threads in external cortical cells and the initiation of nodule primordium (D'Haeze et al., 2003). Ethylene inhibits lateral and basipetal auxin transport but exogenous auxin stimulates the synthesis of ethylene (Woodward, Bartel, 2005).
The synergistic effect with respect to ethylene is shown by jasmonic acid (JA) the main effect of which is related to the increase of plant resistance to extreme effects (Kolupaev, Karpets, 2010). In the legume-rhizobial symbiosis, exogenous JA inhibits the transcription of the noduline protein ENOD11 and RIP1, which are induced in epidermal cells in the functioning of NF-signaling. Inhibition of Ca2+-spiking by the action of JA adversely affects infection and nodulation (Sun et al., 2006). However, the activation of protective reactions in the root hairs of alfalfa during the rhizobial infection is rapidly suppressed with the participation of jasmonate and auxin signaling (Breakspear et al ., 2014). Guinel and Geil (2002) in their review emphasize the difficulty in determining the role of ethylene in the processes of symbiotic and mycorrhizal symbiosis due to its multifarious action in plants.
Abscisic acid (ABA) is a stress hormone and a broad-based inhibitor. Especially a lot of it is synthesized in plants under adverse external influences (Shakirova, 2001). In most cases, ABA inhibits plant growth and acts as an antagonizer of auxins, cytokinins, gibberellins, brassinosteroids. As a negative regulator of legume-rhizobial interactions, ABA (along with ethylene and JA) blocks the transduction of the NF-signal which appears in disorders of root hairs deformation, infection threads formation, Ca2+-spiking, early noduline proteins synthesis and the number of nodules formed (Suzuki et al., 2004; Ding et al., 2008). However, in spite of the similarity of the action of ABA and ethylene in establishing the legume-rhizobial symbiosis, their influence on nodulation can be independent (Ding et al., 2008; Ding, Oldroyd, 2009). Thus, with suppressed ABA signaling in alfalfa, there is an increased induction of the ENOD11 gene in the epidermal cells and an increased level of nodule formation (Ding et al., 2008).
ABA is a cytokinin antagonist: unlike cytokinin which initiates nodule primordium formation in the cortex ABA promotes primordium formation of lateral roots in pericycle indicating a certain ratio of ABA / cytokinin for these processes (Suzuki et al ., 2004; Liang, Harris, 2005; Ding et al ., 2008). In general, ABA is a hormone that functions both in epidermal and cortical root cells. It regulates Nod factor signaling in the epidermis and cytokinin signaling in the cortex. According to Ding et al. (2008), ABA modulation can determine the nature of processes in the epidermal (acting on Nod factor signaling) and in cortical cells (acting on cytokinin signaling). In other words: the formation of nodules or lateral roots depends on the balance of cytokinin, auxin and ABA in cortex and pericycle cells. According to some data, exogenous ABA inhibits the formation of lateral roots in Arabidopsis (De Smet et al., 2003) and reduces the level of free auxin in melon flowers (Dunlap, Robacker, 1990).
Systemic acquired resistance in plants is formed with the participation of salicylic acid (SA) (Molodchenkova, 2001, 2008; Kolupaev, Karpets, 2010). The possibility of the participation of this phytohormone in the legume-rhizobial symbiosis was studied by several authors (Martinez-Abarka et al ., 1998; Blilou et al ., 1999; Garcia-Garrido, Ocampo, 2002; Glyan’ko et al ., 2005; Stacey et al . 2006). It was shown that SA which was exogenously given to the legumes negatively influenced the formation and functioning of the legume-rhizobial symbiosis (Martinez-Abarka et al ., 1998). But at the same time it was found that under normal physiological conditions inoculation of pea seedling with rhizobia helps reduce the content of SA in the roots (Glyan’ko et al., 2005).
Proceeding from the fact that SA is a component of the immune system of plants and the invasion of rhizobia into root tissues is associated with the suppression of protective reactions, this fact is quite understandable from such positions. However, the mechanism of the effect of rhizobia on the level of SA is not known but it can be assumed that this mechanism is also associated with Nod factor signaling (Blilou et al ., 1999; Stacey et al ., 2006). Rhizobia undoubtedly modulate the protective system of a symbiotic plant by including or suppressing some protective reactions associated with the action of phytohormones (Naseem et al ., 2015). But nevertheless, the question is unsettled: how do these 4 hormones negatively affecting the formation of LRS integrate together to suppress Nod factor signaling? (Ding, Oldroud, 2009).
