Получение авермектинов: биотехнологии и органический синтез
Автор: Джафаров М.Х., Василевич Ф.И., Мирзаев М.Н.
Журнал: Сельскохозяйственная биология @agrobiology
Рубрика: Обзоры
Статья в выпуске: 2 т.54, 2019 года.
Бесплатный доступ
В предлагаемом обзоре проанализированы результаты исследований по различным аспектам совершенствования технологии получения авермектинов - 16-членных макроциклических лактонов, обладающих широким спектром противопаразитарного действия при высоком терапевтическом индексе и безвредности для млекопитающих (W.C. Campbell, 2012). Согласно опубликованным данным, уникальная способность авермектинов подавлять развитие насекомых, нематод и клещей связана с возможностью блокировать передачу нервного импульса в нервно-мышечном синапсе. Сущность этого механизма действия, приводящего к параличу и гибели паразитов, заключается в стимуляции выброса ионов хлора, деполяризации мембраны клеток и патологическом нарушении ее функций (A.J. Wolstenholme с соавт., 2016). Из известных 8 компонентов (А1a, А1b, А2а, А2b, B1a, B1b, В2a и В2b) авермектинового комплекса, продуцируемого микроорганизмом Streptomyces avermitilis , наиболее активны против возбудителей паразитозов авермектины группы В1 (S...
Авермектины, мильбемицины, немадектины, дорамектин, абамектин, ивермектин, моксидектин, оксим мильбемицина, 5-о-сукциноилавермектин в1, соединение с2017, оксимы авермектинов, органический синтез, антипаразитарные препараты, нематоциды, инсектоакарициды
Короткий адрес: https://sciup.org/142220097
IDR: 142220097 | УДК: 619+61]:615.28 | DOI: 10.15389/agrobiology.2019.2.199rus
Текст обзорной статьи Получение авермектинов: биотехнологии и органический синтез
Авермектины (16-членные макролиды, продуцируемые Streptomyces avermitilis ) (1, 2) обладают широким спектром нематицидного и инсекто-акарицидного действия и уже более 35 лет успешно применяются в терапии и профилактике паразитарных болезней человека, животных, в защите растений (3-7). Ежегодный объем продаж авермектиновых субстанций превышает 850 млн USD (8, 9). Интегральное антипаразитарное действие субстанций этого класса определяется их способностью взаимодействовать с глутаматзависимыми (основная мишень) Сl - -ионными каналами, специфичными для беспозвоночных животных (10), и ГАМКА ( γ -аминомасляная кислота)-зависимыми рецепторами (11). Кроме того, авермектины имеют
Работа выполнена при финансовой поддержке РНФ (Соглашение ¹ 15-16-00019).
аффинность к разным ионным каналам и рецепторам из Cys-loop-супер-семейства, P2X4 и фармезоидным рецепторам, G-белок-связанным калиевым каналам внутреннего выпрямления (G protein-coupled inwardly-rectifying potassium channels, GIRK receptors) и другим, что имеет фармакотерапев-тические перспективы (12, 13). У представителя авермектинов ивермектина выявлена способность блокировать PAK1-зависимый рост клеток доброкачественных и злокачественных новообразований (14, 15). Противоопухолевое действие имеют и другие представители 16-членных антипара-зитарных макролидов (16-19). Недавно обнаружено ингибирование репликации вируса желтой лихорадки (20) и спорогонии у Plasmodium falciparum в Anopheles gambiae (21) ивермектином, антитуберкулезное действие авермектинов (22), снижение авермектинами поглощения этанола клетками (23), лечебное действие ивермектина при экспериментальных патологических состояниях, например ремиелинизация при аутоиммунном энцефалите в результате аллостерической активации и восстановления нарушенных функций АТФ-зависимых (пуринэргических) ионофорных рецепторов P2X4Rs (24-27).
В представляемом обзоре основное внимание уделено способам получения природных авермектинов и их полусинтетических производных.
Технологии получения авермектинов традиционно (2) предполагают получение высокопродуктивных штаммов, синтезирующих предпочтительно авермектины В1, оптимизацию питательных сред для культивирования продуцента и производство полусинтетических аналогов авермектинов В1 с улучшенными физико-химическими и фармакологическими свойствами (28-30). В последние годы развивается еще одно направление — синтез желаемых продуктов (например, ивермектина, мильбемицинов) методами синтетической биологии (31-34). В 1980-1990-х годах выполнялись исследования по полному химическому синтезу некоторых авермектинов — В1а и А1а (35), однако предложенные схемы включали много стадий при низком выходе целевого продукта (не более 0,08 %), что свидетельствует о преимуществе микробиологического способа получения субстанций этого класса. В настоящее время ведутся исследования по разработке эффективных методик полного химического синтеза авермектинов (36-39).
Селекция продуцентов, микробиологический синтез, биотехнологии. Основное направление модернизации продуцента авермектинов Streptomyces avermitilis (ex Burg et al. 1979) Kim and Goodfellow, 2002 (40) — получение высокопроизводительных штаммов, образующих авермектиновый комплекс или какой-либо его компонент, в основном В1, с подавленным синтезом олигомицинов, отрицательно влияющих на рост и развитие продуцента. Современные промышленные штаммы генеалогически происходят от образцов дикого типа S. avermitilis MA-4680 (штамм NRRL 8165; NCIMB 12804; , японского изолята из почвы, обладающего антигельминтным действием. Этот штамм депонирован под разными номерами в коллекциях микроорганизмов некоторых стран (АТСС 31267, ВКМ Ac-1301 и др.) (41). Прародителем российских продуцентов авермектинов служит штамм ВКМ Ac-1301 из Всероссийской коллекции микроорганизмов (42, 43). В дальнейшем проводили отбор спонтанных и индуцированных физическими (УФ-, рентгеновское излучения и др.) и химическими (азотистый иприт, метилметанолсульфонат и др.) агентами мутантов, а также усовершенствование продуцента методами генной инженерии (44, 45). Один из таких штаммов — производный S. avermitilis MA-4848, продуцирующий восемь известных авермектинов, получен в США посредством УФ-мутагенеза с 200
использованием лиофилизированной сузпензии родительского штамма MA-4680 (АТСС 31267) и оптимизации состава питательной среды и условий культивирования. В результате выход авермектинового комплекса увеличился с 9 до 500 мкг/мл с относительным содержанием В1 около 35 %. Эта композиция, получившая название С-076, обладает нематоцидным, акарицидным и инсектицидным действием. Штамм MA-4848 в лиофилизированной и замороженной формах депонирован под названиями соответственно АТСС 31271 и АТСС 31272 (патент US 4285963; 1981), в дальнейшем его производительность была повышена до > 9000 мкг/мл при содержании авермектина В1 до 95 % и более (33, 46, 47). В России также получены штаммы S. avermitilis , производящие полный 8-компоненентный авермектиновый комплекс (А1a, А1b, А2а, А2b, B1a, B1b, В2a и В2b) с высокой биоцидной активностью (48, 49). Продуктивность штамма S. aver-mitilis ВНИИСХМ 56 по авермектиновому комплексу в среднем 500 мкг/мл, на долю группы B (В1 + В2) приходится до 50-70 % (патенты РФ ¹ 2087535, ¹ 2125609). Полученные при селекции продуценты не синтезировали токсичный олигомицин, который содержался в значительном количестве в экстракте мицелия исходного штамма ВКМ Ас-1301. Первый отечественный препарат Аверсект-1 (МГП «Бифидум» при НПО «Биотехнология», г. Москва), зарегистрированный Главным управлением ветеринарии МСХ РФ (1992 год), включал авермектины штаммов S. avermitilis 198 (ВНИИСХМ 50) и ВНИИСХМ 51, отобранных при ступенчатой селекцией из S. avermitilis ВКМ Ас 1301 (патент РФ ¹ 2087535). Из этих штаммов при отборе получили ВНИИСХМ 54 (патент РФ ¹ 2054483) и ВНИИСХМ 56 (патент РФ ¹ 2087535) с производительностью 400-500 мкг/мл. Упомянутые штаммы составили основу для отбора более активных продуцентов и продолжают использоваться. В частности, при направленной селекции штамма S . avermitilis ВНИИСХМ 54 через ряд промежуточных вариантов получен S. avermitilis ССМ 4697 (выход авермектинов до 2300 мкг/мл, относительное содержание компонента В1 около 50 %; патент РФ ¹ 2156301). У штамма НИЦБ 132 (патент РФ ¹ 2147320) биосинтез авермектинов не менее 3500 мкг/мл, в том числе В1 — 1500 мкг/мл с содержанием В1а около 80 %. Учеными из Украины и Белоруссии выделены продуценты авермектинов S . avermitilis УКМ Ас-2179 и S . avermitilis X-1 (50, 51). Сообщалось о S. avermitilis — продуцентах натуральных (обычно так называют авермектины из состава С-076) и ненатуральных авермектинов на основе рекомбинантных штаммов (патент РФ ¹ 2096462). Как было обнаружено, биосинтез авермектинов у S. avermitilis УКМ Ас-2179 резко усиливается в присутствии пирувата, L-треонина или L-метионина, при этом в культуральной жидкости накапливаются также аминокислоты, липиды, фитогормоны (52), что согласуется с ранее полученными данными (53) и может быть использовано при создании безотходной технологии биосинтеза авермектинов.
