Potato viruses of 7 commercial cultivars grown in field Primorsky krai of Russia
Автор: Sobko Olga A., Fisenko Petr V., Kim Irina V., Matsishina Nathalia V.
Журнал: Овощи России @vegetables
Рубрика: Луговодство, лекарственные и эфиромасличные культуры
Статья в выпуске: 1 (63), 2022 года.
Бесплатный доступ
Scientific relevance. Plant viruses cause a significant economic loss to potato production, especially if plants are infected at early growth stages and infections are mixed. Viral diseases reduce both yield and quality of harvested crops. Detection and identification of plant viruses are key important to prevent their spreading and to achieve potential yield predetermined by characteristics of varieties. Research methods. Seven potato varieties, bred in Russia and overseas, were used in the field experiment: Smak, Avgustin, Yantar, Laperla, Labella, Red Lady, Sante, Belmonda. Viral infection rate was measured by the percent of plants with symptoms to the total number of plants. In addition to infection frequency, a disease rate was described after visual estimation. Total RNA was isolated from the collected leaves according to Bekesiova I. et al. 1999 [13]. Qualitative and quantitative estimation of plant viruses in the samples were conducted by single-step real-time RT-PCR with fluorescent detection with the Applied Biosystems QuantStudio 5 and commercial kits “Potato Virus X, Y, M, L, S, A, PSTVd-RT” (Syntol Company) according to the official protocol of the kits. Results. As a result of our research, symptoms of mixed viral infection were described for potato varieties depending on concentrations and proportions of these viruses in a plant. Mixed viral infection in the potato field in Primorsky Krai comprised PVY, PVX, PVA, PVS, PVM, also PLRV and PSTVd.
Plant viruses, insect vectors, mixed viral infections, solanum tuberosum
Короткий адрес: https://sciup.org/140290730
IDR: 140290730 | УДК: 632.38:635.21 | DOI: 10.18619/2072-9146-2022-1-79-85
Текст научной статьи Potato viruses of 7 commercial cultivars grown in field Primorsky krai of Russia
D amage, inflicted on the agricultural sector by viral diseases, is enormous. Viruses reduce both yield and quality of harvested crops. Ways and means of virus distribution vary significantly and depend on host-organism characteristics. Thick cellulose cell membranes make flowering plants impenetrable for viruses, and invasion occurs only through wounds. Insects are the most important virus carriers and serve both as vectors and as hosts. Relations between viruses and host plants or vectors are very specific. Viruses can accumulate on the stylet while an insect feeds on infected plants. When a vector feeds on healthy plants, viruses penetrate damaged cells, vascular fluids and cause infection. Ability of an insect vector to infect plants with viruses depends not only on cell permeability in its alimentary canal and salivary glands but also on a possibility to infect the vector organism. Virulence of non-persistent or semi-persistent viruses predetermines their need for time (from a few minutes to several hours) and temporary attachment (usually) to the stylet or the foregut of an insect. On the contrary, persistent viruses, that move through the barrier of the midgut and accumulate in the salivary glands, can cause infection for a period from a few days to several months [1].
Potatoes are the main non-cereal food product. Potato production faces such obstacles as pests and diseases, including viral ones. Viral diseases play a significant role among factors that limit potato production [2]. Virus infection symptoms appear at the germination stage and predominantly at the stages of growth, flowering and fruiting [3]. Viruses of the following families are widespread in Primorsky Krai: Bromoviridae, Potyviridae, Flexviridae, Luteoviridae, and Pospiviroida e. PVY (potato virus Y, fam. Potyviridae ) poses the main problem for solanaceous crops. Apart from potato, it infects pepper and tomato. PVY reduces total yield and negatively affects the quality of harvested crops. Seeds infected by PVY can serve as an infection source and present a problem for certification because symptoms appear depending on potato varieties and sometimes cannot be estimated visually [4]. The following factors determine epidemiological importance of PVY:
-
1) non-persistent transmission by more than 50 species of aphids, potato ladybird Henosepilachna vigintioctomaculata, and grass bug Lygus pratensis [5];
-
2) high genetic variability, conditioned by several strains;
-
3) a wide circle of host-plants comprising weeds that grow in potato fields and at field borders.