CONCLUSIONS
The experimental data obtained indicate that cytokinin and auxin are key regulators in the initiation of the nodule organogenesis in the root cortex. However, unlike the formation of the root apical meristem or meristem of the lateral root, a high level of cytokinin and a low level of auxin is required to initiate the nodule. Modulation of these hormones occurs under the initial influence of rhizobial Nod factors which are activated on the plasmalemma of epidermal root cells by receptor-like kinases (RLK). The subsequent cascade of reactions including, in particular, the activation of plant signal systems, the expression of symbiotic genes, the synthesis of early noduline proteins (ENODs) and transcription factor proteins (NIN, NSP1/2), leads to the infection of root tissues with rhizobia and root nodules formation (Fig.1). The positive or negative influence of other phytohormones in nodule formation has been proved but the mechanisms of their influence are still unclear.
As it was already noted, some rhizobia species (eg, Bradyrhizobium) do not need Nod factors to initiate nodule morphogenesis. That seems to indicate the direct regulation of the level of cytokinin in the cells of the host plant. However, most rhizobia use the Nod factor as an evolutionarily fixed way of symbiotic structures formation. There are questions: how does the perception of the rhizobial Nod factor by the epidermal cells of the root hair lead to the accumulation of cytokinin and the subsequent nodule primordium formation in cortex cells; how does the Nod factor coordinate two developing processes in two different tissues – the epidermis and the cortex? It is possible that the coordination of reactions in the epidermis and cortex during nodulation can be associated with not yet known signals that regulate the hormones flux. In this respect, flavonoids as well as other biologically active compounds (for example, NO, ROS) local accumulation or reduction of which in the root can change the balance of hormones initiating lateral roots formation in the pericycle or nodules in the cortex are of interest. The role of a mobile signal between the cells of the epidermis, cortex and pericycle is given to ABA (Ding, Oldrayd, 2009). The concentration, ratio and physiological role of these hormones undoubtedly vary at different stages of the nodule meristem formation (Libbenga et al., 1973). On the other hand, the mechanisms of the negative influence of protective and stress hormones on the nodules formation are not completely understood. In this regard, it is assumed that there is the basis for treating ROS as key mediators in hormonal regulation of NF-signaling transduction (Ding, Oldroyd, 2009) since all these phytohormones directly or indirectly increase the production of ROS (Schaw, Long, 2003, Ramu et al., 2002, Oldroyd, Downie, 2004). Attention should be paid to the interaction of ethylene as an inhibitor of rhizobial invasion in root hairs with auxin and cytokinin that affects the concentration and ratio of these hormones (Liu et al., 2015). There are a sufficient number of scientific facts in support of these assumptions but the mechanisms of their action are insufficiently disclosed.
At present, the study of the gene regulation of the auxin response that causes gene expression and as a result of changes in various physiological processes under the influence of auxin is of great interest (Gray et al., 2001). As it was already noted, the auxin receptors (F-box proteins): TIR1 (Transport Inhibitor Response 1) and AFB (Auxin Signaling F-Box) as well as transcriptional repressors Aux / IAA proteins (Auxin / Indole-3-Acetic Acid) were marked in Arabidopsis. The binding of the auxin to the receptor promotes the inactivation of the repressor and the expression of the corresponding genes (Terrili et al., 2012). With regard to the rhizobial infection, these issues have not been studied.
ACKNOWLEDGEMENT
The author is grateful to Ishenina A.S. for the translation of the article into English.