При селекции высокоактивных продуцентов применялось мутагенное воздействие на споры стрептомицетов короткоимпульсным рентгеновским излучением с энергией квантов 80-160 КэВ (патент РФ ¹ 2074256), УФ-облучение, азотистую кислоту, N-метил-N ″ -нитро-N-нитрозогуанидин, этилметансульфонат, другие традиционные и новые мутагены (54, 55).
В промышленном производстве препаратов на основе авермектинов в настоящее время используются штаммы S. avermitilis — G8-17, SA-01, AV-LP, A-144, A-178, NA-108 (Китай), продуцирующие абамектин, ВКПМ S-1440, ВНИИСХМ 56 (Россия), синтезирующие известный авермектиновый комплекс, и др. Таким образом, при весьма интенсивной селекционной работе с продуцентами авермектинов практическое применение нашли лишь единичные штаммы.
Результатом разработок российской технологии биосинтеза авермектинов стало создание и применение препарата Аверсект-1 (ТУ 10.07090-92. Аверсект-1) на основе авермектинового комплекса (штамм S . avermitilis ВНИИСХМ 51, патент РФ ¹ 2048520). Отличительная особенность биосинтеза авермектинов — их накопление в биомассе стрептомицетов, а не выделение в среду. Если не контролировать процесс, особенно на стадии, когда начинается лизис мицелия, возможны потери целевого продукта (56).
Типичная технология получения авермектинового комплекса, отработанная для отечественных штаммов S. avermitilis ВНИИСХМ 50, ВНИ-ИСХМ 51 и ВНИИСХМ 56, предусматривает культивирование в качалоч-ных колбах (250 и 750 мл), а также в ферментерах (250 л) (56). Традиционную технологию усовершенствуют, направленно изменяя геном штаммов (45-47) или внося в среду компоненты, влияющие на метаболизм продуцента (52, 57-59). Так, в присутствии антиметаболита синефунгина, ингибирующего превращение авермектинов B в авермектины A, доля авермектинов B, продуцируемых культурой S. avermitilis NRRL 8165, от их общего количества достигала 77 % (60, 61). Регуляторная роль аминокислот в биосинтезе авермектинов и изменении соотношения компонентов В и А в авермектиновом комплексе показана при культивировании ряда штаммов S. avermitilis (62, 63). Биосинтез, выделение и очистка авермектинов описаны в ряде работ: концентрат продукта экстрагируют органическим растворителем, не смешивающимся с водой (например, этилацетатом), или смесью растворителей, состоящей из воды и низко- (этанол или пропанол) и высококипящих (например, ПЭГ-200) растворителей, смешивающихся с водой без ограничений (64, 65). Основные принципы биосинтеза авермектинов установлены с помощью изотопных методов и мутагенеза (64).
Проведено клонирование генов авермектинового комплекса и секвенирование генома S. avermitilis, что позволило, в частности, предсказать (с последующим экспериментальным подтверждением) биосинтез других вторичных метаболитов (например, полиенового макролида филипина III) (66). Размер генома S. avermitilis — 9025608 п.н., он содержит не менее 7582 потенциальных открытых рамок считывания и 38 кластеров генов биосинтеза вторичных метаболитов (67). Биосинтез авермектинов детерминирован 17 генами. Четыре из них ( aveA1-aveA4 ) кодируют мультифункци-ональные белковые субъединицы (АveA1-АveA4), состоящие соответственно из 3973, 6239, 5532 и 4681 аминокислотного остатка и образующие авермектиновый поликетидсинтазный комплекс (48). AveA — это поликетид-синтаза I типа, состоящая из 12 модулей (48). Ферменты AveBI-AveBVIII (соответственно гликозилтрансераза, тимидилилтрансфераза, ТДФ-4-кето-6-дезокси-L-гексоз-3-кеторедуктаза, ТДФ-4-кетогексулоз-редуктаза, ТДФ-ТДФ-4-кето-6-дезоксиглюкоз-3-эпимераза, ТДФ-4-кето-6-дезокси-глюкоз-2,3-де-гидратаза, ТДФ-6-дезокси-L-гексоз-3-О-метилтрансфераза, ТДФ-4-кето-6-дезокси-L-гексоз-3-кеторедуктаза) (33) осуществляют синтез дисахарида L-олеандрозы из D-глюкозо-6-фоcфата и присоединение к агликону, AveE и AveF — формирование фуранового цикла, остальные участвуют в образовании спирокетального фрагмента (AveC), 5-О-метилировании (AveD); AveR — фактор положительной регуляции биосинтеза (64, 67).
При образовании авермектинов происходит биосинтез мономерных структурных единиц, используемых в поликетидном синтезе авермектинов, сборка предшественника пентациклического структурного каркаса авермектинов — тридекакетида по поликетидному механизму и постполикетидные превращения (1). Последние включают превращение тридекакетида в ин- термедиат авермектина с 16-членным лактонным кольцом — 6,8а-секо-6,8а-дезокси-5-оксоавермектин; преобразование 6,8а-секо-6,8а-дезокси-5-оксоавермектина в авермектиновый агликон (при окислительной циклизации, восстановлении и/или метилировании); синтез модифицированной L-олеандрозы; гликозилирование агликона дезокситимидин-дифосфат-L-олеандрозой (dTDP-L-Ole) с образованием авермектинов (64).
На стадии инициации вначале происходят биохимические события по настройке (зарядка/перезарядка стартовой единицей) модуля загрузки (модуль 0) для поликетидного синтеза: субстратный центр с ацилтрансферазной активностью (домен АТ0) полифункциональной синтазы захватывает доступный в ферментационной среде остаток монокарбонов ой кислот ы из пула ацил - S-CoA посредством ацилирования тиольной группы цистеина этого домена фермента (68-72). Захваченный АТ0 ацильный (2-метилбутирильный или изобутирильного) остаток переносится на тиольную группу (замещение водорода в - SH) фосфопантетеинильного фрагмента (Ppant), связанного с остатком серина указанного домена, обладающего свойствами ацилпереносящих белков (АПБ, acyl carrier protein ACP0) и выполняющего функцию Ppant «рукава», или АПБ-манипулятора. Так стартовая единица подготавливается для приема модулем 1, в котором аналогичным образом при участии АТ1 для конденсации активируется дикарбоновая метилмалоновая кислота (метилмалонил ∼ S-Ppant-ACP). При конденсации β -кетосинтазный домен (КS1) модуля 1 катализирует образования С-С-связи между ацильными остатками из модуля 0 и 1 по принципу «голова к хвосту» (механизм Клайзена) (1). При этом сопряженно происходит декарбосилирование остатка дикарбоновой кислоты (73) с образованием дикетида (68, 70), заякоренного у домена АСР1 модуля 1. Этот дикетид восстанавливается кеторедуктазным доменом (KR1) до β -гидрокси-дикетида, готового для дальнейшей конденсации в модуле 2. Структурное разнообразие продуктов конденсации определяется набором каталитически активных доменов в каждом модуле (74, 75). Удлинение поликетидной цепи происходит шаг за шагом в 12 модулях (одна конденсация в одном модуле) в 12 последовательных сложноэфирных реакциях конденсации 7 единиц малоновой и 5 единиц метилмалоновой кислот, активированных в форме ацил ∼ S-CoA, в результате чего образуется тридекакетидный предшественник авермектинового агликона ( 1 ) . В каждом цикле конденсации метилмалонильный или малонильный остатки из соответственно метилма-лонил-СоА и малонил-СоА переносятся на фосфопантетеинильную группу ацилпереносящего белка (АСР) под стереохимическим контролем аци-лтрансферазы (AT) следующего модуля (68, 76). В конце последнего цикла роста цепи (12-й модуль, стадия терминации синтеза поликетидной цепи) происходят биохимические реакции (их последовательность до конца не выяснена), приводящие к отделению ациклического агликона от АСР12 и образованию 16-членного лактона — 6,8а-секо-6,8а-деокси-5-оксоавермек-тина и агликона. Показано (77, 78), что спирокетализация происходит после замыкания циклогексенового и 16-членного макролидного циклов, но до образования гексагидробензофуранового фрагмента. Затем после ряда превращений образуются компоненты А и В авермектинового комплекса (64).
Есть значительное сходство в сборке линейных тридекакетидных предшественников авермектинов и близких к ним по структуре и антипа-разитарным свойствам мильбемицинов (79). Однако для ацилтрансферазы модуля загрузки мильбемицинсинтазы (MilA, также состоит из 12 модулей) актиномицета S. hygroscopicus ssp. aureolacrimosus, ssp. noncyanogenus, в отличие от AveA, характерна специфичность к ацетил∼S-CoA, пропионил∼S-CoA и изобутирил∼S-СоА (73). Кроме того, есть некоторые различия в наборе каталитических активностей авермектинсинтазы и мильбемицинсинтазы: во 2-м и 7-м модулях AveA, в отличие от MilA, отсутствует домен еноилредук-тазы (ER), а имеющийся домен дегидратазы (DH) неактивен, что определяет некоторые структурные различия агликонов авермектинов и мильбеми-цинов. Мильбемицины отличаются наличием раскрытого 5-членного тетрагидрофуранового цикла) в положениях С22, С23 и С25 (1, 80) (рис. 1, см. приложение на сайте .
