Повторная лучевая терапия на фоне приема бевацизумаба в лечении рецидивов глиобластомы
Автор: Белоконь С.В., Гулидов И.А., Гоголин Д.В., Медведева К.Е., Иванов С.А., Каприн А.Д.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Обзоры
Статья в выпуске: 1 т.23, 2024 года.
Бесплатный доступ
Введение. Глиобластома остается заболеванием с плохим прогнозом. Ввиду высокой агрессивности опухоли, несмотря на комплексный подход к лечению заболевания, практически неизбежен рецидив. До сих пор не существует единого стандарта лечения рецидивов глиобластомы, многие руководства рекомендуют ведение данных пациентов в рамках клинических исследований. Имеется множество подходов, таких как хирургическое вмешательство, лучевая терапия, системная или локальная химиотерапия или лечение таргетными препаратами, различные стратегии иммунотерапии, применение низкоинтенсивных среднечастотных электрических полей, а также их комбинации. Сочетание двух неинвазивных методик: повторной лучевой терапии и таргетной терапии бевацизумабом, остается наиболее часто применяемым подходом у данной группы пациентов, потенциал которого до конца не раскрыт. Цель исследования - анализ литературных данных по использованию повторной лучевой терапии с бевацизумабом в качестве лечебной опции у пациентов с глиобластомой. Материал и методы. Поиск литературы производился в системах Medline, Cochrane Library, E-library, Scopus, PubMed и Google Scholar.
Глиобластома, повторная лучевая терапия, протонная терапия, таргетная терапия, рецидив высокозлокачественной глиомы
Короткий адрес: https://sciup.org/140303741
IDR: 140303741 | УДК: 616-006.484.04-036.65-08:615.849.1+615.277.3 | DOI: 10.21294/1814-4861-2024-23-1-142-154
Текст обзорной статьи Повторная лучевая терапия на фоне приема бевацизумаба в лечении рецидивов глиобластомы
Глиобластома является самой распространенной и агрессивной опухолью головного мозга у взрослых [1]. В отношении первичной ГБ, несмотря на мультимодальный подход к лечению (протокол Stupp) [2], включающий максимально полную резекцию, последующую лучевую терапию (ЛТ) на фоне приема темозоломида (TMZ) с адъювантной химиотерапией TMZ до 12 циклов, прогноз заболевания остается неутешительным: медиана общей выживаемости (ОВ) не превышает 14 мес, 2- и 5-летняя выживаемость составляют 27 и 3 % соответственно [2–4].
Ввиду высокой агрессивности опухоли практически неизбежен рецидив ГБ. Несмотря на комбинированное лечение, средняя выживаемость без прогрессирования (ВБП) составляет около 7 мес с момента постановки диагноза [2], так как такое лечение, хотя и эффективно снижает агрессивность опухоли на начальном этапе, не приводит к суще- ственному изменению течения заболевания [5]. Рецидивы ГБ ассоциируются с плохим прогнозом (медиана выживаемости после прогрессирования составляет менее 1 года). Рецидивы ГБ встречаются практически на любом удалении от первоначальной локализации [6], однако большинство рецидивов возникает в пределах 2 см от первоначального края опухоли [7], а в некоторых случаях ГБ способна рецидивировать в контралатеральном полушарии, другой доле и инфратенториально [8]. Лечение рецидивирующей глиобластомы (рГБ) остается сложной задачей, поскольку до сих пор не утвержден стандарт лечения, а существуют лишь эмпирические подходы. Только 20–30 % пациентов удается выполнить резекцию рГБ [9], но даже для этой когорты нет единого мнения о том, в каком случае повторное вмешательство принесет наибольшую пользу.
В связи с этим последние исследования направлены в большей степени на неинвазивные или комбинированные подходы к лечению рГБ, такие как повторная лучевая терапия (пЛТ), системное лечение алкилирующими агентами, ингибиторами тирозинкиназы (в частности, бевацизумабом (BEV)), иммунотерпия, использование низкоинтенсивных среднечастотных электрических полей и их сочетание. Одной из наиболее доступных и перспективных нам представляется комбинация ЛТ с BEV.
Повторная лучевая терапия
Роль пЛТ при рГБ остается неопределенной. Применение пЛТ чаще всего ставится под вопрос, поскольку большинство рецидивов ГБ, как уже упоминалось, возникает в поле действия высокой дозы облучения (90–95 %). Соответственно, при пЛТ увеличивается риск возникновения постлучевых осложнений, например радиационного некроза, возникающего при кумулятивной суммарной очаговой дозе стандартного фракционирования (EQD2) >100 Гр, >105 Гр для гипофрационирован-ного и >135 Гр для стереотаксического режимов ЛТ [10]. Ключевым вопросом является и правильный отбор пациентов для лечения. Факторами, которые чаще всего рассматриваются в качестве показаний к проведению лучевой терапии, являются индекс Карновского, возраст, время до прогрессирования, тип прогрессирования, объем мишени и место рецидива.
Оптимальные дозы и методики пЛТ при рГБ не установлены. В некоторых сообщениях [11, 12] предлагается ориентироваться на облучаемый объем: для радиохирургии он составляет <12,5 см3 с отступом ≤5 мм на планируемый объем мишени (PTV) или 4–10 см3 без дополнительного отступа. S. Scoccianti et al. [12] предлагают следующие ограничения: 12,5–35 см3 – для режима гипофракционирования суммарная очаговая доза (СОД) 25 Гр, разовая очаговая доза (РОД) 5 Гр; при объеме 35–50 см3 – традиционное фракционирование СОД 36 Гр за 20 фракций с отступом ≤5 мм на PTV; при объеме 8,5–12,5 см3 – ультрагипофракционирование (5–7 Гр х 5 фр., например). Другие авторы [11] изучали переносимость и эффективность пЛТ с такими ограничениями: 33–100 см3 – умеренное гипофракционирование (например, 2,5–3,5 Гр × 10–15 фр.); при объеме >100 см3 – традиционное фракционирование (2 Гр × 18 фр.).
Учитывая незначительное количество значимых проспективных исследований, на основании опыта применения различных схем пЛТ проведены несколько метаанализов. В метаанализе ретроспективных исследований F. Kazmi et al. [13], включив 50 несравнительных исследований с участием 2095 пациентов, которым проводилась пЛТ при рГБ, получили следующие результаты: ОВ через 6 и 12 мес составила 73 и 36 %, ВБП через 6 и 12 мес – 43 и 17 % соответственно. Примечательно, что более высокие показатели ОВ через 12 мес
(44 %) наблюдались при использовании брахитерапии, а тенденция к улучшению ВБП через 6 мес – при умеренном режиме гипофракционирования (≤5 фр.). В группе дистанционной лучевой терапии не было различий между эквивалентными дозами по EQD2 между группами с высокой (≥36 Гр) и низкой (<36 Гр) дозами облучения. Токсичность G3≥ наблюдалась у 7 % пациентов.