Список литературы Phytohormones and morphogenesis of root nodules and lateral roots of a legume plant
- Adamowski M., Friml J. (2015) PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell. 27, 20-32
- Akimova G.P., Sokolova M.G. (2012) Cytokinin content during early stages of legume-rhizobium symbiosis and effect of hypothermia. Russ. J. Plant Physiol. 59, 656-661
- Bauer P., Ratet P., Crespi M.D., Kondorosi A. (1996) Nod factors and cytokinins induce similar cortical cell division, amyloplast deposition and MsEnod12A expression patterns in alfalfa roots. Plant J. 10, 91-106
- Blilou I., Ocampo J., Garcia-Garrido J. M. (1999) Resistance of pea roots to endomycorrhizal fungus or Rhizobium correlates with enhanced levels of endogenous salicylic acid. J. Exp. Bot. 50, 1663-1668
- Breakspear A., Liu C., Roy S., Stacey N., Rogers C., Trick M., Morien G., Mysore K.S., Wen J., Olroyd G.E.D., Downie J.A., Murray J. D. (2014) The root hair "Infectome" of Medicago trucatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell. 26, 4680-4701
- Сhandler J.W., Werr W. (2015) Cytokinin-auxin crosstalk in cell type specification. Trends Plant Sci. 20, 292-300
- Cooper J.B., Long S.R. (1994) Morphogenetic rescue of Rhizobium meliloti nodulation mutants by trans-zeatin secretion. Plant Cell. 6, 215-225
- Champion A., Lucas M., Tromas A., Vaissayre V., Crabos A., Diedhiou I., Prodjinoto H., Moukouanga D., Pirolles E., Cissoko M., Bonneau J., Gherbi H., Franche C., Hocher V., Svistoonoff S., Laplaze L. (2015) Inhibition of auxin signaling in Frankia species-infected cells in Casuarina glauka nodules leads to increased nodulation. Plant Physiol. 167, 1149-1157
- D’Haeze W., De Rycke R., Mathis R., Goormachtig S., Pagnotta S., Verplancke C., Capoen W., Holsters M. (2003) Reactive oxygen species and ethylene play appositive role in lateral root base nodulation of a semiaquatic legume. Proc. Natl. Acad. Sci. USA. 100, 11789-11794
- De Long A., Mockaitis K., Christensen S. (2002) Protein phosphorylation in the delivery of and response to auxin signals. Plant Mol. Biol. 49, 285-303
- De Smet I., Signora L., Beekman T., Inze D., Foyer C.H., Zhang H. (2003) An abscisic acid -sensitive checkpoint in lateral root development of Arabidopsis. Plant J. 39, 543-555
- Ding Y.L., Kalo P., Yendrek C., Sun J.H., Liang Y., Marsh J.F., Harris J.M., Oldroyd G.E.D. (2008)) Abscisic acid coordinates Nod factor and cytokinin signaling during the regulation of nodulation in Medicago trancatula. Plant Cell. 20, 2681-2685
- Ding Y., Oldroyd G.E.D. (2009) Positioning the nodule, the hormone dictum. Plant Signaling Behavior. 4, 89-93
- Downie J.A. (2007) Infectious heresy. Science. 316, 1296-1297
- Dunlap J.R., Robacker K.M. (1990) Abscisic acid alters the metabolism of indole-3-acetic acid in senescing flowers of Cucumis melo. Plant Physiol. 94, 870-874
- Fang Y., Hirsh A.M. (1998) Studying early nodulin gene ENOD40 expression and induction by nodulation factor and cytokinin in transgenic alfalfa. Plant Physiol. 116, 53-68
- Ferreira F.J., Kieber J.J. (2005) Cytokinin signaling. Curr. Opin. Plant Biol. 8, 518-525
- Ferguson B.J., Ross J.J., Reid J.B. (2005) Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiol. 138, 2396-2405
- Ferguson B.J., Indrasumunar A., Hayashi S., Lin Y-H., Reid D.E., Gresshoff P.M. (2010) Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 52, 61-76
- Fernandez-Marcos M., Sanza L., Lewis D.R., Muday G.K., Lorenzo O. (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-Formed 1 (PIN1)-dependent acropetal auxin transport. Proc. Natl. Acad. Sci. USA. 108, 18506-18511
- Gage D.J., Margolin W. (2000) Hanging by a thread: invasion of legume plants by rhizobia. Curr. Opin. Microbiol. 3, 613-617