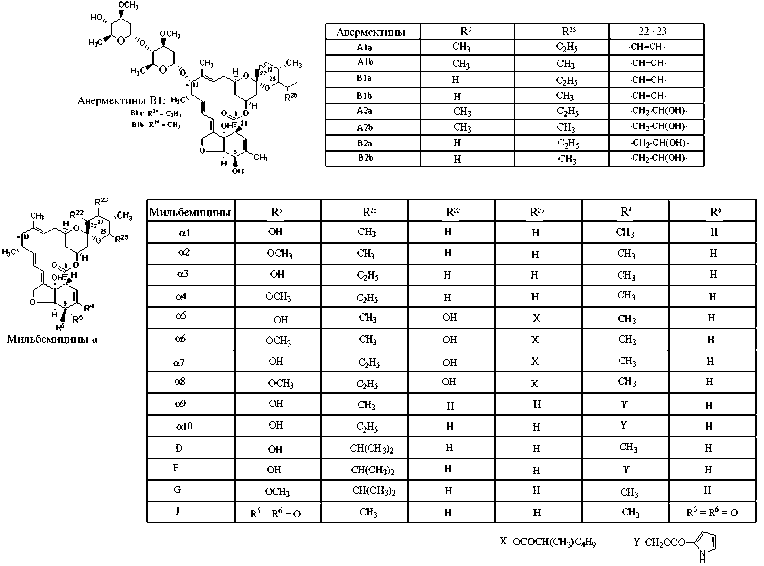
Рис. 1. Разнообразие молекулярных структур авермектинов и мильбемицинов α (мильбемицины β с раскрытым 5-членным циклом не приведены).
Регуляция образования авермектинов в целом происходит в соответствии с общими закономерностями биосинтеза поликетидов (69) у стрептомицетов (81, 82). Факторы регуляции биосинтеза авермектинов делятся на общие и специфические (83-87). В кластере генов синтеза этого класса макроциклических лактонов aveR, детерминирующий образование специфического регуляторного белка AveR, выполняет функцию специфического положительного регулятора и контролирует экспрессию как генов поликетидной конденсации, так и генов постполикетидной модификации (85): мутант с делецией в aveR не синтезирует авермектины, но продуцирует олигомицины, причем в больших количествах, чем дикий штамм. Полагают, что aveR кодирует специфический активатор, необходимый для биосинтеза авермектинов (86). Ген aveI идентифицирован как негативный регулятор биосинтеза этого класса макролидов, так как его инактивация приводит к увеличению продукции авермектина B1a у S. aver-mitilis NRRL 8165 примерно в 16 раз (33). Также установлено, что повышенная экспрессия генов aveT и sav_4189 , кодирующих соответственно регуляторные факторы — SAV3619 (AveT) из семейства репрессорных белков TetR (Tet Repressor Protein) и SAV4189, гомологичный белкам-регуляторам семейства MarR (multiple antibiotic resistance regulator), увеличивает выход авермектинов (87, 88). Среди общих регуляторов биосинтеза поликетидов, найденных также у других представителей актиномицетов рода Streptomyces (например, у S. coelicolor M145, синтезирующего актинородин), факторы SAV3818 и AvaR3 — положительные регуляторы, а AvaR1 — отрицательный регулятор биосинтеза авермектинов (33).
Группу описанных авермектиноподобных природных субстанций пополнили вещества, обладающие, как и все такие соединения, немато-цидной и инсектоакарицидной активностью — мелингмицин, продуцируемый S. nanchangensis (по структуре близок к мильбемицину α 11) (89), тетрациклическое мильбемициноподобное соединение у S. microflavus neau3 Y-3 (90), гомологи авермектинов В1 у горгониевого коралла Anthogorgia caerulea (бухта Beibu, Китай) (91).
Разнообразие продуцентов авермектиноподобных соединений (авермектинов, мильбемицинов, других аналогичных субстанций) свидетельствует о распространенности в природе комбинаторного синтеза по поли-кетидному механизму (89-91). В основе структур агликонов этих природных лактонов лежит один тот же тридекакетид. Многообразие природных авермектиноподобных соединений образуется благодаря вовлечению в биосинтез разных исходных единиц наращивания углеродной цепи (2-R-про-изводные малоновой кислоты (73) и набору каталитически активных доменов поликетидсинтаз у разных стрептомицетов (92, 93).
Использование методов синтетической биологии (32, 34, 73) и органического синтеза (93, 94) — важный тренд в расширение номенклатуры и усовершенствование производства авермектиноподобных субстанций (32). Как известно, большинство коммерческих субстанций этого класса — это полусинтетические производные нативного абамектина (1, 2). Основным подходом при усовершенствовании производства абамектина и других авермектинов служит оптимизация условий культивирования и направленный биосинтез у отобранных высокопроизводительных штаммов (32). Так, при замене участка ДНК aveDH2-KR2 в кластере генов биосинтеза авермектинов у промышленного штамма S. avermitilis NA-108 фрагментом milDH2-ER2-KR2 из кластера биосинтеза мильбемицинов у штамма S. bingchenggensis создан высокопроизводительный штамм S. avermitilis AVE-T27 с выходом биосинтетического ивермектина 3450±65 мкг/мл (95). Известный полусинтетический ивермектин получают реакцией гидрирования 22,23-двойной связи абамектина в присутствии катализатора Уилкинсона [(PH3P)3RCl] (1, 2). При замене aveLAT-ACP и aveDH2-KR2 соответственно на milLAT-ACP и milDH2-ER2-KR2 сконструирован штамм S. av-ermitilis AVE-H39, который синтезирует два новых ивермектиноподобных метаболита, содержащих метильный (выход 2093±61 мкг/мл) и этильный (выход 951±46 мкг/мл) радикалы в положении С25. Их биоцидная активность против Caenorhabditis elegans в 2,5 раза выше, чем у мильбемектина (95). Высокопроизводительный мутантный мильбемицинсинтезирующий штамм S. avermitilis SAMA1M7 получили в результате замены генов aveA1 и aveA3 (7-й модуль AveA3) у высокопроизводительного промышленного штамма S. avermitilis SA-01 темплатами для генов milA1 и milA3 (7-й модуль MilA3) из продуцента мильбемицинов S. hygroscopicus subsp. aureolacrimosus NRRL 5739 (79). Штамм S. avermitilis SAMA1M7 производил мильбемици-ны α3, α4, D (в небольших количествах) и их 5-О-метил-производные (около 292 мкг/мл) (79). Последующей инактивацией 5-О-метилтрансферазы (AveD) у S. avermitilis SAMA1M7 и введением стоп-кодона aveD с плазмидой pΔAveD получили штамм S. avermitilis SAMA1M7ΔD, синтезирующий мильбемицины α3 и α4 (основные компоненты коммерческого продукта — мильбемектина) с выходом 377 мкг/мл (79). Успешно осуществлена гетерологичная экспрессия кластера генов биосинтеза авермектинов ave у стрептомицета S. lividans 1326, при этом получены A2a, B1a и A1a (96). Стрептомицет S. avermitilis или его мутант, лишенный фермента дегидрогеназы α-кетокислот с разветвленной углеродной цепью (bkdF) из-за инак- тивации гена bdkF, способны синтезировать авермектиноподобные соединения, различавшиеся строением радикала в положении С25, при включении в питательную среду карбоновых кислот — предшественников стартовых единиц для модуля загрузки синтазы. В присутствии циклогексанкарбоновой кислоты (ЦГК) продуцент синтезирует аналог авермектина В1 (отличается от него наличием циклогексильного радикала в положении С25), известный как субстанция дорамектин (1, 96, 97). Мутантный штамм S. avermitilis TG2002 сконструирован посредством замены модуля загрузки авермектинсинтазы (aveATL-ACPL) штамма S. avermitilis M1 на ЦГК-син-тезирующий модуль (pnATL-ACPL) фослактомицинсинтазы (Pn) из S. platensis SAM-0654 с использованием плазмиды pTG2002 (99). У рекомбинантного S. avermitilis TG2002 выход дорамектина (58±2 мкг/мл) в 6 раз превышал таковой при ферментации родительского штамма S. avermitilis M1 (9±1 мкг/мл), а соотношение дорамектина и авермектина было 300-кратным (99).
Структура авермектинов, механизмы действия и развитие резистентности. Все известные авермектины и близкие к ним миль-бемицины, а также обнаруженные недавно мелингмицин (89), 28-гомо-авермектин В1а и 28-изопропил-авермектин В1а (91) обладают высокой ан-типарзитарной активностью при чрезвычайно низкой концентрации (порядка 1 нмоль/л) (100). Тем не менее эти соединения не идентичны, а вариация заместителей в разных участках пентациклического ядра (С4, С5, С13, С22-С23, С25) модулирует их биологическую активность лишь в той или иной степени. В ряду авермектинов удаление одного (дальнего) остатка олеандрозы снижает антинематодную активность в некоторой степени, дисахаридного остатка (агликоны авермектинов с 13-ОН-группой) — значительно, причем инсектоакарицидное действие в этом случае сохраняется. При замене 13-ОН-группы на водород (подобное имеет место у миль-бемицина) антипаразитарная активность вновь восстанавливается (76), а производное мильбемицина — лепимектин, имеющий полярный структурный фрагмент в положении С13, — эффективный паразитицид. В целом авермектины и мильбемицины с липофильными группами в положении С13 более активны, а полярные заместители снижают активность. Аналогичную зависимость между структурой и активностью против насекомых и клещей наблюдают у авермектина В1: замена 4 ″ -ОН-группы на 4 ″ -эпиметиламиногруппу заметно усиливает действие на различных чешуекрылых, но снижает — на клещей (101, 102).