С учетом завершенных к 2023 г. исследований R. Marwah et al. [14] проведен последний на данный момент систематический обзор и метаанализ выживаемости и токсичности облучения в сравнении с системной терапией и комбинированной терапией при рецидивирующей глиоме высокой степени злокачественности. Анализ включал 31 исследование (3 рандомизированных, 28 нерандомизированных) и 2084 участника. Группа пЛТ сравнивалась с группами системной и комбинированной терапии, в том числе на основе BEV. Группа пЛТ не показала преимуществ по сравнению ни с системной терапией, где разницы по ОВ и ВБП не наблюдалось, ни с комбинированной терапией с/без BEV, где показатели ОВ и ВБП были выше, а токсичность G3≥ ниже в группе комбинированной терапии в целом.
Отдельно стоит упомянуть о повторной протонной ЛТ, обладающей, по сравнению с фотонной пЛТ, большей конформностью, относительной биологической эффективностью и высоким градиентом дозы на границе с нормальными тканями [15, 16]. Американскими учеными проведен анализ реестра многоинституциональной протонной совместной группы [17], в которой 45 пациентов с рГБ были повторно облучены протонами. Медианы ОВ и ВБП составили 14,2 и 13,9 мес соответственно, у 1 пациента наблюдалась острая токсичность G3, у 4 – поздняя токсичность G3.
В МРНЦ им. А. Ф. Цыба проведено исследование 44 пациентов, получавших повторное лечение на комплексе протонной терапии «Прометеус» [18]. При этом медиана ОВ составила 12 мес, 1- и 2-летняя ОВ – 49,6 и 35,1 % соответственно. Медиана ВБП составила 9 мес, 1- и 2-летняя ВБП – 30,5 и 10,5 % соответственно. Данному вопросу посвящены и другие публикации, демонстрирующие конкретные клинические примеры эффективности повторной протонной терапии [19, 20].
Опцией ЛТ при рГБ является также интерстициальная брахитерапия. Согласно некоторым данным [21, 22], применение такой методики коррелирует с высокой частотой радиационного некроза. В качестве альтернативной формы брахитерапии используется надувной баллонный катетер с жидким радиоизотопом 125I (GliaSite), вводимый во время хирургического вмешательства, что позволяет доставлять в ткани высокую дозу излучения [23]. Однако с повышением конформности дистанционной лучевой терапии со времени проведения данных исследований роль брахитерапии значительно снизилась.
Анти-VEGF терапия: Бевацизумаб
Важность применения анти-VEGF терапии при ГБ связана с тем, что ангиогенез играет ключевую роль в развитии и прогрессировании ГБ [24]. Процесс образования новых сосудов запускается в основном гипоксией и повышением HIF-1. Гипоксия также вызывает увеличение количества проангиогенных факторов, таких как VEGF, FGFs и ангиопоэтин-1 [25, 26]. Многие из этих проан-гиогенных факторов повышены при ГБ и связаны с ухудшением прогноза.
VEGF стал отправной точкой в разработке анти-ангиогенной терапии ГБ. Более высокий уровень мРНК VEGF описан в некротических участках опухолевых образцов ГБ, что способствует образованию новых сосудов и дальнейшей пролиферации клеток [27]. Более того, сверхэкспрессия VEGF-R1 в низкозлокачественных астроцитомах связана с худшим прогнозом, аналогичным тому, который демонстрируют высокозлокачественные глиомы [28]. Показано, что терапия анти-VEGF нормализует сосудистую систему опухоли и снижает давление интерстициальной жидкости [29, 30], а позже выявлена цитотоксичность терапии анти-VEGF для стволовых клеток глиомы, которые, как полагают, более устойчивы к традиционной химиотерапии [31, 32]. На основании этих фактов проведены глубокие исследования антиангиогенных методов лечения. Среди них BEV, представляющий собой рекомбинантные гиперхимерные моноклональные антитела, которые связываются с циркулирующим фактором роста эндотелия сосудов (VEGF), препятствуя его взаимодействию с рецепторами на поверхности эндотелиальных клеток, который остается наиболее изученным антиангиогенным препаратом при ГБ.
За последние два десятилетия проведено множество исследований по применению BEV при ГБ. Одобрение Управлением по контролю качества пищевых продуктов и лекарственных средств (FDA) BEV для лечения рГБ в 2009 г. основано на результатах исследования BRAIN фазы II, которое включало пациентов, у которых отмечено прогрессирование на фоне терапии темозоломидом [33]. В данном исследовании 167 пациентов рандомизированы для получения BEV 10 мг/кг отдельно или в комбинации с иринотеканом 340 мг/м2 (или 125 мг/м2 в случае приема фермент-индуцирующих противоэпилептических препаратов) каждые 2 нед. Показатели объективного ответа для группы, получавшей BEV отдельно, составили 28,2 %, для группы, получавшей комбинацию, – 37,8 %. При этом 6-месячные показатели ВБП составили 42,6 и 50,3 % соответственно, медиана ОВ при монотерапии – 9,2 мес, при комбинированной терапии – 8,7 мес. Однако препарат не был одобрен Европейским агентством лекарственных средств (EMA) для лечения рГБ. Отсутствие одобрения со стороны
EMA было связано, прежде всего, с отсутствием контрольной группы в основном исследовании.
Проведено несколько метаанализов с целью определения эффективности BEV у пациентов с рГБ. G. Lombardi et al. анализировали 14 рандомизированных клинических исследований с участием 4 330 пациентов с целью оценки применения BEV при ГБ и при рГБ. Анализ не показал улучшения ОВ по сравнению с цитотоксической терапией. Более того, при использовании BEV в качестве единственного препарата, по сравнению с химиотерапией, получены менее благоприятные результаты [34]. Метаанализ библиотеки Cochrane вариантов лечения прогрессирования или рГБ от 2021 г. также не выявил преимуществ в ОВ при использовании комбинации с BEV для лечения первого рецидива ГБ, но при этом ВБП улучшена при использовании BEV [35]. T. Zhang et al. в своем метаанализе подтвердили отсутствие выигрыша в ОВ для этой группы пациентов, хотя были отмечены положительный эффект по объективному ответу и возможное преимущество в отношении ВБП [36].
Повторная лучевая терапия на фоне приема бевацизумаба
Комбинация пЛТ с BEV стала перспективным методом лечения рГБ. Отчасти это связано с развитием методик лучевой терапии, позволяющих более щадяще воздействовать на критические структуры и, следовательно, снижать токсичность в отношении центральной нервной системы [37]. Несмотря на то, что повторное облучение может осложняться поздними токсическими эффектами, тщательный отбор пациентов и индивидуализированное планирование ЛТ позволяют минимизировать риски развития радиационного некроза [38, 39].
Лучевая терапия может стимулировать выработку VEGF в опухолях, что парадоксальным образом приводит к усилению ангиогенеза [40, 41]. Но в то же время высказываются предположения о том, что BEV повышает чувствительность эндотелия опухоли к ЛТ и индуцирует апоптоз [42]. Кроме того, установлено, что BEV снижает частоту радиационного некроза у пациентов с рГБ, получавших повторное облучение [43]. Также стоит упомянуть об эффекте стероидных препаратов, свойственных BEV, который, как предполагается, улучшает качество жизни, потому что позволяет отказаться от сопутствующей терапии стероидами [44]. Характеристики исследований, изучавших применение пЛТ на фоне приема BEV, представлены в таблице.