- Garcia-Garrido J.M., Ocampo J.A. (2002) Regulation of the plant defense response in arbuscular mycorrhizal symbiosis. J. Exp. Bot. 53, 1377-1386
- Giraud E., Moulin L., Vallenet D., Barbe V., Cytryn E., Sadowsky M. et al. (2007) Legumes symbioses: absence of Nod genes in photosynthetic Bradyrhizobia. Science. 316, 1307-1312
- Glyan’ko A.K., Makarova L.E., Vasil’eva G.G., Mironova N.V. (2005) Possible involvement of hydrogen peroxide and salicylic acid in the legume-Rhizobium symbiosis. Biology Bulletin. 32, 245-249
- Glyan’ko A.K., Akimova G.P., Sokolova M.G., Makarova L.E., Vasil’eva G.G. (2007) The defense and regulatory mechanisms during development of legume-Rhizobium symbiosis. Applied Biochem. Microbiol. 43, 260-267
- Glyan’ko A.K. (2014) Significance of Nod factors Rhizobium in induction of signaling systems of formation of legume-Rhizobium symbiosis (Znachenie Nod factora Rhizobium v indukcii signal’nykh system pri obrasovanii bobovo-rizobial’nogo simbioza).The Bulletin Кharkiv National Agrarian University. Series Biology. 3 (33), 6-14. (Вicн. Харкiв. нац. аграрн. ун-ту. Серiя Бiологiя) (in Ukrainian)
- Gonzalez-Rizzo S., Crespi M. and Frugier F. (2006) The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell. 18, 2680-2693
- Gourion B., Berrabah F., Ratet P., Stacey G. (2015) Rhizobium-legume symbioses: the crucial role of plant immunity. Trends Plant Sci. 20, 186-194
- Gray W.M., Kepinski S., Rouse D., Leyser O., Estelle M. (2001) Auxin regulates SCF (TIR1)-dependent degradation of AUX/IAA proteins. Nature. 414, 271-276
- Grobbelaar N., Clarke B., Hough M.C. (1971) The nodulation and nitrogen fixation of isolated roots of Phaseolus vulgaris L. III. The effect of carbon dioxide and ethylene. Plant Soil, 35, 215-223
- Guinel F.C., Geil R.D. (2002) A model for the development of the rhizobial and arbuscular mycorrhizal symbioses in legumes and its use to understand the roles of ethylene in the establishment of these two symbioses. Can. J. Bot. 80, 695-720
- Heidstra R., Yang W.C., Yalcin Y., Peck S., Emons A.M., van Kammen A., Bisseling T. (1997) Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development. 124, 1781-1787
- Hirsch A.M., Bhuvaneswari T.V., Torrey J.G., Bisseling T. (1989) Early nodule genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc. Natl. Acad. Sci. USA. 86, 1244-1248
- Hirsch A.M. (1992) Developmental biology of legume nodulation. New Phytol. 122, 211-237
- Hirsch A.M., Fang Y. (1994) Plant hormones and nodulation: what’s the connection? Plant Mol. Biol. 26, 5-9
- Hirsch A.M., Fang Y., Asad S., Kapulnik Y. (1997) The role phytohormones in plant-microbe symbioses. Plant Soil. 194, 171-184
- Ioio R.D., Linhares F.S., Scacchi E., Casamitjana-Martinez E., Heidstra R., Costantino P., Sabatini S. (2007) Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 17, 678-682
- Jacobs M., Rubery P. H. (1988) Naturally occurring auxin transport regulators. Science. 241, 346-349
- Kiseleva A.A., Tarachovskaya E.R., Shishova M.F. (2012) Biosynthesis of phytohormones in alga. Russ. J. Plant Physiol. 59, 595-610
- Kim S.K., Chang S.C., Lee E.J., Chung W.S., Kim Y.S., Hwang S., Lee J.S. (2000) Involvement of brassinosteroids in the gravitropic response of primary root of maize. Plant Physiol. 123, 997-1004
- Kolupaev Yu.Ye., Karpets Yu.V. (2010) Formation of plants adaptive reactions to abiotic stressors influence (Formirovanie adaptivnykh reakcij rastenij na dejstvie abioticheskikh stressorov). Kiev, Osnova, 350 p. (in Ukrainian)
- Krugova E.D. (2009) Specific strategies of nodule and phytopathogenic bacteria in plant infection. Physiology and Biochemistry Cult. Plants (Fiziologiya i biokhimiya kulturnykh rastenij). 41, 3-15 (in Ukrainian)
- Kulaeva O.N. (1973) Citokinins, their structure and function (Citokinins, ikh struktura i funkciya). Moskva: Nauka, 264 p.
