Пористость и сорбционные свойства цеолитов, синтезированных из угольной золы уноса
Автор: Шушков Д.А., Шуктомова И.И., Рачкова Н.Г., Харжа М.
Журнал: Вестник геонаук @vestnik-geo
Рубрика: Научные статьи
Статья в выпуске: 3 (279), 2018 года.
Бесплатный доступ
Зола уноса, образующаяся при сжигании угля для производства энергии и тепла, относятся к промышленным отходам, огромные скопления которых представляют серьезную экологическую угрозу. Разработка технологий её утилизации является актуальной проблемой. В рамках возможных экологических и экономических рисков использование сорбентов на основе золы уноса для очистки сточных вод представляет собой альтернативу индустриальным материалам. В работе представлены результаты экспериментальных исследований цеолитов, полученных гидротермальным автоклавным способом из золы уноса ТЭЦ (г. Воркута). Исходная зола и синтезированные материалы характеризовались с помощью сканирующей электронной микроскопии, рентгеновской дифрактометрии и др. Выявлены характеристики синтезированных цеолитов: удельная площадь поверхности, объем микро- и мезопор, общий объем пор. Экспериментальные результаты показали, что новые материалы могут быть успешно использованы для извлечения из водных растворов аммония, бария, стронция и естественных радиоактивных элементов: урана, радия, тория.
Цеолиты, сорбция, радионуклиды, аммоний, барий, стронций, зола уноса
Короткий адрес: https://sciup.org/149129302
IDR: 149129302 | УДК: 549 | DOI: 10.19110/2221-1381-2018-3-32-37
Текст научной статьи Пористость и сорбционные свойства цеолитов, синтезированных из угольной золы уноса
Rational use of water resources and their effective treatment are urgent problems of modern society. Selective extraction of heavy metals and radionuclides from solutions remains a challenge, for which various methods are used: ion exchange, adsorption, biosorption, chemical precipitation, coagulation, electrodialysis. Among the mentioned processes, sorption methods with the use of inorganic sorbents, possessing high selectivity, chemical and radiation resistance, are efficient and economically justified. Many papers have been published, in which natural and modified clays and zeolites, porous titanosilicates, phosphates, carbonates, technogenic waste — coal fly ash and sorbents, based on it, red mud and clay-salt mud, etc., were tested as sorbents [4—7, 11, 13—15, 17].
Zeolites are widely used in the environmental protection, for example, for soil regeneration, treatment of acid mine waters, removal of sulfur dioxide from industrial emissions, purification of drinking and industrial wastewater, especially for the sorption of ammonium, heavy metals, radionuclides and organic substances. In addition to their high ion-exchange and sorption properties, zeolites have thermal, chemical, radiation resistance, mechanical 32
strength. These properties depend on the type of zeolite and features of its structure: pore size, pore volume, Si/Al ratio, composition and position of cations. The ability of zeolites to effectively absorb and firmly retain radionuclides draws a special interest [10]. Earlier, we synthesized zeolites from fly ash from thermal power plants of Vorkuta town [8, 9], which use hard coal of gas fat, semi-lean mark from the mine «Vorgashorskaya» (Fig. 1).
The aim of this paper is to reveal features of the specific surface area and porosity of synthesized zeolites depending on the conditions of hydrothermal reaction and to test their sorption properties for ammonium, barium, strontium and natural radioactive elements (uranium, thorium and radium).
Objects and methods
The object of our study was reaction products obtained from fly ash from thermal power plants of Vorkuta town by hydrothermal synthesis.