Повторная радиохирургия + BEV
Высокие дозы радиохирургии обладают существенным эффектом абляции эндотелиальных клеток сосудов, который может быть гораздо более
Таблица/Table
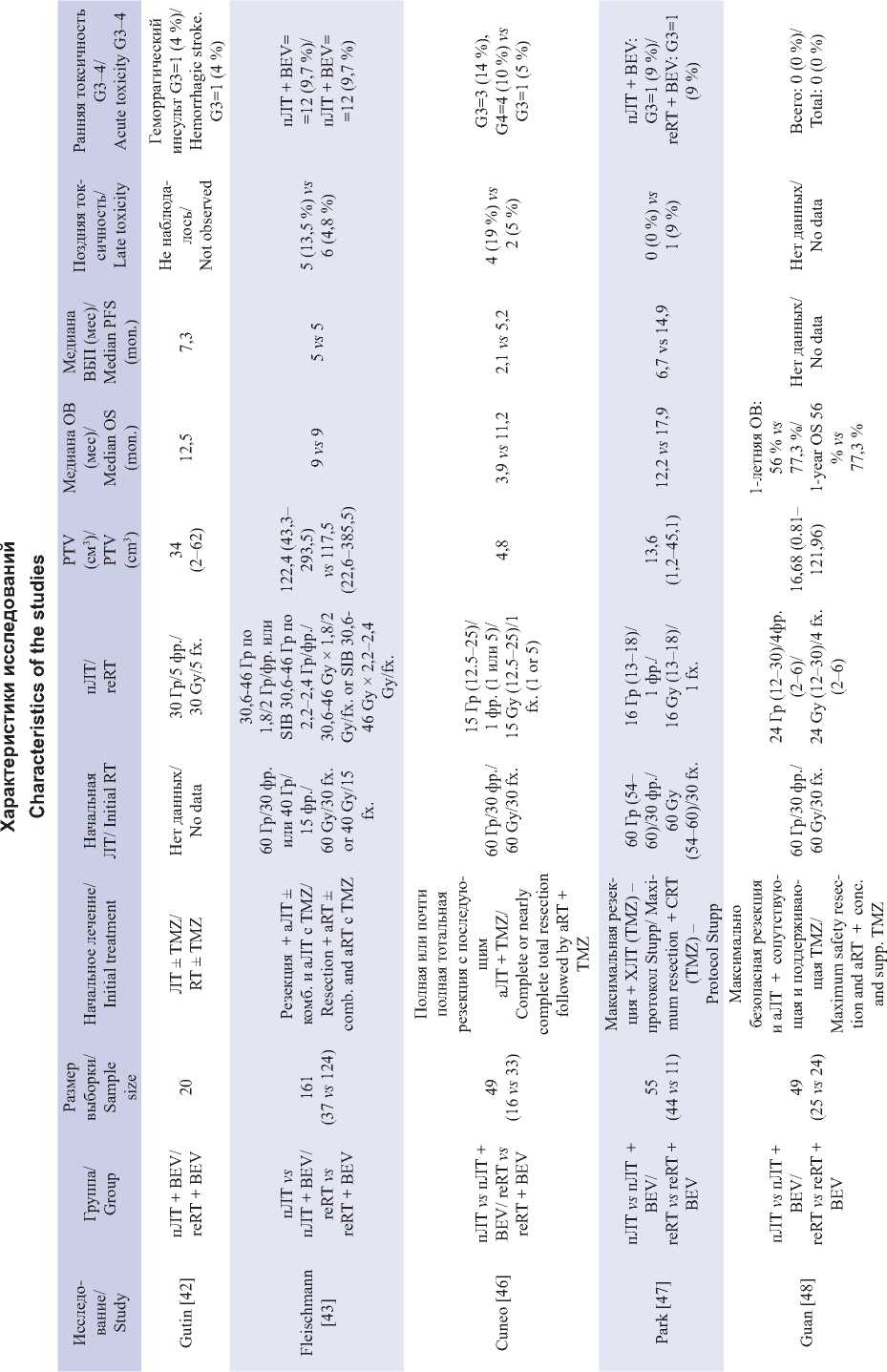
Окончание таблицы/End of Table
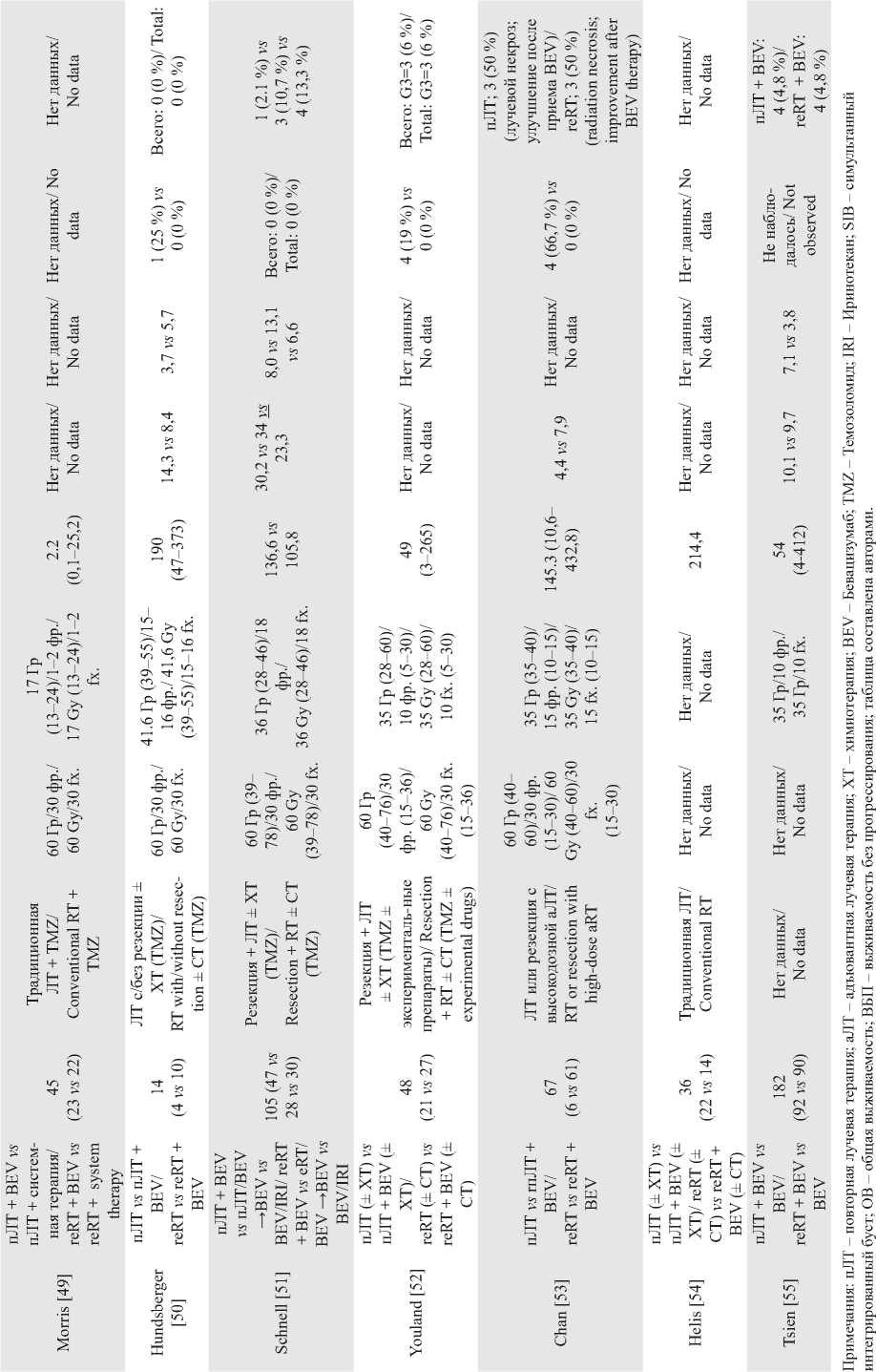
Notes: reRT – re-irradiation; aRT – adjuvant radiation therapy; CT – chemotherapy; BEV – Bevacizumab; TMZ – Temozolomide; IRI – Irinotecan; SIB – Simultaneous Integrated Boost; OS – overall survival; PFS progression-free survival; created by the authors.