It all proves that effective virus transmission depends not only on specific traits of vectors and the virus but also on accessibility of the virus to a carrier [6]. Plant viruses can lead to a significant economic loss to potato production, especially if plants are infected at early growth stages and infections are mixed [2]. Mixed infection implies that there are more than one virus coexisting in a plant. It causes appearance of different symptoms. Presence of more than one virus always hampers disease etiology understanding. Most viral diseases are not diagnosed due to their latency or weak symptomatic expression, or similarity of symptoms with fungal and bacterial infections. It can be true for a plant infected with one specific virus. In case of mixed infection, more severe symptoms usually appear [7]. Viruses can infect one host-plant simultaneously (co-infection) or subsequently (superinfection). Plants, attacked by viruses, activate complex protection mechanisms, which work on different levels and often require considerable inner resources. It decreases the yield. Due to all these factors, PVY can cause significant loss of potato yield with mixed infections with PVX, PVM, PVS and some other viruses [6]. Symptom appearance depends on a type of interactions among viruses in a host-organism. Unrelated viruses usually interact with each other in a synergistic way whereas interactions among related viruses are predominantly antagonistic [8]. Detection and identification of viruses, causing infection, are crucial for successful treatment of viral diseases, especially with mixed infections [7]. All these reasons determinedthe purpose ofthis study. The research purpose is to study viral infection load in the agricultural ecosystem of potato fields, estimate the influence of qualitative and quantitative interrelation among viruses in plants on symptom appearance, and determine the plant virus concentration, which causes expression of symptoms on potato plants in field.
Methods
Seven potato varieties (cvs. Smak, Avgustin, Yantar, Laperla, Labella, Red Lady, Sante, Belmonda) were used in the field experiment. Smak is a medium late variety (breeder – FSBSI “FSC of Agricultural Biotechnology of the Far East named after A.K. Chaika”, Russia), moderately resistant to late blight and Alternaria leaf spot, resistant to potato wart disease and susceptible to nematode Globodera rostochiensis Wollenweber. Cv. Avgustin is a medium variety (breeder – FSBSI “FSC of Agricultural Biotechnology of the Far East named after A.K. Chaika” Russia), susceptible to Globodera rostochiensis. Cv. Yantar is a medium late variety (breeder – “FSC of Agricultural Biotechnology of the Far East”, Russia), resistant to potato wart disease ( Synchytrium endobioticum (Schilberszky) Percival) and susceptible to Globodera ros-tochiensis, with leaves, stems and tubers susceptible to late blight. Cvs. Laperla and Labella are early varieties (breeder – Den Hartigh BV, Netherlands), resistant to potato wart disease, pathotype I and Globodera rostochiensis (R01). According to the breeder, they are resistant to leaf curl. Cv. Red Lady is an early variety (breeder – SOLANA GMBH & CO KG, Germany), resistant to potato wart disease, pathotype I and Globodera rostochiensis (R01), with leaves and stems susceptible and roots moderately susceptible to late blight Phytophthora infestans Mont. de Bary. Cv. Sante is a medium early variety (breeder – AGRICO U.A., Netherlands, “FSC of Agricultural Biotechnology of the Far East”), resistant to potato wart disease, pathotype I and Globodera rostochiensis (R01), late blight and viruses . Cv. Belmonda is a medium early variety (breeder – SOLANA GMBH & CO KG, Germany), resistant to potato wart disease, pathotype I and Globodera ros-tochiensis (R01) [9]. The selected potato varieties are resistant forms in breeding programs.
Plants were planted with 50 tubers per plot. The site is located in the Southern taiga agricultural soil and climate zone (43.850516, 131.960421). The area of the plot is 40 sq. m. Potato is planted on ridges of 70x40 cm at the rate of 37 thousand plants per 1 ha. The size of a plot is 25 sq. m. The plot soil is meadow-brown podzolized. Soil preparation: underwinter plowing to a depth of 22 cm, early spring harrowing, pre-sowing cultivation, and two cultivations for vegetation were used.
For virus identification, symptomatic potato leaves were collected in filter paper bags, folded in polyethylene packaging and frozen at - 20°C for the following PCR analysis of the viruses. The leaf surface was cleaned with non-woven materi- al moistened in alcohol before sample preparation [10].
Virus infection rate was measured by the percent of plants with symptoms to the total number of plants. In addition to infection frequency, disease rate was described after visual estimation. To estimate the degree of disease development on individual plants, we used 9-point scale of virus-resistance evaluation, described in “The broad unified CMEA classifier and international classifier of CMEA for potato varieties of section Tuberarium (Dun.) Buk. of genus Solanum L.” [11, 12]. Petunia sp. was used as a bio-indicator [13].