The morphology and chemical composition of the fly ash was studied by SEM (TESCAN VEGA 3 LMH) equipped with an EDS (Oxford Instruments X-Max). The
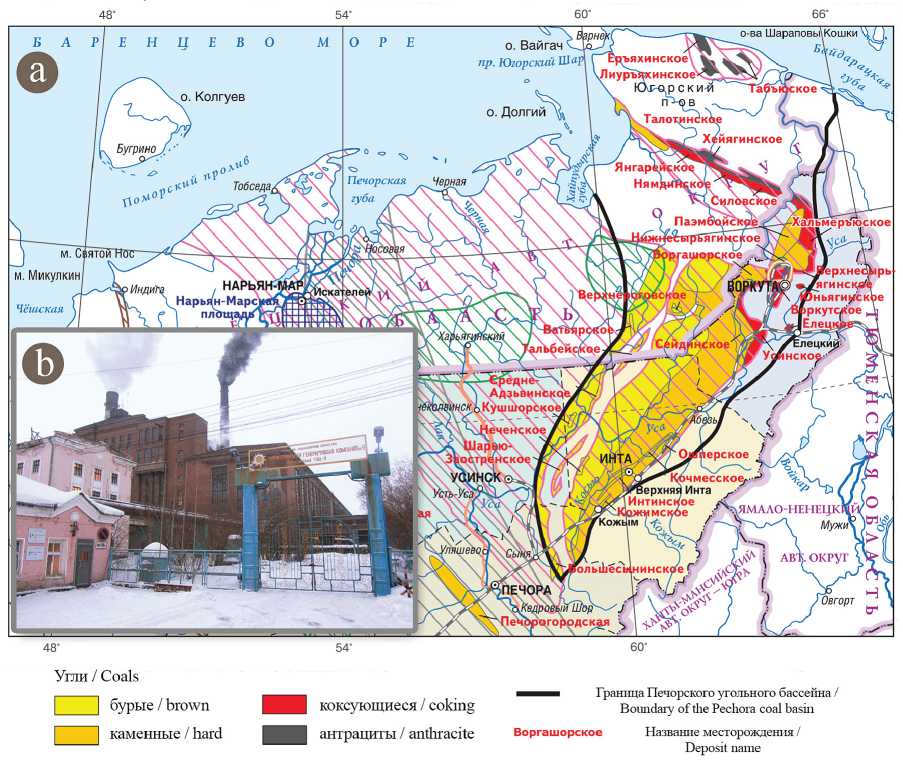
Fig. 1. Deposits of the Pechora coal basin (a) [1]; thermal power plant ¹ 2 of Vorkuta town (b)
Риñ. 1. Ìåñòîðîæäåíèÿ Ïå÷îðñêîãî óãîëüíîãî áàññåéíà (à) [1]; Âîðêóòèíñêàÿ ÒÝÖ-2 (b)
mineral composition was determined by XRD (Shimadzu XRD 6000, CuKa radiation) within range 2—65° 2θ with rate 2° 2θ/min. Identification of zeolites and other phases was carried out using MINCRYST and International Zeolite Association databases. The specific surface area, micro- and mesopore volume, total pore volume were determined using a surface area and pore size analyzer (NOVA 1200e, Quantachrome) at –196 °Ñ with preliminary degasification at 350 °Ñ in vacuum for 2 h. The specific surface area was calculated by the BET method (nitrogen adsorption curve), the mesopore volume by the BJH method (nitrogen desorption curve), the micropore volume by the Dubinin-Astakhov method.
The sorption capacity for cation NH4+ was determined by a photometric method with a Nessler reagent using KFK-2 spectrophotometer at a wavelength 440 nm. The concentration of ammonium chloride was 100 mg/l, the sample weight was 0.02 g, pH of the solution was 5.55, duration of the experiment was 1 hour.
To study Sr2+ sorption, a zeolite sample 0.02 g was filled with 0.01 l of an aqueous solution SrCl2 with a concentration 500 mg/l (the solution was produced by dissolving SrCO3 in HCl) and held for 24 hours at room temperature. Sorption of Ba2+ was carried out as follows: 0.02 g of zeolite was filled with 0.01 l of an aqueous solution of BaCl2 at a concentration of 500 mg/l and held for 24 hours at room temperature. The initial and equilibrium concentrations of cations of strontium and barium in the solution were de- termined by atomic-emission spectroscopy with inductively coupled plasma on the spectrometer Vista MPX Rad.