мощным, чем тот, который наблюдается при традиционной ЛТ [45], что потенциально может суммироваться в сочетании с нейтрализацией VEGF бевацизумабом. Это послужило предпосылкой к изучению данного режима лечения.
В исследовании 2011 г. K. Cuneo et al. [46] с целью определения влияния факторов, связанных с пациентом и лечением, на выживаемость и токсичность провели стереотаксическую радиохирургию по 12,5–25 Гр (медиана PTV – 4,8 см3) и системную терапию BEV по поводу рГБ у 49 пациентов. Медиана ОВ была выше в группе с BEV, чем у пациентов, не получавших BEV (11,2 vs 3,9 мес), как и ВБП (5,2 vs 2,1 мес). Случаев радиационного некроза было значительно меньше в группе с BEV (19 и 5 %). Кроме того, анализ группы, которая получала BEV до прогрессирования и продолжала его прием на фоне пЛТ, показал, что и в этом случае медианы ОВ и ВБП были выше, чем в группе без продолжения приема BEV.
Годом позже K. Park et al. [47] оценивали эффективность и безопасность радиохирургии на аппарате Гамма-нож с медианой дозы 16 Гр (медиана PTV – 13,6 см3) с последующим назначением бевацизумаба в среднем через 5 нед после радиохирургии BEV в дозе 10 мг/кг каждые 2 нед в 28-дневном цикле в сочетании с химиотерапией у 11 пациентов с рГБ, у которых наблюдалось прогрессирование, несмотря на агрессивное начальное лечение. В группе, получавшей BEV, наблюдалось значительное увеличение медиан ВБП до 15 мес и ОВ до 18 мес со снижением вероятности развития радиоационного некроза (9 vs 46 %).
Y. Guan et al. [48] также изучали эффективность и безопасность стереотаксической радиохирургии Гамма-ножом по 12–30 Гр × 2–6 фр. (медиана PTV – 16,68 см3) с одновременным режимом BEV 10 мг/кг у 49 пациентов с рГБ. Годичная выживаемость после радиохирургии у пациентов, получавших BEV в одновременном и адъювантном режиме, и у тех пациентов, которые не получали BEV, составила 77,3 и 56,0 % соответственно. Токсических явлений ≥G3 не зарегистрировано.
В ретроспективном анализе S. Morris et al. [49] 45 пациентам с рГБ проводили радиохирургию с дозой 17 Гр (медиана PTV – 2,2 см3), 23 из которых вводился BEV – 10 мг/кг впервые сразу после лечения и далее каждые 2 нед в течение 9 циклов. Медианы ОВ и ВБП составили 13,3 мес и 5,2 мес для всей когорты пациентов. Отдельно выборка с BEV не оценивалась.
Повторная гипофракционированная и традиционная лучевая терапия + BEV
В 2009 г. P. Gutin et al. [42] проанализировали безопасность и эффективность комбинации BEV 10 мг/кг каждые 14 дней в 1 и 15-й дни c гипофрак-ционированной пЛТ 6 Гр × 5 фр. (медиана PTV – 34 см3), c 7–10-го дня второго цикла у 20 пациентов с рГБ. Медиана ОВ составила 12,5 мес, ВБП – 7,3 мес. Из токсических эффектов G3 отмечалось одно кровоизлияние в ЦНС.
Несколько позже, в 2013 г., T. Hundsberger et al. [50] изучили в ретроспективном когортном исследовании с участием 14 пациентов (8 с рГБ), прошедших пЛТ по 2,7 Гр × 15–16 фр. (медиана PTV – 190 см3) с индукцией 2 курсами BEV по 5 мг/кг каждые 28 дней. Были получены следующие данные: медиана ОВ составила 9,0 мес, ВБП – 5,1 мес, наблюдался один случай радиационного некроза в группе без BEV.
O. Schnell et al. [51] в 2016 г. проанализировали результаты двух протоколов пЛТ 2 Гр ×18 фр. (медиана PTV – 136 см3 и 105 см3) на основе BEV 10 мг/кг в 1 и 15-й дни: с последующим адъювантным приемом BEV у 47 и без него у 28 пациентов с рецидивами ГБ высокозлокачественных глиом. Медиана ОВ была практически одинакова для групп пЛТ/BEV и пЛТ/BEV→BEV, она составила 34,0 и 37,9 мес, однако медиана ВБП составила 8,0 и 16,1 мес соответственно. Авторы пришли к выводу, что режим пЛТ/BEV→BEV ассоциируется с наиболее длительным ВБП, независимо от ВБП после первичного лечения.
R. Youland et al. [52] в 2018 г. ретроспективно проанализировали данные 48 пациентов с рГБ, получавших повторное облучение преимущественно по 3,5–4,0 Гр × 10 фр. (медиана PTV – 49 см3) на фоне сопутствующей химиотерапии, из которых 27 получали BEV 10 мг/кг каждые 2 нед. Авторы не приводят данные ОВ и ВБП по группе BEV, однако они установили, что у пациентов, получавших пЛТ после неэффективности бевацизумаба, медиана ОВ и ВБП была ниже, чем у пациентов, ранее не принимавших BEV. Также у пациентов, одновременно получавших BEV с повторным облучением, случаев радионекроза не наблюдалось.
В 2019 г. D. Fleischmann et al. [43] анализировали результаты лечения комбинацией пЛТ симультанным интегрированным бустом 30,6–46 Гр по 1,8/2 Гр/фр. или 2,2–2,4 Гр/фр. (медиана PTV – 117,45 см3) на фоне приема BEV в 1 и 15-й дни пЛТ по 10 мг/кг у 124 пациентов с рГБ. Медианы ОВ и ВБП составили 5 и 9 мес соответственно. Радиационный некроз наблюдался чаще в группе только пЛТ (13,5 vs 4,8 %). Однако исследователи не обнаружили увеличения ОВ после сопутствующей терапии BEV и подчеркивают ее ограниченное влияние на прогноз заболевания.