Total RNA was isolated from the collected leaves according to Bekesiova I. et al. 1999 [14]. The extracted RNA was additionally cleaned with the mixture of chloroform/ amyl alcohol (24/1) and/or chroloform/ phenol (1/1). Efficiency of isolation was measured by electrophoresis in 1 % agarose gel stained with ethidium bromide. The result of electrophoresis was documented by GelDoc XR+ (BioRad). RNA concentration in the preparation was determined with the usage of the Invitrogen Qubit 4 Fluorometer and the RNA BR Assay Kit with subsequent dilution to 50 nanograms/ microlitre. Qualitative and quantitative estimation of plant viruses in the samples were conducted by single-step real-time RT-PCR with fluorescent detection with the Applied Biosystems QuantStudio 5 and commercial kits “Potato Virus X, Y, M, L, S, A and PSTVd-RT” (Syntol Company, Russia) according to the official protocol of the kits. 250 ng of total RNA were used in each RT-PCR reaction. Qualitative estimation of infection level was conducted with the comparative Ct method (ΔΔСt) [15]. Inner control of the reaction served as the endogenous control. Positive control samples of the reagent kits were used as a reference sample.
The statistical data processing was conducted with the IBM SPSS Statistics software (Version).
Research results and discussion
To study the process of virus accumulation, we performed an experiment on artificial infection with petunias as indicatorplants. The plants were grown from seeds and divided in two groups – infected test and control. The juice from potato leaves with a high PVY concentration established by PCR analysis was applied to the leaves of the test indicator-plants. The plants of the control and infected groups were further grown separately. Necessary arrangements were made to prevent accidental infection. As a result of the experiment, when the concentration of PVY was increased by 1.8 *104 times compared to the asymptomatic Petunia sp. plants, the following symptoms were observed: red and purple strokes and spots appeared on the petals depending on their color, the leaf veins brightened up, the leaf blades remained green. A lower concentration of the plant virus did not lead to development of visible symptoms. It indicates the latent infection (fig. 1 a, b). Consequently, there is a clear correlation between quantitative plant virus load and symptoms of the infection.
To continue the laboratory experiment with the indicatorplants, we made field records and measurements of disease progress. The following symptoms could be observed on potato plants in experimental field plots with natural infection in 2021: mosaics and mottling, chlorosis, curled and rippled leaf edges, distorted and withered flower petals, unopened buds at the flowering stage, red border on leaves, and dwarf plants. The symptoms varied on plants of different varieties and on the plants of the same variety. After visual estimation, the average disease rate on cv. Smak (I) was about 2.4±0.12. The disease appeared as mosaics, leaf necrosis, chlorosis of leaf veins, unopened flower buds. The plants of this variety in the second and third repetitions had the disease rate equal 1.1±0.52 (14 out of 25 plants). Infected plants were characterized by the presence of mosaics on their leaves (fig. 2). This fact can be explained by special isolation and unequal distribution of vectors.
Virus infection on plants of cv. Laperla was the same in all three repetitions and included mosaics, leaf edge necrosis, leaf galls, short height of green parts (dwarf plants). The disease rate was from 3.0±0.12 to 4.0±0.12. Virus infection symptoms on plants of cv. Labella included chlorosis and mosaics ranging from barely discernible yellow spots to pronounced lesions. The disease rate was in average 2.0±0.25. After visual estimation, the number of damaged plants of cv.