The sorption of radionuclides was carried out under static conditions at room temperature and a ratio of solid and liquid phases 1:10 (2 g of sorbent and 20 ml of solution) from aqueous solutions of uranyl nitrate, radium chloride and thorium chloride, in which radionuclides were represented by a natural mixture of isotopes. The initial concentration of radionuclides in solution: radium — 28.31 ⋅ 10–12 g/ml; uranium 1.35 ⋅ 10–6 g/ml; thorium 0.87 ⋅ 10–6 g/ml. The contact time was 24 h, pH of the solution — 6. The acidity of the liquid phase was adjusted to the required level by alkalinization with concentrated (13 mole/l) ammonium hydroxide solution. After sorption, the sorbents were separated from the liquid phase by filtration. In the filtrate, the content of radionuclides was determined. The extraction of uranium, radium and thorium was calculated by the radionuclide decrease. The uranium content was measured by a photometer «LUF-57» based on the luminescence intensity of pearls with NaF at the sensitivity 2.0 ⋅ 10–8 g/g. Thorium was determined pho-tocolorimetrically with arsenazo III with separation of impurities on cation-exchange KU-2 at a detection limit 1.0 ⋅ 10–8 g/g. The radium content was calculated from ra-don-222 emanation measured on Alfa-1 device with the sensitivity of the method 2.0–10–12 g/g. The desorption was estimated from the content of radionuclides in the extracts obtained by sequential treatment with radionucliderich sorbent with distilled water, solutions of ammonium 33
acetate (1M CH3COONH4) and hydrochloric acid (1M HCl) for 24 hours.
Results and discussion
Two sets of experiments were carried out:
-
— in the first set the effect of temperature of hydrothermal reaction on zeolite synthesis was studied (reaction temperature 95, 140 and 180 °C, reaction duration 12 hours, NaOH concentration 3.0 mol/l).
-
— in the second set of experiments the effect of reaction time and concentration of alkali on synthesis process was studied (reaction temperature 140 °C, reaction duration 2, 4, 6 and 8 hours, concentration NaOH 1.5, 3.0 and 4.5 mol/l).
The synthesis results in powders consisting of the mixture of zeolite/zeolites and unreacted residue in different proportions.
The effect of reaction temperature on morphology and porosity of synthesized zeolites
At reaction temperatures from 95 to 180 °C several species of zeolites were obtained [6], different by their morphology and porosity (Fig. 2, a—c). It was found that increasing temperature leads to reducing pore size of the zeolites. Thus, at 95 °C zeolites X (a synthetic analogue of natural faujasite, pore size 0.74 nm [3]) formed with an admixture of zeolite P (a synthetic analogue of natural gis-mondine, pore size 0.31x0.45 and 0.28x0.48 nm [3]). At 140 °C zeolites P formed with an admixture of analcime (pore size 0.26 and 0.42x0.16 nm [3]). At 180 °C, analcime crystallizes with an admixture of cancrinite (a non-zeolit-ic phase). According to the classification of zeolites by pore size [2], analcime and zeolite P refer to narrow-porous, zeolite X — to wide-porous species.
Nitrogen adsorption-desorption isotherms of fly ash and reaction products (Fig 3, a, b) are related to IV(a) type according to IUPAC classification [16], characteristic for mesoporous sorbents. Isotherms have a hysteresis loop close to H3 type, which indicates the existence of slotted capillaries with parallel plates.