Мишени действия авермектинов и других 16-членных макроциклических лактонов — глутамат-зависимые каналы для хлорид-ионов (GluCl-каналы), широко распространенные у беспозвоночных (нематод, членистоногих — насекомых, клещей) в отличие от позвоночных животных. Эти каналы активируются наномолярными концентрациями лактонов. Необратимая активация GluCl-каналов приводит к гиперполяризации мембран, несовместимой с нервной проводимостью в нейромышечных синапсах, и вызывает сильный и стойкий паралич мышц глоточной системы, кожномускульного мешка и органов кладки яиц (103, 104). У беспозвоночных широко распространены родственные, хотя и эволюционно удаленные от GluCl-каналообразующих белков протеины GABA-рецепторов (подвид А) (g-butyric acid; ГАМКА) (ГАМКА-зависимые Cl--каналы), которые также служат мишенями авермектинов (ГАМК — важнейший тормозный нейромедиатор в центральной нервной системе млекопитающих, включая человека). Однако авермектины безопасны для млекопитающих, так как не могут преодолеть гематоэнцефалический барьер и достичь ГАМКА-чув- ствительные Cl-каналы в центральной нервной системе (105). Как известно, GluCl-каналы и GABAA-рецепторы входят в семейство Cys-петлевых рецепторов, включающее также глициновые, никотиновые и серотониновые (5-HT3) ионотропные рецепторы (101, 106, 107), с которыми авермектины и мильбемицины также взаимодействуют, проявляя, однако, меньшую аффинность. Обнаружено также взаимодействие ивермектина с рецептором P2X4 (108).
Анализ чувствительноcти и устойчивости к ивермектину показал, что у паразитов резистентность к ивермектину связана с мутацией генов, детерминирующих синтез субъединиц GluCl ( glc-1 , avr-14 и avr-15 ), и повышенной экспрессии генов P-гликопротеина (109). Особая чувствительность собак породы колли к ивермектину и моксидоктину обусловлена мутацией гена MDR1 , отвечающего за образование Р-гликопротеина — обязательного компонента гематоэнцефалического барьера, играющего важную роль в сохранении его целостности и предотвращении проникновения препарата в головной мозг. Мутация в гене этого белка приводит к проникновению лактонов через гематоэнцефалический барьер млекопитающих (110).
В ряду 16-членных макроциклических лактонов имеется определенная специфичность в формировании резистентности в зависимости от структуры соединения. Например, возможна перекрестная резистентность к ивермектину и дорамектину, но во многих случаях дорамектин проявляет высокую активность при резистентности к авермектинам (101). Обнаружено интересное явление (111): увеличение концентрации нейромедиатора ГАМК при кормлении паутинного клеща Tetranychus cinnabarinus экзогенным ГАМК или подавлении экспрессии гена ГАМК-трансаминазы (GABA-T) определяет резистентность подопытных особей вредителя к абамектину. Другой интересный феномен, описанный совсем недавно, — факт прямого взаимодействия авермектинов с эпидермальным фактором роста (EGFR, epidermal growth factor receptor). Этот фактор активирует EGFR/AKT/ERK-пути и индуцирует сверхэкспериссию Р-гликопротеина в утолщенных хитиновых слоях у личинок Drosophila melanogaster в резистентной популяции (112).
Полусинтетические авермектины. Часто вторичные метаболиты применяют в качестве действующего вещества после химической модификации с целью повышения биодоступности, качества, придания необходимых физико-химических свойств, снижения побочных эффектов и т.д. Так создаются наиболее эффективные аналоги природного родоначальника, в том числе 16-членные авермектины (2, 113-115).
С химической точки зрения авермектины можно представить как производных соответствующих компонентов мильбемицинового комплекса, полученных наращиванием последних 4- α -L-олеандрозил-L-олеандро-зилокси-группой в положении С13 лактонного ядра. Рассмотрим примеры создания практически важных фармацевтических субстанций, а также перспективные направления, например получение 5-О-производных, разрабатываемое авторами настоящей работы с середины 1990-х годов.
Стратегия химической модификации определяется сведениями о биологической активности компонентов авермектинового и в какой-то мере мильбемицинового комплексов, обладающих противопаразитарной активностью. Примером могут служить данные о контактном действии производных авермектина В1 против половозрелых самок паутинного клеща (116). Так, смертность через 96 ч (при концентрации действующего вещества 0,05 ppm) составила для авермектина В1 (абамектина), 8,9-эпок-сидавермектина В1, 10,11-дигидроавермектина В1 и 10-фторо-10,11-дигид- роавермектина В1 100 %, для 22,23-дигидроавермектина В1 (ивермектина) 92 %, 10-гидрокси-10,11-дигидроавермектина В1 — 72 %, 3,4-циклопро-пилавермектина В1 и 8,9-эпоксидмильбемицина (25-втор-бутила) — 20 %, 3,4,8,9,10,11,22,23-октагидроавермектина В1 — 18 %, 8,9-циклопропилавер-мектина В1 — 15 % и для 3,4,10,11,22,23-гексагидроавермектина В1 — 11 % (116). Получение производных авермектинов и мильбемицинов, имеющих практическое значение благодаря эффективности, антипаразитар-ному спектру и экологической безопасности, включают этапы микробиологического синтеза и химической модификации (1) (рис. 2, см. приложение на сайте . Нами ведутся исследования по оценке пригодности 5-О- и 5-С-производных авермектина В1, ивермектина и других авермектинов и мильбемицинов в качестве антипаразитарных субстанций (некоторые уже запатентованы) (117):
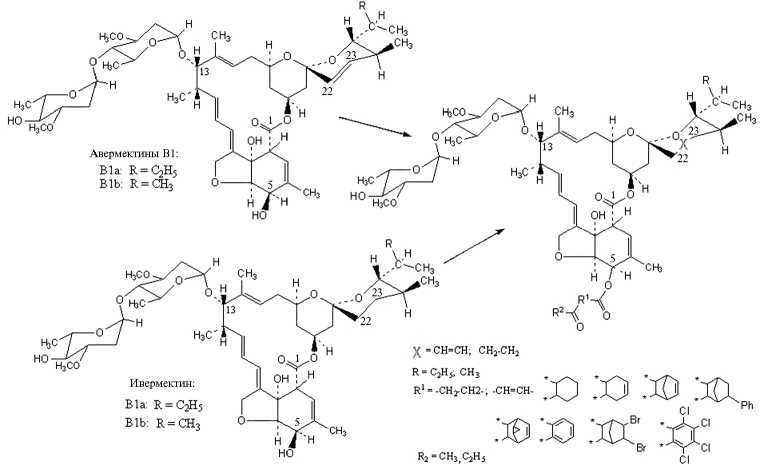
Рис. 2. Схема получения 5-О-производных авермектина В1 (22 и 23 — соответственно структурные единицы -СH2- или =СH- в случае одинарной или двойной связи для X).
Также нами получена серия 5-О-, 5-О,4 ″ -О- и 4 ″ -О-ацилпроиз-водных и их эфиров, метилкарбаматов, натриевой соли 5-О-сульфата и ряда других (118-122). Установлено, что среди этих производных 5-О-сук-циноилавермектин В1 (сумектин) и соединение под рабочим названием С2017 обладают выраженным антипаразитарным действием, и на их основе разработаны жидкие и твердые формы лекарственных препаратов для накожного и перорального применения (патенты РФ ¹ 2629600, 2661615). Учеными других стран также предпринимаются попытки получить аналогичные соединения, в частности 5-оксимпроизводные и производное хитозана (123, 124). При сравнении антипаразитарных свойств сумектина, С2017 и абамектина на лабораторных мышах, зараженных нематодой Aspiculuris tetraptera , мы показали, что при пероральном введении в дозе 0,25 мг/кг все субстанции обладают 100 % антигельминтной активностью, кроме того, С2017 (в отличие от абамектина и сумектина) проявляет также репеллентное действие (неопубликованные данные). Также установлено, что соединение С2017 превосходит абамектин по влиянию на связывание радиолиганда [G-3H]SR 95531 с мембранами, содержащими ГАМКA-рецеп-торы коры мозга у крыс, повышая максимальное ингибирование специфического связывания Imax на 86 % (собственные неопубликованные данные).
Суммируя, можно констатировать, что натуральные и полусинтети-ческие авермектины нашли широкое применение для лечения и профилактики нематодозов и арахноэнтомозов животных, человека и растений.
Наиболее часто применяются абамектин, ивермектин (1), дорамектин (2, 3), селамектин (2), бензоат авермектина В1 (4), эприномектин (1), а также близкие к ним мильбемектин (смесь мильбемицинов α 3 и α 4) (1). На основе этих субстанций выпускаются многочисленные лекарственные ветеринарные и медицинские препараты под разными торговыми названиями для лечения онхоцеркоза, дерматитов и др. В последние годы в России активно патентуются кремы для лечения розацеи на основе ивермектина (125), мазей и жидких форм для лечения и профилактики арахноэнтомозов, на основе гемисукцината авермектина В1 и др., включая российские препараты для перорального применения (126-129), а также гранулы «ВЭИС приманки для тараканов» (для борьбы с синантропными насекомыми, Свидетельство ¹ RU.77.99.88.002. Е007964.09.14).