- Kuppusamy K.T., Ivashuta S., Bucciarelli B., Vance C.P., Gantt J.S., VandenBosch K.A. (2009) Knockdown of cell division cycle16 reveals an inverse relationship between lateral root and nodule numbers and a link to auxin in Medicago truncatula. Plant Physiol. 151, 1155-1166
- Laplaze L., Lucas M., Champion A. (2015) Rhizobial root hair infection requires auxin signaling. Trends Plant Sci. 20, 332-334
- Liang Y., Mitchell D.M., Harris J.M. (2007) Abscisic acid rescues the root meristem defects of the Medicago truncatula lard mutant. Dev. Biol. 304, 297-307
- Leyser O. (2006) Dynamic integration of auxin transport and signaling. Curr. Biol. 16, 424-433
- Libbenga K.R., van Iren F., Bogera R.J., Schraag-Lamera M.F. (1973) The role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativum L. Planta. 114, 29-39
- Liu C-W., Breakspear A., Roy S., Murray J.D. (2015) Cytokinin responses counterpoint auxin signaling during rhizobial infection. Plant Signal. Behavior. 10, e1019982
- Liu W-Z, Kong D-D., Gu X-X., Gao H-B., Wang J-Z., Xia M., Gao Q., Tian L-L., Xu Z-H., Bao F., Hu Y., Ye N-S., Pei Z-M., He Y-K. (2013) Cytokinins can act as suppressors of nitric oxide in Arabidopsis. Proc. Natl. Acad. Sci. USA. 110, 1548-1553
- Ljung K., Bhalerao R.P., Sandberg G. (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 21, 465-474
- Lohar D.P., Schaff J.E., Laskey J.G., Kieber J.J., Bilyeu K.D., Bird D.M. (2004) Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant J. 38, 203-214
- Lomax T.L., Muday G.K., Rubery P.H. (1995) Auxin transport. In: Plant hormones. Dordrecht: Kluwer, pp. 509-530
- Martinez-Abarka F., Herrera-Cervera J.A., Bueno P., Sanjuan J., Bisseling T., Olivares J. (1998) Involvement of salicylic acid in the establishment of the Rhizobium meliloti-alfalfa symbiosis. Mol. Plant-Microbe Interac. 11, 153-155
- Mathesius U., Schlaman H.R.M., Spaink H.P., Sautter C., Rolfe B.G., Djordjevic M.A. (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 14, 23-34
- Mathesius U., Charon C., Rolfe B.G., Kondorosi A., Crespi M. (2000) Temporal and spatial order of events during the induction of cortical cell divisions in white clover by Rhizobium leguminosarum bv. trifolii inoculation or localized cytokinin addition. Mol. Plant-Microbe Interac. 13, 617-628
- Mathesius U. (2001) Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J. Exp. Bot. 52, 419-426
- Moubayidin L., Mambro R., Sabatini S. (2009) Cytokinin-auxin crosstalk. Trends Plant Sci. 14, 557-562
- Muller B., Sheen J. (2008) Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature.453, 1094-1097
- Mulligan J.T., Long S.K. (1989) A family of activator genes regulates expression of Rhizobium meliloti nodulation genes. Genetics. 122, 7-18
- Molodchenkova O.O. (2001) Assumed functions of salicylic acid in plants. Physiology and Biochemistry Cult. Plants (Fiziologiya i biokhimiya kulturnykh rastenij). 33, 463-473 (in Ukrainian)
- Molodchenkova O.O. (2008) Influence of salicylic acid on the response of corn seedlings under abiotic stress (Vliyanie salicylic kisloth na otvetnye reakcii prorostkov kukuruzy pri abioticheskih stressah). The Bulletin Kharkiv National Agrarian University. Series Biology. 3 (15), 24-32 (Вicн. Харкiв. нац. аграрн. ун-ту. Серiя Бiологiя) (in Ukrainian)
- Murray J.D., Karas B.J., Sato S., Tabata S., Amyot L., Sczczyglowski K. (2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science.315, 101-104
- Murray J.D. (2011) Invasion by invitation: rhizobial infection in legumes. Mol. Plant-Microbe Interac. 24, 631-639
- Naseem M., Kaltdorf M., Dandekar T. (2015) The nexus between growth and defense signaling: auxin and cytokinin modulate plant immune response pathways. J. Exp. Bot. 66, 4885-4896
- Nomura T., Nakayama M., Reid J.B., Takeuchi Y., Yokota T. (1997) Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol. 113, 31-37
- Nordström A., Tarkowski P., Tarkowska D., Norbaek R., Astot C., Dolezal K., Sanberg G. (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin -regulated development. Proc. Natl. Acad. Sci. USA. 101, 8039-8044
- Olah B., Briere C., Becard G., Denarie J., Gough C. (2005) Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula. Plant J. 44, 195-207
- Oldroyd G.E., Engstrom E.M., Long S.R. (2001) Ethylene inhibits the Nod factor signal transduction pathway of Medicago trancatula. Plant Cell. 13, 1835-1849
- Oldroyd G.E., Downie J.A. (2004) Calcium, kinases and nodulation signaling in legumes. Nat. Rev. Mol. Cell Biol. 5, 566-576
- Oldroyd G.E. (2007) Nodules and hormones. Science. 315, 52-53
- Oldroyd G.E., Downie J.A. (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 59, 519-546
- Oldroyd G.E., Murray J.D., Poole P.S., Downie J.A. (2011) The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 45, 119-144
- Penmetsa R.V., Cook D. R. (1997) A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 275, 527-530
- Peters N.K., Crist-Estes D.K. (1989). Nodule formation is stimulated by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 91, 690-693
- Peer W.A., Murphy A.S. (2007) Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 12, 556-563
- Plet J., Wasson A., Ariel F., Le Signor C., Baker D. (2011) MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago trancatula. Plant J. 65, 622-633
- Polevoy V.V. (1982) Phytohormones (Fitogormony). Leningrad: Izdatel’stvo LGU, 248 p.