В 2020 г. J. Chan et al. [53] в когортном исследовании изучали пЛТ 2,7 Гр × 15 фр. в комбинации с BEV по 5 мг/м2 каждые 2–3 нед на большие объемы опухоли (медиана PTV – 145,3 см3) у 51 пациента с рГБ и 16 с ВСГ. Медиана ОВ составила 7,5 мес для пациентов с рГБ. Радиологические признаки радионекроза выявлены у 4 пациентов (G 2–4), не получавших терапию BEV, назначение которого после данного осложнения у всех привело к рент- генологическому и клиническому улучшению. Примечательно, что при многофакторном анализе всей когорты статус мутации IDH1 и статус ECOG до повторной ЛТ были достоверно связаны с ОВ, что согласуется с данными других исследований [11, 38, 43].
C. Helis et al. [54] в 2022 г. представили ретроспективный обзор пациентов, получавших повторное облучение в различных режимах (медиана BED10 53,1 Гр) на фоне приема BEV 10 мг/м2 1 раз в 2 нед у 36 пациентов срГБ (медиана PTV – 208,5 см3). Медиана ОВ составила 6,7 мес. Ни у одного пациента, получавшего BEV одновременно с пЛТ, не развилась токсичность уровня G3 или выше.
В том же году опубликованы результаты первого крупного проспективного многоцентрового исследования II фазы NRG Oncology/RTOG1205 [55] по оценке безопасности и эффективности пЛТ по 3,5 Гр × 10 фр. (медиана PTV – 54 см3) при рГБ с одновременным применением BEV в дозе 10 мг/кг 1 раз в 2 нед против только BEV до прогрессирования. Медиана ОВ для контрольной группы составила 9,7 мес и 10,1 мес для основной группы; ВБП при использовании критериев MacDonald и RANO для контрольной и основной групп составили 3,8 и 7,1 мес соответственно; показатель 6-месячной ВБП – 29,1 % против 54,3 % в пользу BEV + пЛТ. Зафиксировано 2 случая острой токсичности G5, вероятно, связанных с лечением, – внутриопу-холевое кровоизлияние и внезапная смерть. При этом ни у одного пациента не зарегистрировано проявлений поздней токсичности ЦНС, класса G3+, связанных с лечением. Таким образом, исследование NRG Oncology/RTOG1205 подтвердило улучшение показателей ВБП и 6-месячной ВБП в группе с BEV + ЛТ по сравнению с применением только BEV. Исследователи расценивают показатель 6-месячной ВБП в качестве важной конечной точки для рГБ, так как ни одно из предыдущих терапевтических исследований не продемонстрировало преимущества ОВ.
Систематические обзоры и сетевые метаанализы
Учитывая достаточное количество, но в то же время значимую разнородность проведенных исследований по изучению сочетания различных режимов пЛТ с конкурентным/адъювантным приемом BEV, для определения места данного подхода в лечении рГБ предпринимались и предпринимаются попытки обобщить имеющиеся данные путем проведения метаанализов и систематических обзоров.
Авторы сетевого метаанализа вариантов лечения прогрессирования или рецидива глиобластомы [35] на основании ограниченных данных трех разнородных исследований [56–58] (разные контрольные группы и популяции с разным уров- нем рецидивов ГБ) пришли к выводу, что данные об улучшении ВБП или ОВ при комбинировании пЛТ с BEV для всех или для отдельных кандидатов нуждаются в дополнительном подтверждении.
R. Marwah et al. в метаанализе [14] среди прочих отдельно оценивали группу комбинированной терапии на основе BEV в сравнении с повторным облучением с/без системной терапии без BEV в 8 отобранных исследованиях. В группе комбинированной терапии на основе BEV медиана ВБП составила от 5 до 14,9 мес, медиана ОВ – от 7,9 до 17,9 мес. В группе пЛТ без бевацизумаба медиана ВБП составила от 2,1 до 6,7 мес, медиана ОВ – от 3,9 до 14,3 мес. Частота радиационного некроза в группе комбинированной терапии на основе BEV составляла от 0 до 9 % и от 13,5 до 66,7 % в группе пЛТ без BEV. Частота токсичности G3+ варьировала от 0 до 10 % в группе комбинированной терапии на основе BEV и от 0 до 50 % в группе пЛТ без BEV. В группе комбинированной терапии на основе BEV был зарегистрирован 1 случай смерти, связанный с лечением.
Комбинация повторной ЛТ с системной терапией на основе BEV улучшила ВБП – 2 исследования [46, 47], 104 участника (низкая достоверность) и ОВ – 5 исследований [46–48, 53, 54], 256 участников (низкая достоверность), снижая при этом развитие радиационного некроза (5 исследований [43, 46, 48, 50, 53], 353 участника). На основании полученных данных авторы пришли к выводу, что комбинированная терапия может улучшить ОВ и ВБП при приемлемой токсичности у отдельных пациентов с рГБ по сравнению с повторным облучением или системной терапией. Также они акцентируют внимание на необходимости дальнейших исследований для подтверждения целесообразности эскалации дозы пЛТ с целью улучшения локального контроля и выживаемости.
Обсуждение
Несмотря на то, что определенного подхода к лечению рГБ на настоящий момент нет, пЛТ остается одной из основных методик, применяемой у таких пациентов. Этому способствуют доступность данного вида помощи, возможность неинвазивного лечения и независимость от состояния гематоэнцефалического барьера, в отличие от лекарственной терапии. Однако, как показывают последние метаанализы, эффективность пЛТ в отношении как ОВ, так и ВБП значимо возрастает при комбинировании с системной терапией на основе BEV. Это объясняется тем, что BEV является в таком случае радиомодификатором благодаря синергетическому взаимодействию с ЛТ [59]. Данный эффект имеет под собой клинико-экспериментальную основу. Имеющиеся экспериментальные данные свидетельствуют о том, что подавление экспрессии VEGF анти-VEGF терапией ассоциируется со значимым уменьшением пролиферации клеток глии [60]. Также доказана способность анти-VEGF терапии нормализовать тканевой кровоток и за счет кислородного эффекта усиливать действие ионизирующего излучения [32, 42, 61]. Кроме того, наличие порочного круга, заключающегося в стимуляции роста сосудистой сети стволовыми клетками ГБ, нуждающимися в ангиогенезе для собственной пролиферации, дает основания полагать, что стволовые клетки ГБ сами становятся мишенью для анти-VEGF терапии [62, 63]. Наконец, BEV обладает и свойствами стероидов, что способствует уменьшению выраженности воспалительных явлений, приводящих к радиационному некрозу. Эти данные подтверждаются многочисленными исследованиями, обзорами и метаанализами, сравнивающими комбинированный подход с системной терапией или ЛТ в монорежиме. Однако применение BEV как компонента радиохирургии или ультрагипофракционированной терапии, по сути, использует в основном его профилактическое действие в отношении радиационного некроза, так как за время курса ЛТ, составляющего от 1 до 5 фракций, учитывая схему введения BEV 10 мг/м2 1 раз в 2 нед, последний не успевает реализовать свое синергетическое действие. Демонстрация обоих эффектов возможна при его введении в рамках умеренного гипофракционирования (по 2,5–3 ГрЕ) или традиционного фракционирования (по 2 ГрЕ). К сожалению, крупных исследований или метаанализов, которые бы сравнивали различные режимы фракционирования пЛТ на основе BEV, нет.