Итак, продуцируемые микроорганизмом Streptomyces avermitilis 16членные макролиды (авермектины) и другие близкие к ним макроциклические лактоны обладают высокой нематоцидной и инсектоакарицидной активностью вследствие взаимодействия с глутаматзависимыми каналами для Cl-ионов у беспозвоночных, а также в некоторой степени с GABA-зависимыми рецепторами из семейства цис-петлевых рецепторов. Исследования, ориентированные на разработку технологии полного химического синтеза авермектинов пока не дали существенных результатов из-за низкого выхода целевого продукта и сложности схемы синтеза. Благодаря химической модификации природных макролидов — авермектина В1 (продуцент S. avermitilis ), мильбемицинов α 3/ α 4 ( S. hygroscopicus ssp. aureolac-rimosus ), немадектина ( S. hygroscopicus ssp. noncyanogenus ), других биосинтетических аналогов (например, дорамектина, продуцируемого мутантным штаммом S. avermitilis с дефектным геном дегидрогеназы разветвленных α -кетокислот) получены субстанции-аналоги для преимущественного применения в ветеринарии (ивермектин, эприномектин, селамектин, мокси-дектин), медицине (ивермектин), защите растений и урожая (абамектин, бензоат эмамектина, оксим мильбемина α 3/ α 4), как перспективные рассматриваются соединения 5-О-сукциноилавермектин В1 и С2017.
Список литературы Получение авермектинов: биотехнологии и органический синтез
- Macrolide antibiotics. Chemistry, biology and practice. 2nd ed./S. Omura (ed.). Elsevier Science, NY, 2002.
- Campbell W.C. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr. Pharm. Biotechnol., 2012, 13(6): 853-865 ( ) DOI: 10.2174/138920112800399095
- Omura S. Ivermectin: 25 years and still going strong. Int. J. Antimicrob. Agents, 2008, 31(2): 91-98 ( ) DOI: 10.1016/j.ijantimicag.2007.08.023
- Crump A., Omura S. Ivermectin, ‘Wonder drug’ from Japan: the human use perspective. Proc. Jpn. Acad., Ser. B, 2011, 87(2): 13-28 ( ) DOI: 10.2183/pjab.87.13
- Goodman and Gilman’s the pharmacological basis of therapeutics. 13th ed./L. Brunton, R. Hilal-Dandan, B.C. Knollman (eds.). McGraw Hill Medical, NY, 2018.
- Сафиуллин Р.Т. Авермектины на российском ветеринарном рынке. Российский ветеринарный журнал, 2006, 2: 6-8.
- Долженко Т.В. Инсектоакарициды на основе абамектина. Агрохимия, 2017, 4: 34-40.
- Kitani S., Miyamoto K.T., Takamatsu S., Herawati E., Iguchi H., Nishitomi K., Uchida M., Nagamitsu T., Omura S., Ikeda H., Nihira T. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc. Natl. Acad. Sci. USA, 2011, 108: 16410-16415 ( )
- DOI: 10.1073/pnas.1113908108
- Corey E.J., Czako B., Kurti L. Molecules and medicine. Wiley-VCH Verlag, Weinheim, 2007.
- Macrocyclic lactones in antiparasitic therapy/J. Vercruysse, R.S. Rew (eds.). Wallingford, CABI Publishing, NY, 2002.
- Lynagh T., Lynch J.W. Molecular mechanisms of Cys-loop ion channel receptor modulation by ivermectin. Front. Mol. Neurosci., 2012, 5: 60 ( )
- DOI: 10.3389/fnmol.2012.00060
- Chen I.S., Kubo Y. Ivermectin and its target molecules: shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J. Physiol., 2018, 596(10): 1833-1845 ( )
- DOI: 10.1113/JP275236
- Chen I.S., Tateyama M., Fukata Y., Uesugi M., Kubo Y. Ivermectin activates GIRK channels in a PIP2-dependent, Gβγ-independent manner and an amino acid residue at the slide helix governs the activation. J. Physiol., 2017, 595(17): 5895-5912 ( )
- DOI: 10.1113/JP274871
- Hashimoto H., Messerli S.M., Sudo T., Maruta H. Ivermectin inactivates the kinase PAK1 and blocks the PAK1-dependent growth of human ovarian cancer and NF2 tumor cell lines. Drug Discov. Ther., 2009, 3(6): 243-246.
- Gallardo F., Mariamé B., Gence R., Tilkin-Mariamé A.F. Macrocyclic lactones inhibit nasopharyngeal carcinoma cells proliferation through PAK1 inhibition and reduce in vivo tumor growth. Drug Des. Devel. Ther., 2018, 12: 2805-2814 ( )
- DOI: 10.2147/DDDT.S172538
- Melotti A., Mas C., Kuciak M., Lorente-Trigos A., Borges I., Altaba A.R. The river blindness drug Ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Molecular Medicine, 2014, 6(10): 1263-1278 ( )
- DOI: 10.15252/emmm.201404084
- Drinyaev V.A., Mosin V.A., Kruglyak E.B., Novik T.S., Sterlina T.S., Ermakova N.V., Kublik L.N., Levitman M.Kh., Shaposhnikova V.V., Korystov Y.N. Antitumor effect of avermectins. Eur. J. Pharmacol., 2004, 501(1-3): 19-23 ( )
- DOI: 10.1016/j.ejphar.2004.08.009
- Kwon Y.J., Petrie K., Leibovitch B.A., Zeng L., Mezei M., Howell L., Gil V., Christova R., Bansal N., Yang S., Sharma R., Ariztia E.V., Frankum J., Brough R., Sbirkov Y., Ashworth A., Lord C.J., Zelent A., Farias E., Zhou M.M., Waxman S. Selective inhibition of SIN3 corepressor with avermectins as a novel therapeutic strategy in triple-negative breast cancer. Mol. Cancer Ther., 2015, 14(8): 1824-1836 ( )
- DOI: 10.1158/1535-7163.MCT-14-0980-T
- Juarez M., Schcolnik-Cabrera A., Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer. Res., 2018, 8(2): 317-331 (https://www.ncbi.nlm.nih.gov/pubmed/29511601).
- Mastrangelo E., Pezzullo M., De Burghgraeve T., Kaptein S., Pastorino B., Dallmeier K., de Lamballerie X., Neyts J., Hanson A. M., Frick D.N., Bolognesi M., Milani M. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J. Antimicrob. Chemother., 2012, 67(8): 1884-1894 ( )
- DOI: 10.1093/jac/dks147
- Kobylinski K.C., Foy B.D., Richardson J.H. Ivermectin inhibits the sporogony of Plasmodium falciparum in Anopheles gambiae. Malaria Journal, 2012, 11: 381 ( )
- DOI: 10.1186/1475-2875-11-381
- Lim L.E., Vilchèze C., Ng C., Jacobs W.R. Jr., Ramón-García S., Thompson C.J. Anthelmintic avermectins kill M. tuberculosis, including multidrug resistant clinical strains. Antimicrob. Agents Chemother., 2013, 57(2): 1040-1046 ( )
- DOI: 10.1128/AAC.01696-12
- Khoja S., Huynh N., Warnecke A.M.P., Asatryan L., Jakowec M.W., Davies D.L. Preclinical evaluation of avermectins as novel therapeutic agents for alcohol use disorders. Psychopharmacology (Berl.), 2018, 235(6): 1697-1709 ( )
- DOI: 10.1007/s00213-018-4869-9
- Zabala A., Vazquez-Villoldo N., Rissiek B., Gejo J., Martin A., Palomino A., Perez-Samartín A., Pulagam K.R., Lukowiak M., Capetillo-Zarate E., Llop J., Magnus T., Koch-Nolte F., Rassendren F., Matute C., Domercq M. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis EMBO Mol. Med., 2018, 10(8): e8743 ( )
- DOI: 10.15252/emmm.201708743
- Di Virgilio F., Sarti A.C. Microglia P2X4 receptors as pharmacological targets for demyelinating diseases. EMBO Mol. Med., 2018, 10(8): e9369 ( )
- DOI: 10.15252/emmm.201809369
- Davies D.L., Bortolato M., Finn D.A., Ramaker M.J., Barak S., Ron D., Liang J., Olsen R.W. Recent advances in the discovery and preclinical testing of novel compounds for the prevention and/or treatment of alcohol use disorders. Alcohol Clin. Exp. Res., 2013, 37(1): 8-15 ( )
- DOI: 10.1111/j.1530-0277.2012.01846.x
- Pasqualetto G., Brancale A., Young M.T. The Molecular determinants of small-molecule ligand binding at P2X receptors. Front. Pharmacol., 2018, 9: 58 ( )
- DOI: 10.3389/fphar.2018.00058
- Van Voorhis W.C. Profile of William C. Campbell, Satoshi Omura, and Youyou Tu, 2015 Nobel Laureates in Physiology or Medicine. Proc. Natl. Acad. Sci. USA, 2015, 112(52): 15773-15776 ( )
- DOI: 10.1073/pnas.1520952112
- Wang S.-Y., Bo Y.-H., Zhou X., Chen J.H., Li W.J., Liang J.P., Xiao G.Q., Wang Y.C., Liu J., Hu W., Jiang B.L. Significance of heavy-ion beam irradiation-induced avermectin B1a production by engineered Streptomyces avermitilis. BioMed. Research. Int., 2017, 2017: 5373262 ( )