Table 1 presents characteristics of the fly ash and reaction products synthesized at temperatures 95, 140 and 180 °C. The specific surface area of the initial fly ash was 5.63 m2/g. The conversion of ash to zeolites is accompanied by increasing specific surface area (2.8—45.4 times), volume of micro- (4—68 times) and mesopores (3.8—16.1 times) and total pore volume (3.7—29.4 times), in accordance with [12]. Increasing temperature of the hydrothermal

Fig. 2. SEM-images of hydrothermal reaction products synthesized at 95 (a), 140 (b), 180 °Ñ (c): a — octahedral crystals of zeolite X, b — skeleton crystal of zeolite P, c — tetragontrioctahedron of analcime covered with columnar cancrinite
Риñ. 2. ÑÝÌ-èçîáðàæåíèÿ ïðîäóêòîâ ãèäðîòåðìàëüíîé ðåàêöèè, ïîëó÷åííûõ ïðè òåìïåðàòóðàõ 95 (à), 140 (b) è 180 °Ñ (c): a — êðèñòàëëû öåîëèòà Õ îêòàýäðè÷åñêîé ôîðìû, b — ñêåëåòíûé êðèñòàëë öåîëèòà P, c — òåòðàãîíòðèîêòàýäð àíàëüöèìà, ïîêðûòûé ñòîëá÷àòûì êàíêðèíèòîì
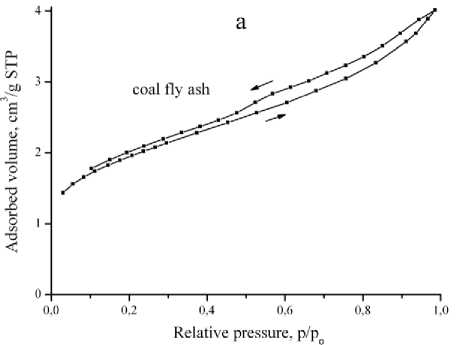
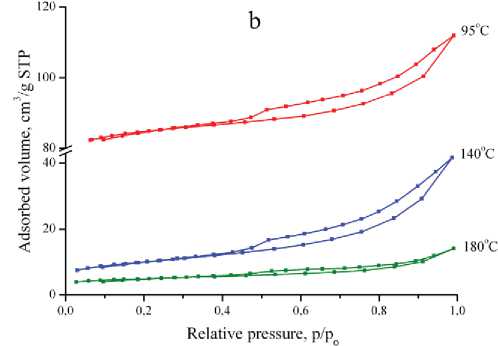
Fig 3. Nitrogen adsorption-desorption isotherms of coal fly ash (a) and reaction products synthesized at 95, 140, 180 °Ñ (b)
Риñ. 3. Èçîòåðìû àäñîðáöèè-äåñîðáöèè àçîòà çîëû óíîñà (à) è ïðîäóêòîâ ðåàêöèè, ïîëó÷åííûõ ïðè òåìïåðàòóðàõ 95, 140 è 180 °Ñ (b)
reaction leads to decreasing specific surface area and pore volume parameters of the synthesis products, which is related to formation of less porous zeolites. Wide-porous zeolites X, produced at 95 °C, are characterized by a high specific surface, micropore volume and total pore volume. The values of these parameters for zeolite P and analcime, synthesized at 140 and 180 °C, respectively, are much lower.
The effect of reaction duration and NaOH concentration on specific surface area and porosity of synthesized zeolites
Table 2 characterized products obtained at temperature 140 °C, NaOH concentration 1.5—4.5 mol/l, and reaction time 2 to 8 hours. Both single zeolites and a mixture of zeolites are diagnosed in the synthesized samples. The specific surface area of the synthesis products, containing zeolites of different types, varies within 27.9 to 261.9 m2/g. A sample, containing a wide-porous zeolite X (261.9 m2/g) has the largest specific surface. The corresponding characteristic for medium porous zeolites P varies within 35.43—45.32 m2/g. The synthesis products, in which no newly formed phases are detected, show increasing specific surface area in comparison to the initial ash in 3.9—5.9 times. Perhaps this is due to the disintegration (dissolution) of the original globules of ash in an alkaline medium.
The total pore volume of synthesized materials (0.0297—0.1526 cm3/g), the volume of their meso- (0.0217— 0.0506 cm3/g) and micropores (0.0104—0.1323 cm3/g) also increase in comparison with the initial fly ash (0.0059, 0.0041 and 0.0020 cm3/g respectively). They depend both on the
Table 1. Specific surface area and porosity of coal fly ash and reaction products synthesized at 95,140 and 180 °C Таблица 1. Удельная площадь поверхности и пористость золы уноса и продуктов реакции, синтезированных при температурах 95,140 и 180 °C
|
Reaction temperature, ° |
Reaction products* |
Specific surface area, m2/g |
Total pore volume, cm3/g |
Mesopore volume, cm3/g |
Micropore volume, cm3/g |
|
fly ash |
5.63 |
0.0059 |
0.0041 |
0.0020 |
|
|
95 |
zeolite Õ, zeolite Ð |
255.6 |
0.1732 |
0.0503 |
0.1362 |
|
140 |
zeolite Ð, analcime |
38.77 |
0.0744 |
0.0662 |
0.0211 |
|
180 |
analcime |
16.07 |
0.0218 |
0.0156 |
0.0080 |
Note: * — the phase composition of the hydrothermal reaction products was determined by X-ray diffraction, the data were presented in [9];
Ïðèìå÷àíèå: * — ôàçîâûé ñîñòàâ ïðîäóêòîâ ãèäðîòåðìàëüíîé ðåàêöèè îïðåäåëåí ñ ïîìîùüþ ðåíòãåíîâñêîé äèôðàêöèè, äàííûå ïðåäñòàâëåíû â ðàáîòå [9].