Достоверным является и тот факт, что комбинация пЛТ с BEV значимо снижает риск как нежелательных явлений, так и радиационного некроза G3≥. Возникновение последнего зависит, в первую очередь, от кумулятивной дозы (так как рецидивы ГБ возникают сравнительно рано и в пределах полей предшествующего облучения, что обусловливает перекрытие PTV) и объемов облучения. Немаловажным в этом свете, помимо введения BEV, представляется оптимизация физико-технических факторов, таких как стандартизация оконтуривания целевых объемов мишени, точное планирование распределения и подведения дозы, а также увеличение градиента дозы на границе с нормальными тканями. В отно- шении последнего, особенно касательно рецидивов с большим объемом PTV, решением может быть использование протонной терапии, обладающей по сравнению с фотонной ЛТ увеличением линейной передачи энергии по мере замедления протонов в тканях вплоть до появления пика Брэгга, относительной биологической эффективностью около 1,1, а также методикой активного сканирующего пучка. Крупных исследований по применению протонной терапии в комбинации с сопутствующим приемом BEV, по нашим данным, не проводилось.
Список литературы Повторная лучевая терапия на фоне приема бевацизумаба в лечении рецидивов глиобластомы
- Grochans S., Cybulska A.M., Simińska D., Korbecki J., Kojder K., Chlubek D., Baranowska-Bosiacka I. Epidemiology of Glioblastoma Multiforme - Literature Review. Cancers (Basel). 2022; 14(10): 2412. https://doi.org/10.3390/cancers14102412.
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., Curschmann J., Janzer R.C., Ludwin S.K., Gorlia T., Allgeier A., Lacombe D., Cairncross J.G., Eisenhauer E., Mirimanoff R.O.; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10): 987-96. https://doi.org/10.1056/NEJMoa043330.
- Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Nikšić M., Bonaventure A., Valkov M., Johnson C.J., Estève J., Ogunbiyi O.J., Azevedo E Silva G., Chen W.Q., Eser S., Engholm G., Stiller C.A., Monnereau A., Woods R.R., Visser O., Lim G.H., Aitken J., Weir H.K., Coleman M.P.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018; 391(10125): 1023-75. https://doi.org/10.1016/S0140-6736(17)33326-3.
- Ostrom Q.T., Price M., Neff C., Cioffi G., Waite K.A., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019. Neuro Oncol. 2022; 24(s5):1-95. https://doi.org/10.1093/neuonc/noac202.
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., Hau P., Brandes A.A., Gijtenbeek J., Marosi C., Vecht C.J., Mokhtari K., Wesseling P., Villa S., Eisenhauer E., Gorlia T., Weller M., Lacombe D., Cairncross J.G., Mirimanoff R.O.; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10(5): 459-66. https://doi.org/10.1016/S1470-2045(09)70025-7.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Central Nervous System Cancers [Internet]. Version 1.2023 - March 24, 2023, nccn.org. [cited 2023 Dec 19]. URL: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- De Bonis P., Anile C., Pompucci A., Fiorentino A., Balducci M., Chiesa S., Lauriola L., Maira G., Mangiola A. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg. 2013; 115(1): 37-43. https://doi.org/10.1016/j.clineuro.2012.04.005.
- van Nifterik K.A., Elkhuizen P.H., van Andel R.J., Stalpers L.J., Leenstra S., Lafleur M.V., Vandertop W.P., Slotman B.J., Hulsebos T.J., Sminia P. Genetic profiling of a distant second glioblastoma multiforme after radiotherapy: Recurrence or second primary tumor? J Neurosurg. 2006; 105(5): 739-44. https://doi.org/10.3171/jns.2006.105.5.739.
- Weller M., van den Bent M., Preusser M., Le Rhun E., Tonn J.C., Minniti G., Bendszus M., Balana C., Chinot O., Dirven L., French P., Hegi M.E., Jakola A.S., Platten M., Roth P., Rudà R., Short S., Smits M., Taphoorn M.J.B., von Deimling A., Westphal M., Soffietti R., Reifenberger G., Wick W. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021; 18(3): 170-86. https://doi.org/10.1038/s41571-020-00447-z. Erratum in: Nat Rev Clin Oncol. 2022; 19(5): 357-8.
- Sminia P., Mayer R. External beam radiotherapy of recurrent glioma: radiation tolerance of the human brain. Cancers (Basel). 2012; 4(2): 379-99. https://doi.org/10.3390/cancers4020379.
- Minniti G., Niyazi M., Alongi F., Navarria P., Belka C. Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol. 2021; 16(1): 36. https://doi.org/10.1186/s13014-021-01767-9.
- Scoccianti S., Francolini G., Carta G.A., Greto D., Detti B., Simontacchi G., Visani L., Baki M., Poggesi L., Bonomo P., Mangoni M., Desideri I., Pallotta S., Livi L. Re-irradiation as salvage treatment in recurrent glioblastoma: A comprehensive literature review to provide practical answers to frequently asked questions. Crit Rev Oncol Hematol. 2018; 126: 80-91. https://doi.org/10.1016/j.critrevonc.2018.03.024.
- Kazmi F., Soon Y.Y., Leong Y.H., Koh W.Y., Vellayappan B. Reirradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neurooncol. 2019; 142(1): 79-90. https://doi.org/10.1007/s11060-018-03064-0.
- Marwah R., Xing D., Squire T., Soon Y.Y., Gan H.K., Ng S.P. Reirradiation versus systemic therapy versus combination therapy for recurrent high-grade glioma: a systematic review and meta-analysis of survival and toxicity. J Neurooncol. 2023; 164(3): 505-24. https://doi.org/10.1007/s11060-023-04441-0.
- Kiseleva V., Gordon K., Vishnyakova P., Gantsova E., Elchaninov A., Fatkhudinov T. Particle Therapy: Clinical Applications and Biological Effects. Life (Basel). 2022; 12(12): 2071. https://doi.org/10.3390/life12122071.
- Patyal B. Dosimetry aspects of proton therapy. Technol Cancer Res Treat. 2007; 6(4s): 17-23. https://doi.org/10.1177/15330346070060S403.
- Saeed A.M., Khairnar R., Sharma A.M., Larson G.L., Tsai H.K., Wang C.J., Halasz L.M., Chinnaiyan P., Vargas C.E., Mishra M.V. Clinical Outcomes in Patients with Recurrent Glioblastoma Treated with Proton Beam Therapy Reirradiation: Analysis of the Multi-Institutional Proton Collaborative Group Registry. Adv Radiat Oncol. 2020; 5(5): 978-83. https://doi.org/10.1016/j.adro.2020.03.022.
- Gulidov I., Gordon K., Semenov A., Gogolin D., Lepilina O., Golovanova O., Dujenko S., Medvedeva K., Koryakin S., Ivanov S., Kaprin A. Proton re-irradiation of unresectable recurrent brain gliomas: clinical outcomes and toxicity. J BUON. 2021; 26(3): 970-6.
- Gulidov I.A., Gordon K.B., Gogolin D.V., Mardynskii Yu.S., Lepilina O.G., Neledov D.V., Galkin V.N., Kaprin A.D. Povtornoe obluchenie intrakranial'nykh opukholei aktivnym skaniruyushchim puchkom protonov. Sibirskii onkologicheskii zhurnal. 2017; 16(5): 63-70. https://doi.org/10.21294/1814-4861-2017-16-5-63-70.