Рис.1.Проявления инфекции на Petuniasp.:а) здоровое растение;б)больное растение
Fig.1.Symptoms of PVY infection on Petunia sp.:a) a healthy plant;b)a diseased plant
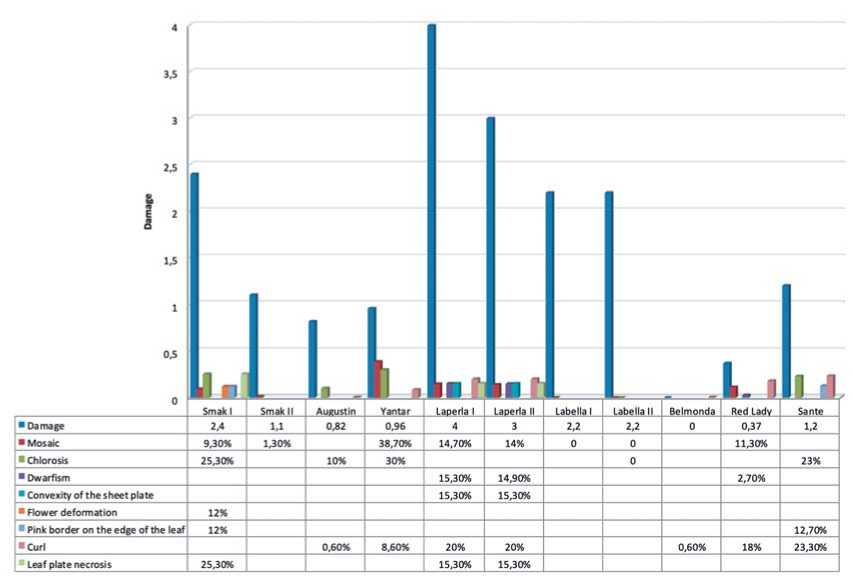
Рис.2.Балл повреждения и распределение по симптомам фитовирусной инфекции на сортах картофеля
Avgustin varied from 6 to 14 depending on a repetition. Appeared symptoms were mild (a slight leaf curl). Only few plants displayed leaf curl and mosaics. The average disease rate was 0.82±0.12. Only few individual plants of cv. Belmonda had curled leaves. The disease rate was zero (fig. 2).
Viral infection on plants of cv. Red Lady was shown as leaf curl, mosaics and dwarf plants. The disease rate was in average 0.37±0.02. Plants of cv. Sante had symptoms of: pink leaf borders, intercostal chlorosis, and leaf curl. The disease rate was 1.20±0.05 (fig. 2).
We selected plants with a minimal and a maximum degree of visible infection symptoms for every variety. Mixed infection was identified by PCR and included viruses of mosaic group: PVX, PVA, PVS, PVM, also PLRV and PSTVd. Viral load differed among varieties. We suppose that appearance of symptoms on potato plants depended both on qualitative and quantitative composition of plant viruses. Thus, symptoms on plants of cv. Smak differed depending on the presence or the absence of a particular virus, also on proportion of viruses (fig. 3, tab.).
Таблица. Количественная оценка фитовирусной нагрузки на сортах картофеля и петунье Table. Quantitative estimation of plant virus load on potato varieties and petunia plants
|
PVY |
PVX |
PVA |
PVM |
PVS |
PLRV |
PSTVd |
||||||||
|
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
Ct |
Rq |
|
|
Labella I |
33,52 ±0,19 |
0,15 ±0,08 |
35,30 ±0,24 |
0,07 ±0,02 |
35,32 ±0,24 |
0,02 ±0,02 |
34,67 ±0,23 |
0,06 ±0,01 |
29,17 ±0,41 |
0,06 ±0,02 |
35,90 ±0,24 |
0,13 ±0,01 |
||
|
Labella II |
20,82 ±0,29 |
1066 ±1,01 |
38,09 ±0,25 |
0,01 ±0,02 |
36,34 ±0,13 |
0,01 ±0,02 |
28,97 ±0,31 |
1,89 ±0,09 |
28,18 ±0,31 |
1,41 ±0,09 |
36,51 ±0,13 |
0,05 ±0,01 |
||
|
Laperla I |
19,78 ±0,27 |
2545 ±1,01 |
33,15 ±0,19 |
0,11 ±0,03 |
25,02 ±0,15 |
12,53 ±0,09 |
37,44 ±0,39 |
0,03 ±0,01 |
||||||
|
Laperla II |
28,79 ±0,31 |
3,61 ±0,09 |
36,64 ±0,13 |
0,07 ±0,02 |
34,10 ±0,23 |
0,05 ±0,02 |
31,70 ±0,63 |
0,26 ±0,03 |
24,72 ±0,15 |
16,30 ±0,10 |
35,29 ±0,24 |
0,11 ±0,01 |
||
|
Smak I |
17,63 ±0,22 |
9407 ±1,03 |
35,55 ±0,24 |
0,02 ±0,02 |
31,86 ±0,63 |