Table 2. Specific surface area and porosity of coal fly ash and reaction products synthesized at 140 °C, NaOH concentration 1.5, 3.0, 4.5 mol/l and reaction duration 2—8 h
Таблица 2. Удельная поверхность и пористость золы уноса и продуктов реакции, синтезированных при температуре 140 °C, концентрации NaOH 1.5, 3.0, 4.5 моль/л и продолжительности реакции 2—8 ч
|
Sample |
Concentration of NaOH, mol/l |
Reaction duration, h |
Reaction products* |
Specific surface area, m2/g |
Pore volume |
||
|
total, cm3/g |
mesopore, cm3/g |
micropore, cm3/g |
|||||
|
fly ash |
5.63 |
0.0059 |
0.0041 |
0.0020 |
|||
|
1.5—2 |
1.5 |
2 |
n. d. |
22.21 |
0.0297 |
0.0231 |
0.0104 |
|
1.5—4 |
4 |
zeolite P** |
27.90 |
0.0502 |
0.0433 |
0.0125 |
|
|
1.5—6 |
6 |
zeolite Ð |
35.43 |
0.0435 |
0.0329 |
0.0158 |
|
|
1.5—8 |
8 |
zeolite Ð, analcime |
44.63 |
0.0641 |
0.0506 |
0.0206 |
|
|
3.0—2 |
3.0 |
2 |
n. d. |
23.86 |
0.0494 |
0.0449 |
0.0111 |
|
3.0—4 |
4 |
zeolite Ð |
45.32 |
0.0685 |
0.0583 |
0.0205 |
|
|
3.0—6 |
6 |
zeolite Ð, analcime |
42.73 |
0.0490 |
0.0361 |
0.0194 |
|
|
3.0—6 |
8 |
zeolite Ð, analcime |
45.61 |
0.0518 |
0.0369 |
0.0207 |
|
|
4.5—2 |
4.5 |
2 |
n. d. |
33.07 |
0.0426 |
0.0314 |
0.0156 |
|
4.5—4 |
4 |
zeolite Õ, zeolite Ð, hydrosodalite |
261.9 |
0.1526 |
0.0246 |
0.1323 |
|
|
4.5—6 |
6 |
zeolite Ð, analcime, hydrosodalite |
44.14 |
0.0388 |
0.0217 |
0.0217 |
|
|
4.5—8 |
8 |
zeolite Ð, analcime, hydrosodalite |
37.15 |
0.0411 |
0.0307 |
0.0170 |
|
Note: * — the phase composition of the hydrothermal reaction products was determined by X-ray diffraction, the data were presented in [9]; Ð** — XRD weak peaks are observed, probably, related to zeolite P (data are not shown); n. d. — zeolite phases are not determined.
Ïðèìå÷àíèå: * — ôàçîâûé ñîñòàâ ïðîäóêòîâ ãèäðîòåðìàëüíîé ðåàêöèè îïðåäåëåí ñ ïîìîùüþ ðåíòãåíîâñêîé äèôðàêöèè, äàííûå ïðåäñòàâëåíû â ðàáîòå [9]; P** — íà äèôðàêòîãðàììå íàáëþäàþòñÿ ñëàáûå ðåôëåêñû, âîçìîæíî ïðèíàäëåæàùèå öåî-ëèòó Ð; n. d. — öåîëèòîâûå ôàçû íå îáíàðóæåíû.
type of zeolite and on its content in the mixture. A sample, containing zeolite X, is characterized by increasing total pore volume and microporous volume in 2—5 and 6—8 times, respectively, compared to other synthesis products.