- Medvedeva K.E., Gulidov I.A., Mardynskii Yu.S., Gogolin D., Gordon K.B., Semenov A.V., Lepilina O.G., Kaprin A.D., Kostin A.A., Ivanov S.A. Vozmozhnosti protonnoi terapii pri povtornom obluchenii retsidivnykh gliom. Meditsinskaya radiologiya i radiatsionnaya bezopasnost'. 2019; 64(2): 70-4. https://doi.org/10.12737/article_5ca607bf670c97.49055999.
- Simon J.M., Cornu P., Boisserie G., Hasboun D., Tep B., Hardiman C., Valery C.A., Delattre J.Y., Dormont D., Baillet F., Mazeron J.J. Brachytherapy of glioblastoma recurring in previously irradiated territory: predictive value of tumor volume. Int J Radiat Oncol Biol Phys. 2002; 53(1): 67-74. https://doi.org/10.1016/s0360-3016(01)02804-8.
- Gutin P.H., Phillips T.L., Wara W.M., Leibel S.A., Hosobuchi Y., Levin V.A., Weaver K.A., Lamb S. Brachytherapy of recurrent malignant brain tumors with removable high-activity iodine-125 sources. J Neurosurg. 1984; 60(1): 61-8. https://doi.org/10.3171/jns.1984.60.1.0061.
- Chan T.A., Weingart J.D., Parisi M., Hughes M.A., Olivi A., Borzillary S., Alahakone D., Detorie N.A., Wharam M.D., Kleinberg L. Treatment of recurrent glioblastoma multiforme with GliaSite brachy-therapy. Int J Radiat Oncol Biol Phys. 2005; 62(4): 1133-9. https://doi.org/10.1016/j.ijrobp.2004.12.032.
- Takano S., Yamashita T., Ohneda O. Molecular therapeutic targets for glioma angiogenesis. J Oncol. 2010. https://doi.org/10.1155/2010/351908.
- Bruna A., Darken R.S., Rojo F., Ocaña A., Peñuelas S., Arias A., Paris R., Tortosa A., Mora J., Baselga J., Seoane J. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007; 11(2): 147-60. https://doi.org/10.1016/j.ccr.2006.11.023.
- Lin J.L., Wang M.J., Lee D., Liang C.C., Lin S. Hypoxia-inducible factor-1alpha regulates matrix metalloproteinase-1 activity in human bone marrow-derived mesenchymal stem cells. FEBS Lett. 2008; 582(17): 2615-9. https://doi.org/10.1016/j.febslet.2008.06.033.
- Phillips H., Armani M., Stavrou D., Ferrara N., Westphal M. Intense focal expression of vascular endothelial growth-factor messenger-RNA in human intracranial neoplasms - association with regions of necrosis. Int J Oncol. 1993; 2(6): 913-9. https://doi.org/10.3892/ijo.2.6.913.
- Zang J., Li C., Zhao L.N., Shi M., Zhou Y.C., Wang J.H., Li X. Prognostic value of vascular endothelial growth factor in patients with head and neck cancer: A meta-analysis. Head Neck. 2013; 35(10): 1507-14. https://doi.org/10.1002/hed.23156.
- Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000; 407(6801): 249-57. https://doi.org/10.1038/35025220.
- Winkler F., Kozin S.V., Tong R.T., Chae S.S., Booth M.F., Garkavtsev I., Xu L., Hicklin D.J., Fukumura D., di Tomaso E., Munn L.L., Jain R.K. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004; 6(6): 553-63. https://doi.org/10.1016/j.ccr.2004.10.011.
- Liu G., Yuan X., Zeng Z., Tunici P., Ng H., Abdulkadir I.R., Lu L., Irvin D., Black K.L., Yu J.S. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006; 5: 67. https://doi.org/10.1186/1476-4598-5-67.
- Bao S., Wu Q., Sathornsumetee S., Hao Y., Li Z., Hjelmeland A.B., Shi Q., McLendon R.E., Bigner D.D., Rich J.N. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006; 66(16): 7843-8. https://doi.org/10.1158/0008-5472.CAN-06-1010.
- Friedman H.S., Prados M.D., Wen P.Y., Mikkelsen T., Schiff D., Abrey L.E., Yung W.K., Paleologos N., Nicholas M.K., Jensen R., Vredenburgh J., Huang J., Zheng M., Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009; 27(28): 4733-40. https://doi.org/10.1200/JCO.2008.19.8721.
- Lombardi G., Pambuku A., Bellu L., Farina M., Della Puppa A., Denaro L., Zagonel V. Effectiveness of antiangiogenic drugs in glioblastoma patients: A systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2017; 111: 94-102. https://doi.org/10.1016/j.critrevonc.2017.01.018.
- McBain C., Lawrie T.A., Rogozińska E., Kernohan A., Robinson T., Jefferies S. Treatment options for progression or recurrence of glioblastoma: a network meta-analysis. Cochrane Database Syst Rev. 2021; 5(1). https://doi.org/10.1002/14651858.CD013579.pub2.
- Zhang T., Xin Q., Kang J.M. Bevacizumab for recurrent glioblastoma: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021; 25(21): 6480-91. https://doi.org/10.26355/eurrev_202111_27092.
- Kazmi F., Soon Y.Y., Leong Y.H., Koh W.Y., Vellayappan B. Reirradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. J Neurooncol. 2019; 142(1): 79-90. https://doi.org/10.1007/s11060-018-03064-0.
- Combs S.E., Niyazi M., Adeberg S., Bougatf N., Kaul D., Fleischmann D.F., Gruen A., Fokas E., Rödel C.M., Eckert F., Paulsen F., Oehlke O., Grosu A.L., Seidlitz A., Lattermann A., Krause M., Baumann M., Guberina M., Stuschke M., Budach V., Belka C., Debus J., Kessel K.A. Re-irradiation of recurrent gliomas: pooled analysis and validation of an established prognostic score-report of the Radiation Oncology Group (ROG) of the German Cancer Consortium (DKTK). Cancer Med. 2018; 7(5): 1742-9. https://doi.org/10.1002/cam4.1425.
- Møller S., Munck Af Rosenschöld P., Costa J., Law I., Poulsen H.S., Engelholm S.A., Engelholm S. Toxicity and efficacy of re-irradiation of high-grade glioma in a phase I dose- and volume escalation trial. Radiother Oncol. 2017; 125(2): 223-7. https://doi.org/10.1016/j.radonc.2017.09.039.
- Moeller B.J., Cao Y., Li C.Y., Dewhirst M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004; 5(5): 429-41. https://doi.org/10.1016/s1535-6108(04)00115-1.
- Heath V.L., Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009; 6(7): 395-404. https://doi.org/10.1038/nrclinonc.2009.52.
- Gutin P.H., Iwamoto F.M., Beal K., Mohile N.A., Karimi S., Hou B.L., Lymberis S., Yamada Y., Chang J., Abrey L.E. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009; 75(1): 156-63. https://doi.org/10.1016/j.ijrobp.2008.10.043.