0,32 ±0,03 |
27,28 ±0,27 |
3,37 ±0,09 |
39,24 ±0,11 |
0,01 ±0,01 |
38,68 ±0,25 |
0,0042 ±0,02 |
||
|
Smak II |
19,55 ±0,27 |
1605 ±1,01 |
40,79 ±0,09 |
0,001 ±0,02 |
35,89 ±0,24 |
0,01 ±0,02 |
17,94 ±0,22 |
3304 ±1,01 |
27,75 ±0,27 |
1,80 ±0,09 |
36,42 ±0,13 |
0,05 ±0,01 |
25,05 ±0,15 |
55,62 ±0,09 |
|
Red Lady |
30,49 ±0,63 |
1,05 ±0,09 |
34,96 ±0,23 |
0,08 ±0,02 |
34,07 ±0,23 |
0,03 ±0,02 |
31,66 ±0,63 |
0,27 ±0,03 |
27,91 ±0,27 |
1,29 ±0,03 |
34,98 ±0,23 |
0,14 ±0,01 |
||
|
Augustin |
32,59 ±0,42 |
0,24 ±0,08 |
35,51 ±0,24 |
0,05 ±0,02 |
36,49 ±0,13 |
0,01 ±0,02 |
16,05 ±0,10 |
14767 |
28,95 ±0,31 |
0,95 ±0,03 |
37,03 ±0,39 |
0,04 ±0,01 |
38,54 ±0,25 |
0,004 ±0,02 |
|
Yantar |
19,44 ±0,27 |
2533 ±1,01 |
38,17 ±0,25 |
0,01 ±0,02 |
28,97 ±0,31 |
2,08 ±0,09 |
28,97 ±0,31 |
0,71 ±0,03 |
38,23 ±0,25 |
0,02 ±0,01 |
||||
|
Belmonda |
19,06 ±0,27 |
2294 ±1,01 |
35,54 ±0,24 |
0,02 ±0,02 |
33,39 ±0,19 |
0,09 ±0,03 |
16,13 ±0,10 |
5510 ±1,01 |
37,80 ±0,39 |
0,02 ±0,01 |
||||
|
Petunia sp. I |
33,56 ±0,19 |
0,15 ±0,08 |
34,50 ±0,23 |
0,02 ±0,01 |
||||||||||
|
Petunia sp. II |
19,65 ±0,27 |
2699 ±1,01 |
36,69 ±0,13 |
0,01 ±0,02 |
35,38 ±0,24 |
0,01 ±0,01 |
37,48 ±0,39 |
0,03 ±0,01 |
- |
|||||

а

b
High PVX concentrations in plants of variety Laperla with almost an equal proportion of other viruses led to appearance ofchlorosis on leafedges, necrosis and leaf galls, dwarf plants and mosaics.
Low concentrations of PVY, PVX, PVA, PVS, PVM and PLRV in plants of cv. Labella were shown only as chlorosis. Leaf chlorosis, mosaics and yellow leaves could be observed on plants when the PVY concentration was increased by 7*103 times, the PVM concentration by 90 times, and the PVS concentration by 2 times, compared to the plants with chlorosis only (fig. 4, tab. 1).
A high PVM concentration was expressed as leaf curl on plants of cv.Avgustin compared to the plants without visible symptoms. Its infection load, being higherthan the one of PVY by 10.5 times in coinfection, did not produce any changes in symptoms. Simultaneously the combination of high PVY and PVS concentrations caused leaf curl on plants of cvs. Belmonda and Red Lady compared to asymptomatic plants. The total viral load of PVY, PVX, PVA, PVS, PVM and PLRV (fig. 5) led to appearance of pink borders on the leaves of potato cv. Sante, mosaics and chlorosis on the leaves of cv. Avgustin compared to the plants, which were infected but did not have visible symptoms.