- DOI: 10.1155/2017/5373262
- Awasthi A., Razzak M., Al-Kassas R., Harvey J., Garg S. An overview on chemical derivatization and stability aspects of selected avermectin derivatives. Chem. Pharm. Bull., 2012, 60(8): 931-944 ( )
- DOI: 10.1248/cpb.c12-00258
- Cummings M., Breitling R., Takano E. Steps towards the synthetic biology of polyketide biosynthesis. FEMS Microbiol. Lett., 2014, 351: 116-125 ( )
- DOI: 10.1111/1574-6968.12365
- Zhuo Y., Zhang T., Wang Q., Cruz-Morales P., Zhang B., Liu M., Barona-Gómez F., Zhang L. Synthetic biology of avermectin for production improvement and structure diversification. Biotechnol. J., 2014, 9(3): 316-325 ( )
- DOI: 10.1002/biot.201200383
- Thuan N.H., Pandey R.P., Sohng J.K. Recent advances in biochemistry and biotechnological synthesis of avermectins and their derivatives. Appl. Microbiol. Biotechnol., 2014, 98(18): 7747-7759 ( )
- DOI: 10.1007/s00253-014-5926-x
- Alper H.S., Avalos J.L. Metabolic pathway engineering. Synth. Syst. Biotechnol., 2018, 3(1): 1-2 ( )
- DOI: 10.1016/j.synbio.2018.01.002
- Brady P.B., Oda S., Yamamoto H. Stereodivergent approach to the avermectins based on "Super Silyl" directed aldol reactions. Org. Lett., 2014, 16(15): 3864-3867 ( )
- DOI: 10.1021/ol501327g
- Hirama M. Total synthesis and related studies of large, strained, and bioactive natural products. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci., 2016, 92(8): 290-329 ( )
- DOI: 10.2183/pjab.92.290
- Yamashita S., Hayashi D., Nakano A., Hayashi Y., Hirama M. Total synthesis of avermectin B1a revisited. J. Antibiot. (Tokyo), 2016, 69(1): 31-50 ( )
- DOI: 10.1038/ja.2015.47
- Pitterna T., Cassayre J. Hüter O.F., Jung P.M., Maienfisch P., Kessabi F.M., Quaranta L., Tobler H. New ventures in the chemistry of avermectins. Bioorg. Med. Chem., 2009, 17(12): 4085-4095 ( )
- DOI: 10.1016/j.bmc.2008.12.069
- Bennett C.S., Galan M.C. Methods for 2-deoxyglycoside synthesis. Chem. Rev., 2018, 118(17): 7931-7985 ( )
- DOI: 10.1021/acs.chemrev.7b00731
- Kim S.B., Goodfellow M. Streptomyces avermitilis sp. nov., nom. rev., a taxonomic home for the avermectin-producing streptomycetes. Int. J. Syst. Evol. Microbiol., 2002, 52(Pt 6): 2011-2014 ( )
- DOI: 10.1099/00207713-52-6-2011
- Wu L., Sun Q., Sugawara H., Yang S., Zhou Y., McCluskey K., Vasilenko A., Suzuki K., Ohkuma M., Lee Y., Robert V., Ingsriswang S., Guissart F., Philippe D., Ma J. Global catalogue of microorganisms (gcm): a comprehensive database and information retrieval, analysis, and visualization system for microbial resources. BMC Genomics, 2013, 14: 933 ( )
- DOI: 10.1186/1471-2164-14-933
- Черменский Д.Н., Аданин В.А., Дриняев В.А., Ковалев В.Н., Головлева Л.А. Авермектины: биотехнологичекие особенности штамма продуцента Streptomyces avermitilis ВКМ Ас 1301. Прикладная биохимия и микробиология, 1991, 6: 838-844.
- Дриняев В.А., Стерлина Т.С., Берёзкина Н.Е., Мосин В.А., Кругляк Е.Б., Есипов С.Е., Кобрин М.Б., Юркив В.А. Авермектины: естественная изменчивость штамма-продуцента Streptomices avermitilis ВКМ Ас 1301. Биотехнология, 1993, 11-12: 21-25.
- Ki S.S., Jeong Y.-S., Kim P.-H., Chun G.-T. Effects of dissolved oxygen level on avermectin B1a production by Streptomyces avermitilis in computer-controlled bioreactor cultures. J. Microbiol. Biotechnol., 2006, 16(11): 1690-1698.
- Gao H. Liu M., Zhou X., Liu J., Zhuo Y., Gou Z., Xu B., Zhang W., Liu X., Luo A., Zheng C., Chen X., Zhang L. Identification of avermectin-high-producing strains by high throughput screening methods. Appl. Microbiol. Biotechnol., 2010, 85(4): 219-1225 ( )
- DOI: 10.1007/s00253-009-2345-5
- Chen J., Liu M., Liu X., Miao J., Fu C., Gao H., Müller R., Zhang Q., Zhang L. Interrogation of Streptomyces avermitilis for efficient production of avermectins. Synth. Syst. Biotechnol., 2016, 1(1): 7-16 ( )
- DOI: 10.1016/j.synbio.2016.03.002
- Meng L., Xiong Z., Chu J., Wang Y. Enhanced production of avermectin by deletion of type III polyketide synthases biosynthetic cluster rpp in Streptomyces avermitilis. Lett. Appl. Microbiol., 2016, 63(5): 384-390 ( )
- DOI: 10.1111/lam.12635
- Дриняев В.А., Стерлина Т.С., Берёзкина Н.Е., Мосин В.А., Кругляк Е.Б., Зиновьев О.А., Юркив В.А. Авермектины: селекция штамма продуцента Streptomices avermitilis. ВКМ Ас 1301. Получение естественного мутанта. Биотехнология, 1994, 12: 16-18.
- Дриняев В.А., Стерлина Т.Е., Берёзкина Н.Е., Мосин В.А., Кругляк Е.Б., Зиновьев О.А., Юркив В.А. Авермектины: получение индуцированных мутантных штаммов Streptomices avermitilis. Биотехнология, 1994, 4: 17-20.
- Белявская Л.А., Козырицкая В.Е., Валагурова Е.В., Иутинская Г.А. Биологически активные вещества препарата аверком. Мikробiол. журн., 2012, 74(3): 10-15.
- Адамович О.Т., Коломиец Э.И. Оптимизация состава питательной среды для глубинного культивирования актиномицета Streptomyces avermitilis X-1. Мат. Межд. науч.-практ. конф. «Перспективы и проблемы развития биотехнологии в рамках единого экономического пространства стран содружества, 25-28 мая 2005 г. Минск-Нарочь». Минск, 2005: 6-7.
- Biliavska L., Kozyrits’ka V., Valaghurova H., Iutynska G. Effect of pyruvate and valine on avermectin biosintesis in Streptomyces avermitilis UCМ Ас-2179. Microbiol. Zhurn., 2007, 69(4): 10-17.
- Мирзаев М.Н., Шерстнев В.В., Буянтогтох Ч. Липиды Streptomyces avermitilis: возможность применения в ветеринарной медицине. Биотехнология, 2004, 3: 75-77.
- Kodym A., Afza R. Physical and chemical mutagenesis. Methods Mol. Biol., 2003, 236: 189-204 ( )
- DOI: 10.1385/1-59259-413-1:189
- Wang L.Y., Huang Z.L., Li G., Zhao H.X., Xing X.H., Sun W.T., Li H.P., Gou Z.X., Bao C.Y. Novel mutation breeding method for Streptomyces avermitilis using an atmospheric pressure glow discharge plasma. J. Appl. Microbiol., 2010, 108(3): 851-858 ( )
- DOI: 10.1111/j.1365-2672.2009.04483.x
- Савченков С.Н., Мирзаев М.Н., Девришов Д.А. Некоторые особенности технологии получения авермектинов. Биотехнология, 1997, 3: 35-38.
- Ikeda H., Kotaki H., Tanaka H., Omura S. Involvent of glucose catabolism in avermectin production by Streptomyces avermitilis. Antimicrob. Agents Chemother., 1988, 32(2): 282-284.
- Cao P., Hu D., Zhang J., Zhang B., Gao Q. Enhanced avermectin production by rational feeding strategies based on comparative metabolomics. Wei Sheng Wu Xue Bao, 2017, 57(2): 281-292.
- Tian P., Cao P., Hu D., Wang D., Zhang J., Wang L., Zhu Y., Gao Q. Comparative metabolomics reveals the mechanism of avermectin production enhancement by S-adenosylmethionine. J. Ind. Microbiol. Biotechnol., 2017, 44(4-5): 595-604 ( )
- DOI: 10.1007/s10295-016-1883-y
- Schulman M.D., Valentino D., Streicher S., Ruby C. Streptomyces avermitilis mutants defective in methylation of avermectins. Antimicrob. Agents Chemother., 1987, 31(5): 744-749 ( )
- DOI: 10.1128/AAC.31.5.744
- Yin P., Li Y.Y., Zhou J., Wang Y.H., Zhang S.L., Ye B.C., Ge W.F., Xia Y.L. Direct proteomic mapping of Streptomyces avermitilis wild and industrial strain and insights into avermectin production. J. Proteomics, 2013, 79: 1-12 ( )
- DOI: 10.1016/j.jprot.2012.11.012
- Миронов В.А., Сергеева A.B., Воронкова В.В., Даниленко В.Н. Биосинтез авермектинов: физиологические и технологические аспекты. Антибиотики и химиотерапия, 1997, 42(3): 31-36.
- Миронов В.А., Сергеева А.В., Гаврилина А.В., Даниленко В.Н. Зависимость состава авермектинового комплекса Streptomyces avermitilis от содержания глюкозы в среде. Прикладная биохимия и микробиология, 2003, 2: 208-212.