- Fleischmann D.F., Jenn J., Corradini S., Ruf V., Herms J., Forbrig R., Unterrainer M., Thon N., Kreth F.W., Belka C., Niyazi M. Bevacizumab reduces toxicity of reirradiation in recurrent high-grade glioma. Radiother Oncol. 2019; 138: 99-105. https://doi.org/10.1016/j.radonc.2019.06.009.
- Kreisl T.N., Kim L., Moore K., Duic P., Royce C., Stroud I., Garren N., Mackey M., Butman J.A., Camphausen K., Park J., Albert P.S., Fine H.A. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009; 27(5): 740-5. https://doi.org/10.1200/JCO.2008.16.3055.
- Kirkpatrick J.P., Meyer J.J., Marks L.B. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008; 18(4): 240-3. https://doi.org/10.1016/j.semradonc.2008.04.005.
- Cuneo K.C., Vredenburgh J.J., Sampson J.H., Reardon D.A., Desjardins A., Peters K.B., Friedman H.S., Willett C.G., Kirkpatrick J.P. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012; 82(5): 2018-24. https://doi.org/10.1016/j.ijrobp.2010.12.074.
- Park K.J., Kano H., Iyer A., Liu X., Niranjan A., Flickinger J.C., Lieberman F.S., Lunsford L.D., Kondziolka D. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: a case-control study. J Neurooncol. 2012; 107(2): 323-33. https://doi.org/10.1007/s11060-011-0744-9.
- Guan Y., Xiong J., Pan M., Shi W., Li J., Zhu H., Gong X., Li C., Mei G., Liu X., Pan L., Dai J., Wang Y., Wang E., Wang X. Safety and efficacy of Hypofractionated stereotactic radiosurgery for high-grade Gliomas at first recurrence: a single-center experience. BMC Cancer. 2021; 21(1): 123. https://doi.org/10.1186/s12885-021-07856-y.
- Morris S.L., Zhu P., Rao M., Martir M., Zhu J.J., Hsu S., Ballester L.Y., Day A.L., Tandon N., Kim D.H., Shepard S., Blanco A., Esquenazi Y. Gamma Knife Stereotactic Radiosurgery in Combination with Bevacizumab for Recurrent Glioblastoma. World Neurosurg. 2019; 127: 523-33. https://doi.org/10.1016/j.wneu.2019.03.193.
- Hundsberger T., Brügge D., Putora P.M., Weder P., Weber J., Plasswilm L. Re-irradiation with and without bevacizumab as salvage therapy for recurrent or progressive high-grade gliomas. J Neurooncol. 2013; 112(1): 133-9. https://doi.org/10.1007/s11060-013-1044-3.
- Schnell O., Thorsteinsdottir J., Fleischmann D.F., Lenski M., Abenhardt W., Giese A., Tonn J.C., Belka C., Kreth F.W., Niyazi M. Reirradiation strategies in combination with bevacizumab for recurrent malignant glioma. J Neurooncol. 2016; 130(3): 591-9. https://doi.org/10.1007/s11060-016-2267-x.
- Youland R.S., Lee J.Y., Kreofsky C.R., Brown P.D., Uhm J.H., Laack N.N. Modern reirradiation for recurrent gliomas can safely delay tumor progression. Neurooncol Pract. 2018; 5(1): 46-55. https://doi.org/10.1093/nop/npx014.
- Chan J., Jayamanne D., Wheeler H., Khasraw M., Wong M., Kastelan M., Guo L., Back M. The role of large volume re-irradiation with Bevacizumab in chemorefractory high grade glioma. Clin Transl Radiat Oncol. 2020; 22: 33-9. https://doi.org/10.1016/j.ctro.2020.03.005.
- Helis C.A., Prim S.N., Cramer C.K., Strowd R., Lesser G.J., White J.J., Tatter S.B., Laxton A.W., Whitlow C., Lo H.W., Debinski W., Ververs J.D., Black P.J., Chan M.D. Clinical outcomes of dose-escalated re-irradiation in patients with recurrent high-grade glioma. Neurooncol Pract. 2022; 9(5): 390-401. https://doi.org/10.1093/nop/npac032.
- Tsien C.I., Pugh S.L., Dicker A.P., Raizer J.J., Matuszak M.M., Lallana E.C., Huang J., Algan O., Deb N., Portelance L., Villano J.L., Hamm J.T., Oh K.S., Ali A.N., Kim M.M., Lindhorst S.M., Mehta M.P. NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma. J Clin Oncol. 2023; 41(6): 1285-95. https://doi.org/10.1200/JCO.22.00164.
- Cuncannon M., Wong M., Jayamanne D., Guo L., Cove N., Wheeler H., Back M. Role of delayed salvage bevacizumab at symptomatic progression of chemorefractory glioblastoma. BMC Cancer. 2019; 19(1): 445. https://doi.org/10.1186/s12885-019-5678-1.
- Modh A., Bergman D., Schultz L., Snyder J., Mikkelsen T., Ryu S., Siddiqui M.S., Walbert T. RTHP-06. Randomized prospective trial of stereotactic radiosurgery versus chemotherapy for recurrent malignant glioma after second-line chemotherapy. Neuro Oncol. 2018; 20(6). https://doi.org/10.1093/neuonc/noy148.938.
- Tsien C., Pugh S., Dicker A.P., Raizer J.J., Matuszak M.M., Lallana E., Huang J., Algan O., Taylor N., Portelance L., Villano J., Hamm J., Oh K.S., Ali A.N., Kim M.M., Lindhorst S., Mehta M.P. Randomized phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRG Oncology/RTOG 1205): initial outcomes and RT plan quality report. Int J Radiat Oncol Biol Phys. 2019; 105(1): 78. https://doi.org/10.1016/j.ijrobp.2019.06.539.
- Kim K.J., Li B., Winer J., Armanini M., Gillett N., Phillips H.S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993; 362(6423): 841-4. https://doi.org/10.1038/362841a0.
- Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005; 307(5706): 58-62. https://doi.org/10.1126/science.1104819.
- Duda D.G., Jain R.K., Willett C.G. Antiangiogenics: the potential role of integrating this novel treatment modality with chemoradiation for solid cancers. J Clin Oncol. 2007; 25(26): 4033-42. https://doi.org/10.1200/JCO.2007.11.3985.
- Calabrese C., Poppleton H., Kocak M., Hogg T.L., Fuller C., Hamner B., Oh E.Y., Gaber M.W., Finklestein D., Allen M., Frank A., Bayazitov I.T., Zakharenko S.S., Gajjar A., Davidoff A., Gilbertson R.J. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007; 11(1): 69-82. https://doi.org/10.1016/j.ccr.2006.11.020.
- Gilbertson R.J., Rich J.N. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007; 7(10): 733-6. https://doi.org/10.1038/nrc2246.
- Matsko M.V., Matsko E.D. Neiroonkologiya, 2021. Kratkii analiz novoi klassifikatsii Vsemirnoi organizatsii zdravookhrane-niya opukholei tsentral'noi nervnoi sistemy. Vestnik Sankt-Peterburgskogo universiteta. Meditsina. 2022; 17(2): 88-100. https://doi.org/10.21638/spbu11.2022.202.