There are several types of interactions among viruses in an infected plant, which are usually called synergistic and antagonistic. They cause more severe symptoms than single viral infection does [7]. Propagation of one virus is supposed to be facilitated by propagation of the other in such systems. Mixed PVS and PVX infection can increase both the titer of PVS and enhance appearance of symptoms on potato leaves [16]. Synergy between potyvirus PVY and flex-ivirus PVX leads to an enhanced propagation rate of the latter increases its titers and consequently aggravates symptoms [17]. Mixed infection of calivirus PVA and luteovirus PLRV allows the later to infect all cell types in leaves, whereas the phloem limits its distribution when infection is single. It must occur due to the fact that movement proteins of PVA can complement PLRV movement deficiency [18]. After examining andcollecting leaf samples, M.S. Kolychikhina et al. (2021) concluded that potato plants of cv. Ramos, grown in an experimental field in Lipetsk oblast, suffered from a multiple infection: PVY, PVM + PVS. They also identified a

а
Рис.4.а)Здоровое растение сорта Labella;б)проявление смешанной вирусной инфекции
PVY,PVX,PVA,PVS,PVM и PLRV на картофеле сорта Labella
Fig.4.a) a healthy plantof the variety Labella;b)symptoms of mixed viralinfection
PVY,PVX,PVA,PVS,PVM and PLRV on plants of potato cv.Labella

b

а
Рис.5.а)Здоровое растение сорта Sante;б)Проявление смешанной вирусной инфекции
PVY,PVX,PVA,PVS,PVM и PLRV на картофеле сорта Sante

b
mixed viral infection in potato plants of cv. Impala in Astrakhan oblast: PVM + PVS, PVM + PVS + PVY [19]. Mixed viral infection was more complex in our experiments and comprised not only plant viruses of mosaic group but also PLRV and PSTVd. Multiple viral infections, caused by heterologous viruses such as PVY and PLRV, are common for potato [20]. PVX when combined with PVY leads to development of potato rugose mosaic [21]. We suppose that single infection of potato plants should not to be studied in field conditions because viruses circulate around the agricultural ecosystem of potato fields and can infect various host-plants, including both wild species and cultivars. Coexistence of several plant viruses and occurrence of mixed infections in plants are conditioned by obligate parasitism. Also most of the vectors are polyphagous. Due to this fact, they can accumulate virus from one plant and transmit it to another that might be already infected. It creates conditions for superinfection [7]. Behavior and physiology of carriers can be affected by changes in plants, caused by viruses, and this factor, in its turn, facilitates distribution and epidemiology of infection. Attraction of carriers to infected plants increases risk of infection. Mixed PVY and PLRV infection enhanced fertility of aphid species Myzus persicae and Macrosiphum euphorbiae , feeding on potato plants, what determined their choice of these plants. According to Chatzivassiliou et al., such behavior can be explained by an increase in the content of sugars and amino acids in the phloem of potato plants which makes them more attractive for insects. Because mixed infections of these viruses often occur in potato plants, qualities of hosts and behavior of carriers can have important epidemiologic consequences in the course of this interaction [21]. Knowledge, acquired as the result of the study on mixed viral infections, can become a rich source of useful information, for example, for development of effective management techniques or even for creation of plants resistant to viruses.
Список литературы Potato viruses of 7 commercial cultivars grown in field Primorsky krai of Russia
- Kolliopoulou A., Kontogiannatos D., Swevers L. The Use of Engineered Plant Viruses in a Trans-Kingdom Silencing Strategy Against Their Insect Vectors. Front. Plant Sci. 2020;(11):917. https://doi.org/10.3389/fpls.2020.00917
- Pourrahim R., Farzadfar Sh., Golnaraghi A.R., Ahoonmanesh A. Incidence and distribution of important viral pathogens in some Iranian potato fields. Plant Dis. 2007;(91):609-615. https://doi.org/10.1094/PDIS-91-5-0609
- Ngoh Dooh J.P., Boydoul F.U., Madjerembe A., Tchoupou Tsouala D.B., Hawaou Adagoro D.B., Kosma P., Ambang Z. Inventory of the potato diseases and impact on growth and yield traits in far North Cameroon. Int. J. Biol. Chem. Sci. 2020;14(8):2826-2836. https://doi.org/10.4314/ijbcs.v14i8.14
- Murphy A. F., Rondon S. I., Moreno A., Fereres A. Effect of Potato virus Y Presence in Solanum tuberosum (Solanales: Solanaceae) and Chenopodium album on Aphid (Hemiptera: Aphididae) Behavior. Environmental Entomology. 2018;XX(X):1- 6. https://doi.org/10.1093/ee/nvy041
- Sobko O.A., Matsishina N.V., Fesenko P.V., Kim I.V., Didora A.S., Boginskay N.G., Volkov D.I. Viruses in the agrobiocenosis of the potato fields. IOP Conference Serie: Earth and Environmental Science. 2021;677(5):052093. https://doi.org/10.1088/1755-1315/677/5/052093. (In Russ.)