- Yoon Y.J., Kim E.-S., Hwang Y.-S., Choi C.-Y. Avermectin biochemical and molecular basis of its biosynthesis and regulation. Appl. Microbiol. Biotechnol., 2004, 63(6): 626-634 ( )
- DOI: 10.1007/s00253-003-1491-4
- Давыдова Е.М., Дриняев В.А., Кругляк Е.Б., Кантере В.М. Изучение условий экстракции авермектинового комплекса из высушенной мицелиальной биомассы Streptomyces avermetilis. Биотехнология, 2000, 6: 66-74.
- Ikeda H., Ishikawa J., Hanamoto A., Shinose M., Kikuchi H., Shiba T., Sakaki Y., Hattori M., Omura S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol., 2003, 21(5): 526-531 ( )
- DOI: 10.1038/nbt820
- Ikeda H., Kazuo S.Y., Omura S. Genome mining of the Streptomyces avermitilis genome and development of genome-minimized hosts for heterologous expression of biosynthetic gene clusters. J. Ind. Microbiol. Biotechnol., 2014, 41(2): 233-250 ( )
- DOI: 10.1007/s10295-013-1327-x
- Fischbach M.A., Walsh C.T. Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev., 2006, 106(8): 3468-3496 ( )
- DOI: 10.1021/cr0503097
- Dutta S., Whicher J.R., Hansen D.A., Hale W.A., Chemler J.A., Congdon G.R., Narayan A.R., Håkansson K., Sherman D.H., Smith J.L., Skiniotis G. Structure of a modular polyketide synthase. Nature, 2014, 510(7506): 512-517 ( )
- DOI: 10.1038/nature13423
- Kwan D.H., Schulz F. The stereochemistry of complex polyketide biosynthesis by modular polyketide synthases. Molecules, 2011, 16(7): 6092-6115 ( )
- DOI: 10.3390/molecules16076092
- Bayly C.L., Yadav V.G. Towards precision engineering of canonical polyketide synthase domains: recent advances and future prospects. Molecules, 2017, 22(2): 235 ( )
- DOI: 10.3390/molecules22020235
- Zhang W., Liu J. Recent advances in understanding and engineering polyketide synthesis. F1000Research, 2016, 5(F1000 Faculty Rev): 208 ( )
- DOI: 10.12688/f1000research.7326.1
- Yuzawa S., Backman T.W.H., Keasling J.D., Katz L. Synthetic biology of polyketide synthases. J. Ind. Microbiol. Biotechnol., 2018, 45(7): 621-633 ( )
- DOI: 10.1007/s10295-018-2021-9
- Smith J.L., Skiniotis G., Sherman D.H. Architecture of the polyketide synthase module: surprises from electron cryo-microscopy. Curr. Opin. Struct. Biol., 2015, (31): 9-19 ( )
- DOI: 10.1016/j.sbi.2015.02.014
- Klaus M., Grininger M. Engineering strategies for rational polyketide synthase design. Nat. Prod. Rep., 2018, 35(10): 1070-1081 ( )
- DOI: 10.1039/c8np00030a
- Wang F., Wang Y., Ji J., Zhou Z., Yu J., Zhu H., Su Z., Zhang L., Zheng J. Structural and functional analysis of the loading acyltransferase from avermectin modular polyketide synthase. ACS Chem. Biol., 2015, 10(4): 1017-1025 ( )
- DOI: 10.1021/cb500873k
- Sun P., Zhao Q., Yu F, Zhang H., Wu Z., Wang Y., Wang Y., Zhang Q., Liu W. Spiroketal formation and modification in avermectin biosynthesis involves a dual activity of Ave C. J. Am. Chem. Soc., 2013, 135(4): 1540-1548 ( )
- DOI: 10.1021/ja311339u
- Tang M.C., Zou Y., Watanabe K., Walsh C.T., Tang Y. Oxidative cyclization in natural product biosynthesis. Chem. Rev., 2017, 117(8): 5226-5333 ( )
- DOI: 10.1021/acs.chemrev.6b00478
- Kim M.S., Cho W.J., Song M.C., Park S.W., Kim K., Kim E., Lee N., Nam S.J., Oh K.H., Yoon Y.J. Engineered biosynthesis of milbemycins in the avermectin high-producing strain Streptomyces avermitilis. Microbial Cell Factories, 2017, 16: 9 ( )
- DOI: 10.1186/s12934-017-0626-8
- Nonaka K., Tsukiyama T., Okamoto Y., Sato K., Kumasaka C., Yammoto T., Maruyama F., Yoshikawa H. New milbemycins from Streptomyces hygroscopicus subsp. aureolacrimosus: fermentation, isolation and structure elucidation. J. Antibiot. (Tokyo), 2000, 53(7): 694-704.
- He H., Ye L., Li C., Wang H., Guo X., Wang X., Zhang Y., Xiang W. SbbR/SbbA, an important ArpA/AfsA-like system, regulates milbemycin production in Streptomyces bingchenggensis. Front. Microbiol., 2018, 9: 1064 ( 2018)
- DOI: 10.3389/fmicb.2018.01064
- van Wezel G.P., McDowall K.J. The regulation of the secondary metabolism of Streptomyces: new links and experimental advances. Nat. Prod. Rep., 2011, 28(7): 1311-1333 ( )
- DOI: 10.1039/c1np00003a
- Sun D., Wang Q., Chen Z., Li J., Wen Y. An Alternative Factor, s8, controls avermectin production and multiple stress responses in Streptomyces avermitilis. Front. Microbiol., 2017, 8: 736 ( )
- DOI: 10.3389/fmicb.2017.00736
- McCormick J.R., Flärdh K. Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev., 2012, 36(1): 206-231 ( )
- DOI: 10.1111/j.1574-6976.2011.00317.x
- Kitani S., Ikeda H., Sakamoto T., Noguchi S., Nihira T. Characterization of a regulatory gene, aveR, for the biosynthesis of avermectin in Streptomyces avermitilis. Appl. Microbiol. Biotechnol., 2009, 82(6): 1089-1096 ( )
- DOI: 10.1007/s00253-008-1850-2
- Guo J., Zhao J., Li L., Chen Z., Wen Y., Li J. The pathway-specific regulator AveR from Streptomyces avermitilis positively regulates avermectin production while it negatively affects oligomycin biosynthesis. Mol. Genet. Genomics, 2010, 283(2): 123-133 ( )
- DOI: 10.1007/s00438-009-0502-2
- Liu W., Zhang Q., Guo J., Chen Z., Li J., Wen Y. Increasing avermectin production in Streptomyces avermitilis by manipulating the expression of a novel TetR-family regulator and its target gene product. Appl. Environ. Microbiol., 2015, 81(15): 5157-5173 ( )
- DOI: 10.1128/AEM.00868-15
- Guo J., Zhang X., Lu X., Liu W., Chen Z., Li J., Deng L., Wen Y. SAV4189, a MarR-family regulator in Streptomyces avermitilis, activates avermectin biosynthesis. Front. Microbiol., 2018, 9: 1358 ( )
- DOI: 10.3389/fmicb.2018.01358
- Sun Y., Zhou X., Liu J., Bao K., Zhang G., Tu G., Kieser T., Deng Z. Streptomyces nanchangensis, a producer of the insecticidal polyether antibiotic nanchangmycin and the antiparasitic macrolide meilingmycin, contains multiple polyketide gene clusters. Microbiology, 2002, 148(Pt 2): 361-371 ( )
- DOI: 10.1099/00221287-148-2-361
- Yang L.Y., Wang J.D., Zhang J., Xue C.Y., Zhang H., Wang X.J., Xiang W.S. New nemadectin congeners with acaricidal and nematocidal activity from Streptomyces microflavus neau3 Y-3. Bioorg. Med. Chem. Lett., 2013, 23(20): 5710-5713 ( )
- DOI: 10.1016/j.bmcl.2013.08.002
- Gao C., Wang Y., Chen Y., He B., Zhang R., Xu M., Huang R. Two new avermectin derivatives from the Beibu Gulf gorgonian Anthogorgia caerulea. Chem. Biodivers., 2014, 11(5): 812-818 ( )
- DOI: 10.1002/cbdv.201300265
- Wong F.T., Khosla C. Combinatorial biosynthesis of polyketides -a perspective. Curr. Opin. Chem. Biol., 2012, 16(1-2): 117-123 ( )
- DOI: 10.1016/j.cbpa.2012.01.018
- Cane D.E. Nature as organic chemist. J. Antibiot. (Tokyo), 2016, 69(7): 473-485 ( )
- DOI: 10.1038/ja.2016.55
- Blakemore D.C., Castro L., Churcher I., Rees D.C., Thomas A.W., Wilson D.M., Wood A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem., 2018, 10(4): 383-394 ( )
- DOI: 10.1038/s41557-018-0021-z
- Zhang J., Yan Y.J., An J., Huang S.X., Wang X.J., Xiang W.S. Designed biosynthesis of 25-methyl and 25-ethyl ivermectin with enhanced insecticidal activity by domain swap of avermectin polyketide synthase. Microbial Cell Factories, 2015, 14: 152 ( )
- DOI: 10.1186/s12934-015-0337-y