- Ramu S.K., Peng H.M., Cook D.R. (2002) Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago trancatula. Mol. Plant Microbe Interac. 15, 522-528
- Ross J.J., O’Neill D.P., Smith J.J., Kerckhoffs L.H.J., Elliott R.C. (2000) Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J. 21, 547-552
- Shakirova F.M. (2001) Non-specific resistance of plants to stress factors and its regulation (Nespecificheskaya ustojchivost’ pastenij k stressovym faktoram i ee pegulyaciya). Ufa, Gilem, 160 p.
- Sakakibara H. (2005) Cytokinin biosynthesis and regulation. Vitam. Horm. 72, 271-287
- Shaw S.L., Long S.R. (2003) Nod factor inhibition of reactive oxygen efflux in a host legume. Plant Physiol. 132, 2196-2204
- Skoog F., Miller C.O. (1957) Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symp. Soc. Exp. Biol. 54, 118-131
- Stacey G., McAlvin C.B., Sung-Yong Kim, Olivares J., Sato M.J. (2006) Effects of endogenous salicylic acid on nodulation in the model legumes Lotus japonicus and Medicago truncatula. Plant Physiol. 141, 1473-1481
- Sun J., Cardoza V., Mitchell D.M., Bright L., Olroyd G., Harris J.M. (2006) Crosstalk between jasmonic acid, ethylene and Nod factor signaling allows integration of diverse inputs for regulation of nodulation. Plant J. 46, 961-970
- Suzuki A., Akune M., Kogiso M., Imagama Y., Osuki K., Uchium T., Higashi S., Han S-Y., Yoshida S., Asami T., Abe M. (2004) Control of nodule number by the phytohormone abscisic acid in the roots of two leguminous species. Plant Cell Physiol. 45, 914-922
- Terrile M.C., Paris R., Calderon L.I.A., Inglesias M.J., Lamattina L., Estelle M., Casalongue C.A. (2012) Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidipsis transport inhibitor Response1 auxin receptor. Plant J. 70, 492-500
- Thimann K.V. (1936) On the physiology of the formation of nodules on legume roots. Proc. Natl. Acad. Sci. USA. 22, 511-514
- Timmers A.C.J. (2008) The role of the plant cytoskeleton in the interaction between legumes and rhizobia. J. Microsc. 231, 247-256
- Tirichine L., Sandal N., Madsen L.H., Radutoiu S., Albrektsen A.S. (2007) A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 315, 104-107
- Truchet G., Barker D.G., Camut S., de Billy F., Vasse J., Huguet T. (1989) Alfalfa nodulation in the absence of Rhizobium. Mol. Gen. Genet. 219, 65-68
- Wasson A.P., Pellerone F.I., Mathesius U. (2006) Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell. 18, 1617-1629
- Werner T., Motyka V., Laucou V., Smets R., Van Onckelen H., Schmuelling T. (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 15, 2532-2550
- Woodward A.W., Bartel B. (2005) Auxin: regulation, action, and interaction. Ann. Bot. 95, 707-735
- Zhang J., Subramanian S., Stacey G., Yu O. (2009) Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 57, 171-183
- Zhao Y. (2010) Auxin biosynthesis and its role in plant development. Annu. Rev.Plant Biol. 61, 49-64