- Cervantes F.A., Alvarez J.M. Within plant distribution of Potato Virus Y in hairy nightshade (Solanum sarrachoides): An inoculum source affecting PVY aphid transmission. Virus Research. 2011;(159):194-200. https://doi.org/10.1016/j.virusres.2011.05.003
- Pankhuri S., Sajad U. N., Manoj K. Y., Abhishek D.. Mixed infection of plant viruses: diagnostics, interactions and impact on host. Journal of Plant Diseases and Protection. 2020;128(4). https://doi.org/10.1007/s41348-020-00384-0
- Syller J., Grupa A. Antagonistic within-host interactions between plant viruses: molecular basis and impact on viral and host fitness. Molecular Plant Pathology. 2016;17(5):769-782. https://doi.org/10.1111/mpp.12322
- Государственный реестр селекционных достижений, Допущенных к использованию. Сорта растений: офиц. изд.: М., 2021;(1):719.
- Рябушкина Н.А., Омашева М.Е., Галиакпаров Н.Н. Специфика выделения ДНК из растительных объектов. Биотехнология. Теория и практика. 2012;(2):9-26. https://doi.org/10.11134/btp.2.2012.1
- Широкий унифицированный классификатор СЭВ и Международный классификатор СЭВ видов картофеля секции Tuberarium (Dun.) Buk. рода Solanum L. Л.: ВИР, 1977. 60 с.
- Панычева Ю.С., Васильев Д.М., Супрунова Т.П., Сахарова А.Н., Игнатов А.Н. Динамика поражения сортов картофеля вирусом Y в полевых условиях. Картофель и овощи. 2019;(5):25-29. https://doi.org/10.25630/PAV.2019.45.82.006
- Kumar R., Tiwari R. K., Jeevalatha A., Kaundal P., Sharma S., Chakrabarti S.K. Potato viruses and their diagnostic techniques: An overview. Journal of Pharmacognosy and Phytochemistry. 2019;8(6):1932-1944
- Bekesiova I., Nap J-P., Mlynarova L. Isolation of high quality DNA and RNA from leaves of the carnivorous plant Drosera rotundifolia. Plant Molecular Biology Reporter. 1999;(17):269- 277.
- Watzinger F., Ebner K., Lion T. Detection and monitoring of virus infections by real-time PCR. Molecular Aspects of Medicine. 2006;(27):254-298. https://doi.org/10.1016/j.mam.2005.12.001
- Nyalugwe E.P., Wilson C.R., Coutts B.A., Jones R.A.C. Biological properties of Potato virus X in potato: Effects of mixed infection with Potato virus S and resistance phenotypes in cultivars from three continents. Plant Dis. 2012;(96):43-54. https://doi.org/10.1094/PDIS-04-11-0305
- Karyeija R.F., Kreuze J.F., Gibson R.W., Valkonen J.P.T. Synergistic Interactions of a Potyvirus and a Phloem-Limited Crinivirus in Sweet Potato Plants. Virology. 2000;(269):26-36. https://doi.org/10.1006/viro.1999.0169
- Savenkov E.I., Valkonen J.P.T. Potyviral helper componentproteinase expressed in transgenic plants enhances titers of Potato leaf roll virus but does not alleviate its phloem-limitation. Virology. 2001;283(2):285-293. https://doi.org/10.1006viro.2000.0838
- Kolychikhina M.S., Beloshapkina O.O., Phiri C. Change in potato productivity under the impact of viral diseases. IOP Conf. Series: Earth and Environmental Science. 2021;(663). https://doi.org/10.1088/1755-1315/663/1/012035 (In Russ.)
- Srinivasan R., Alvarez J. M. Effect of Mixed Viral Infections (Potato Virus Y-Potato Leafroll Virus) on Biology and Preference of Vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). J. Econ. Entomol. 2007;100(3):646-655. https://doi.org/10.1603/0022-0493(2007)100[646:EOMVIP]2.0.CO;2
- Awasthi L.P., Verma H.N. Current status of viral diseases of potato and their ecofriendly management-A critical review. Virol Res Rev. 2017;1(4):1-16. https://doi.org/10.15761/VRR.10001
- Chatzivassiliou1 E.K., Moschos E., Gazi S., Koutretsis P., Tsoukaki M. Infection of potato crops and seeds with potato virus Y and potato leafroll virus in Greece. Journal of Plant Pathology. 2008;90(2):253-261.