- Zhao X., Wang Y., Wang S., Chen Z., Wen Y., Song Y. Construction of a doramectin producer mutant from an avermectin-overproducing industrial strain of Streptomyces avermitilis. Can. J. Microbiol., 2009, 55(12): 1355-1363 ( )
- DOI: 10.1139/W09-098
- Deng Q., Zhou L, Luo M., Deng Z., Zhao C. Heterologous expression of avermectins biosynthetic gene cluster by construction of a bacterial artificial chromosome library of the producers. Synth. Syst. Biotechnol., 2017, 2(1): 59-64 ( )
- DOI: 10.1016/j.synbio.2017.03.001
- Wang X.J., Zhang J., Wang J.D., Huang S.X., Chen Y.H., Liu C.X., Xiang W.S. Four new doramectin congeners with acaricidal and insecticidal activity from Streptomyces avermitilis NEAU1069. Chem. Biodivers., 2011, 8(11): 2117-2125 ( )
- DOI: 10.1002/cbdv.201000295
- Wang J.B., Pan H.X., Tang G.L. Production of doramectin by rational engineering of the avermectin biosynthetic pathway. Bioorg. Med. Chem. Lett., 2011, 21(11): 3320-3323 ( )
- DOI: 10.1016/j.bmcl.2011.04.008
- Wolstenholme A.J., Maclean M.J., Coates R., McCoy C.J., Reaves B.J. How do the macrocyclic lactones kill filarial nematode larvae? Invert. Neurosci., 2016, 16(3): 7 ( )
- DOI: 10.1007/s10158-016-0190-7
- Prichard R., Ménez C., Lespine A. Moxidectin and the avermectins: consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist., 2012, 2: 134-153 ( )
- DOI: 10.1016/j.ijpddr.2012.04.001
- Mounsey K.E., Walton S.F., Innes A., Cash-Deans S., McCarthy J. In vitro efficacy of moxidectin versus ivermectin against Sarcoptes scabiei. Antimicrob. Agents Chemother., 2017, 61(8): e00381-17 ( )
- DOI: 10.1128/AAC.00381-17
- Raymond V., Sattelle D.B. Novel animal-health drug targets from ligand-gated chloride channels. Nat. Rev. Drug Discov., 2002, 1(6): 427-436 ( )
- DOI: 10.1038/nrd821
- Wolstenholme A.J., Rogers A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology, 2005, 131 Suppl: S85-95 ()
- DOI: 10.1074/jbc.R112.406280
- Estrada-Mondragon A., Lynch J.W. Functional characterization of ivermectin binding sites in α1β2γ2L GABA(A) receptors. Front. Mol. Neurosci., 2015, 8: 55 ( )
- DOI: 10.3389/fnmol.2015.00055
- Wolstenholme A.J. Glutamate-gated chloride channels. J. Biol. Chem., 2012, 287(48): 40232-40238 ( )
- DOI: 10.1074/jbc.R112.406280
- Degani-Katzav N., Gortler R., Weissman M., Paas Y. Mutational analysis at intersubunit interfaces of an anionic glutamate receptor reveals a key interaction important for channel gating by ivermectin. Front. Mol. Neurosci., 2017, 10: 92 ( )
- DOI: 10.3389/fnmol.2017.00092
- Stokes L., Layhadi J.A., Bibic L., Dhuna K., Fountain S.J. P2X4 receptor function in the nervous system and current breakthroughs in pharmacology. Front. Pharmacol., 2017, 8: Article 291 ( )
- DOI: 10.3389/fphar.2017.00291
- Godoy P., Che H., Beech R.N., Prichard R.K. Characterisation of P-glycoprotein-9.1 in Haemonchus contortus. Parasites & Vectors, 2016, 9: 52 ( )
- DOI: 10.1186/s13071-016-1317-8
- Mealey K.L., Bentjen S.A., Gay J.M., Cantor G.H. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics, 2001, 11(8): 727-733 ( )
- DOI: 10.1097/00008571-200111000-00012
- Xin-Jun Z., Wen-Cai L., Ya-Ning F., Lin H. High γ-aminobutyric acid content, a novel component associated with resistance to abamectin in Tetranychus cinnabarinus (Boisduval). J. Insect. Physiol., 2010, 56(12): 1895-1900 ( )
- DOI: 10.1016/j.jinsphys.2010.08.011
- Chen L.-P., Wang P., Sun Y.-J., Wu Y.-J. Direct interaction of avermectin with epidermal growth factor receptor mediates the penetration resistance in Drosophila larvae. Open Biol., 2016, 6(4): 150231 ( )
- DOI: 10.1098/rsob.150231
- Driggers E.M., Hale S.P., Lee J., Terrett N.K. The exploration of macrocycles for drug discovery -an underexploited structural class. Nat. Rev. Drug Discov., 2008, 7(7): 608-624 ( )
- DOI: 10.1038/nrd2590
- Wessjohann L.A., Ruijter E., Garcia-Rivera D., Brandt W. What can a chemist learn from nature’s macrocycles? -A brief conceptual view. Mol. Divers., 2005, 9(1-3): 171-186 ( )
- DOI: 10.1007/s11030-005-1314-x
- Yudin A.K. Macrocycles: lessons from the distant past, recent developments, and future directions. Chem. Sci., 2015, 6(1): 30-49 ( )
- DOI: 10.1039/c4sc03089c
- Fisher M.H. Recent advances in avermectin research. Pure App. Chem., 1990, 62(7): 1231-1240 ( )
- DOI: 10.1351/pac199062071231
- Dzhafarov M.Kh. Evolution in chemotherapy of human and animal helminthiases (review). Sel’skokhozyaistvennaya biologiya, 2013, 4: 26-44 ( )
- DOI: 10.15389/agrobiology.2013.4.26eng
- Chernoburova E.I., Danchenko K.V., Shchetinina M.A., Zharov A.A., Kolobov A.V., Dzhafarov M.Kh., Vasilevich F.I., Zavarzin I.V. Synthesis of 5,4-di-O-succinoylavermectin B1. Russ. Chem. Bull., 2016, 65(12): 2952-2955 ( )
- DOI: 10.1007/s11172-016-1684-5
- Chernoburova E.I., Polyukhova E.S., Shchetinina M.A., Kolobov A.V., Dzhafarov M.Kh., Vasilevich F.I., Zavarzin I.V. Synthesis of esters of bile acids and avermectin B. Russ. Chem. Bull., 2016, 65(12): 2956-2964 ( )
- DOI: 10.1007/s11172-016-1685-4
- Chernoburova E.I., Lishchuk V.A., Ovchinnikov K.L., Kolobov A.V., Dzhafarov M.Kh., Vasilevich F.I., Zavarzin I.V. Reaction of 5-O-succinoylavermectin B1 with alkylating agents. Russ. Chem. Bull., 2016, 65(12): 2965-2969 ( )
- DOI: 10.1007/s11172-016-1686-3
- Blinnikov A.N., Chernoburova E.I., Kolotyrkina N.G. Shchetinina M.A., Lishchuk V.A., Ovchinnikov K.L., Kolobov A.V., Dzhafarov M.Kh., Vasilevich F.I., Zavarzin I.V. Synthesis of ivermectin-4″,5-diyl. Russ. Chem. Bull., 2018, 67(5): 833-835 ( )
- DOI: 10.1007/s11172-018-2145-0
- Shchetinina M.A., Chernoburova E.I., Kolotyrkina N.G., Dzhafarov M.Kh., Vasilevich F.I., Zavarzin I.V. Synthesis of sodium 5-sulfate-ivermectin and disodium 4,5-disulfate-ivermectin. Russ. Chem. Bull., 2018, 67(5): 836-839 ( )
- DOI: 10.1007/s11172-018-2146-z
- Zeng X., Tian X., Hong X., Yu Y., Deng Z., Zhao C. Patent CN 103833811 A. Abamectin derivative and preparation method thereof. Appl. 2014. Publ. 2014.
- Li Y., Qin Y., Liu S., Xing R., Yu H., Li K., Li P. Preparation, characterization, and insecticidal activity of avermectin-grafted-carboxymethyl chitosan. Biomed. Res. Int., 2016, 2016: 9805675 ( )
- DOI: 10.1155/2016/9805675
- Дел Россо Д. Розацеа кожи: патогенез, клинические проявления, современные рекомендации по тактике ведения пациентов. Вестник дерматологии и венерологии, 2016, 2: 21-31 ( )
- DOI: 10.25208/0042-4609-2016-0-2-21-31
- Джафаров М.Х., Шемякова С.А., Мирзаев М.Н., Есаулова Н.В., Василевич Ф.И. Исследование нематоцидной и инсектоакарицидной активности препарата сумектин. Медицинская паразитология и паразитарные болезни, 2017, 3: 25-28.
- Джафаров М.Х., Мирзаева К.М., Василевич Ф.И., Мирзаев М.Н., Мельницкая Т.И. Методические положения по применению препарата Гемакс (Сумектин) при стронгилятозах желудочно-кишечного тракта овец. М., 2018.
- Мирзаева К.М., Земцова Л.К., Мирзаев М.Н., Джафаров М.Х., Мельницкая Т.И., Юсуфов Ю.А. Параметры острой и хронической токсичности инсектицидного препарата «ВЭИС-2». Ветеринария, зоотехния и биотехнология, 2017, 2: 16-21.
- Земцова Л.К., Мирзаев М.Н., Джафаров М.Х., Мирзаева К.М. Инсектицидная активность авермектинсодержащего препарата ВЭИС-2 против Tineola bisselliella и Attagenus smirnovi Zhant. Ветеринария, зоотехния и биотехнология, 2017, 3: 73-78.