As can be seen from Table 3, the specific surface area directly and strongly correlates with the total pore volume and the volume of micropores and does not correlate with the mesopore volume. The total pore volume directly and strongly correlates with the volume of micropores and does not correlate with the volume of mesopores. The volume of mesopores does not correlate with any parameter.
Sorption properties
The synthesized zeolites (sample 4.5—4) are characterized by a high sorption activity to long-living natural radionuclides — uranium, radium and thorium. From model solutions with a concentration of radionuclides exceeding their content in natural waters, up to 98 % of radium, more than 89 % of thorium and 80 % of uranium are removed (Table 4).
Analisys of the desorption characteristics shows (Fig. 4) that the sorbents possess a high retain characteristic with respect to radium and uranium. This is demonstrated by their low total desorption, which is about 4.88 è 10.15 % respectively. Thorium is retained by zeolites less firmly, up to 35 % of thorium desorbed into the solution.
Comparison of sorption characteristics of materials for three tested radioactive elements indicates a more pronounced tendency of zeolites to radium removal from aqueous solutions. In this respect, it is natural that the determined sorption capacity of zeolites for other elements of group II of the Periodic Table also shows high values (Table 5). Thus, for barium sorption capacity ranges from 113.3 to 157.5 mg/g (1.65—2.29 meq/g), for strontium it reaches 85.9 mg/g (1.94 meq/g). For comparison, the analogous value for an ammonium ion is 32.2 mg/g (1.78 meq/g). When background electrolyte (NaOH) is added to the solution, the sorption capacity decreases to 57.2 mg/g (1.31 meq/g), which can result from both competing sorption of sodium ions on zeolites, and a significant suppression of activity of their sorption centers under alkaline conditions.
Conclusions
We compared zeolites, produced from fly ash by a hydrothermal reaction, by their specific surface area and porosity.
The determined characteristics of the synthesized materials vary within a wide range depending on type of zeolite and reaction products. Samples with wide-porous zeolites X has the greatest specific surface area (up to 261.9 m2/g)
Synthesized zeolites are characterized by a high sorption activity with respect to long-living natural radionuclides and possess low desorption of uranium and radium. The extraction degree of radium from solutions is 98, thorium — more than 89, uranium — more than 80 %. Thorium is retained in the structure of synthesized sorbents the least strongly. The sorption capacity for barium has values from 113.3 to 157.5 mg/g (1.65—2.29 meq/g), for strontium — 85.9 mg/g (1.94 meq/g), for ammonium 32.2 mg/g (1.78 meq/g). By selectivity of sorption, the above cations can be arranged in a line: Ba2+> Sr2+> NH4+.
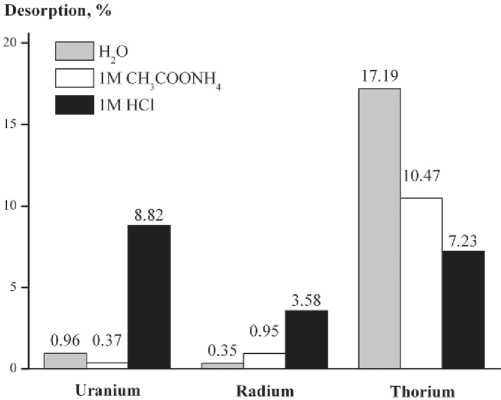
Fig. 4. Desorption of uranium, radium and thorium from synthesized zeolites after treatment by distilled water, ammonium acetate and hydrochloric acid
Рис. 4. Десорбция yра^а, радия и тория из cи^тeзиpoвa^^ыx öåîëèòîâ â ðåçóëüòàòå îáðàáîòêè äèñòèëëèðîâàííîé âîäîé, àöåòàòîì àììîíèÿ è ñîëÿíîé êèñëîòîé
Table 3. Coefficients of pair correlation of specific surface area and porosity of reaction products
Таблица 3. Коэффициенты парной корреляции удельной площади поверхности и пористости продуктов реакции
|
Specific surface area |
Total pore volume |
Mesopore volume |
Micropore volume |
|
|
Specific surface area |
1.00 |
|||
|
Total pore volume |
0.96 |
1.00 |
||
|
Mesopore volume |
–0.29 |
–0.01 |
1.00 |
|
|
Micropore volume |
1.00 |
0.96 |
–0.30 |
1.00 |
Table 4. Uranium, radium and thorium sorption by synthesized zeolites Таблица 4. Сорбция урана, радия и тория синтезированными цеолитами
|
Radionuclide |
Radionuclide concentration in solution, g/ml |
Removal efficiency, % |
Distribution coefficient, ml/g |
|
|
initial |
equilibrium |
|||
|
Uranium |
1.35 x 10—6 |
0.25 x 10—6 |
81.30 |
43.53 |
|
Radium |
28.31 х 10-12 |
0.47 х 10-12 |
98.34 |
592.24 |
|
Thorium |
0.87 х 10-6 |
0.09 х 10-6 |
89.36 |
84.54 |
Table 5. Barium, strontium and ammonium sorption capacity of reaction products
Табёица 5. Сорбционная емкость продуктов реакции по барию, стронцию и аммонию
|
Sample |
Cation concentration in solution, mg/l |
Sorption capacity |
Distribution coefficient, ml/g |
||
|
initial |
equilibrium |
mg/g |
meq/g |
||
|
Barium |
|||||
|
1.5—8 |
500.0 |
180.2 |
157.5 |
2.29 |
1523.6 |
|
4.5—4 |
500.0 |
273.4 |
113.3 |
1.65 |
576.3 |
|
Strontium |
|||||
|
1.5—8 |
500.0 |
155.0 |
85.9 |
1.94 |
1307.3 |
|
4.5—4 |
500.0 |
192.0 |
77.0 |
1.76 |
754.9 |
|
Ammonium |
|||||
|
1.5—8 |
100.0 |
35.6 |
32.2 |
1.78 |
904.5 |
|
4.5—4 |
100.0 |
40.0 |
29.9 |
1.66 |
746.3 |
The combination of a high sorption activity and ability to retain potential pollutants rather firmly is an important characteristic of the sorption material in terms of its practical use, since it avoids secondary (reverse) contamination of the working environment by radionuclides. The produced sorbents can be used for trapping and retaining of radioactive elements in solutions in a wide range of concentrations. It also increases the scope of the sorption material — from low-concentration radionuclides of natural waters to high radioactive liquid wastes from nuclear power plants.
The authors express gratitude to the common use center «Geonauka» for their help in analytical work.
The work was carried out with a partial financial support of UB RAS Programs (project 18-5-5-44).
Список литературы Пористость и сорбционные свойства цеолитов, синтезированных из угольной золы уноса
- Burtsev I. N., Saldin V. A., Ievlev A. A., Anischenko L. A., Protsko O. S. Burye ugly -perspektivniy resurs dlya sozdaniya novyh otrasley promyshlennosti v Timano-Severouralskom regione (Brown coals -a promising resource for the creation of new industries in the Timan-North Ural region). Vestnik of Institute of Geology of Komi SC of UB RAS, 2012, No. 10, pp. 26-31.
- Chica A. Zeolites: promised material for the sustainable production of hydrogen. ISRN Chemical engineering, 2013. Article ID 907425. 19 p.
- Database of zeolite structures. www.iza-structure.org/database (accessed at: 05.02.2018)
- Harja M., Kotova O., Ciobanu G., Litu L. New adsorbent materials on the base of ash and lime for lead removal. Proceedings book: International Symposium "The environment and the industry", Simi 2017, Bucharest, Romania.
- Ivanets A. I., Shashkova I. L., Kitikova N. V., Drozdova N. V., Saprunova N. A., Radkevich A. V., Kulbitskaya L. V. Sorbtsionnaya ochistka rastvorov ot ionov strontsiya fosfatami kaltsiya i magniya (Sorption purification of solutions from strontium ions by calcium and magnesium phosphates). Radiokhimiya (Radiochemistry), 2014, V. 56, No. 1, pp. 30-34.


