Prediction of Future World on The Basis of High Heat conditions on Plant Metabolism
Автор: Malini Bhattacharyya, Babita Patni
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.16, 2021 года.
Бесплатный доступ
Global warming creates temperature elevation with the help of green house gases and anthropogenic activities. Elevated temperatures have been selected as key factors that influence on plant growth and development. CO2 is essential for plant photosynthesis and growth. But CO2 is a green house gas and responsible for temperature elevation. Up to certain level heat is helpful for plant growth, survival and physio – biochemical processes. Above the certain range, heat causes disruption in plant metabolic processes. This is also important for the development of new strategies and biotechnological tools for enhancing plant growth in the future world facing from high heat stress situations. In this review, we have focused on role of high temperature effects on plant metabolisms to illustrate the possible future world.
Climate Change, Crops, Heat Stress, Photosynthesis
Короткий адрес: https://sciup.org/143178315
IDR: 143178315
Текст научной статьи Prediction of Future World on The Basis of High Heat conditions on Plant Metabolism
Earth population is going to increase day by day. This phenomenon indicates that, it is required to enhance crop yield to fulfill the need of heterotrophs. Quality of food should be important because it gather all nutrients to maintain healthy life. Global warming is the reason of temperature increase. Global warming is caused by several anthropogenic activities like, burning of hydrocarbon materials, emission of green house gases. Pollution and green house gases trap heat from sunlight and preserve it in the atmosphere.
Elevated temperature has been recognized as the reason for faster crop development in tropical areas mainly in C3 plants. In vitro condition, increased atmospheric CO 2 concentration is studied as a single factor, crop production tends to increase, but under field conditions, various stress factors can occur simultaneously, like changing climate and high temperature, which could stop the positive effect of high CO 2 on plant yields (Lobell and Gourdji, 2012). CO 2 plays dual role in plants. It is the major green house gas as well as most important for plant photosynthesis, growth and development.
Photosynthetic Metabolism under Changing Environment
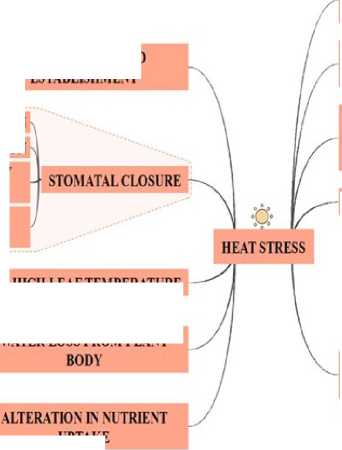
Exposure to heat stress induces several physiological reactions in plant systems. Among the main physiological changes are alterations in photosynthesis and translocation assimilation (Morgan et al. 2004) , changes in capacity of water uptake, evapotranspiration (Rivelli et al. 2002) , effects on nutrient uptake and translocation (Hu and Schmidhalter, 2005, Sanchez-Rodrigues et al. 2010) , antioxidant production (Blokhina et al. 2003, Apel and Hirt, 2004) , programmed cell death (PCD) (Kangasjärvi et al. 2005) , gene expression alteration and enzyme activity (Yamakawa et al. 2007, Guo et al. 2009) . Stress based inhibitory effects on photosynthesis may be for two reasons. These are, limited CO 2 diffusion and metabolic factors. Stomatal closure in heat stress is major response from plants (Cornic 2000, Brestic et al. 1995, Gao et al. 2002, Chaves et al. 2002) .
CO2 availability to plants can vary depending on the changing climate and heat stress. The assimilation is dependent on so many factors like, stomatal closure, changes in Calvin cycle metabolisms and alterations in the thylakoid membrane (Berry and Bjorkman 1980, Weis and Berry 1988). Exposure of plants to high-temperature results in changes at the biochemical and physiological level like, photosynthetic activities (Sharkey et al. 2007).
The effects of extreme high temperatures on crops have been analyzed in several crops like; Triticum sp. (Djanaguiraman 2018) , Vigna sp. (Ismail, 1999), Glycine max (Alsajri et al. 2019) , and in Oryza (Matsui and Omasa 2002) . In Triticum sp., Asseng et al. in 2013, assessed the role of high temperatures on yield and the adverse effects on the crop production. Many responses to heat stress have been discovered in plants. These are growth inhibition, disability in seed establishment, slow transpiration, water loss and low grade crop production (Mathur et al. 2014) . Decline in chlorophyll content in Cucumis sativus and Triticum is also one of the effects present in plants exposed to high-temperature (Kumar Tewary and Tripathy 1998) , degradation of enzymes is also found (Ashraf and Harris 2013) .
Extreme temperatures affect CO2 assimilation at the stomatal level by controlling the closure of stomata under heat stress conditions. The impaired photosynthesis also happens by extreme heat stress. By controlling CO2 diffusion, stomata controls the rate of photosynthesis (Morales et al., 2004). Researchers have analyzed plant behavior at the stomatal conductance level under heat-stressed situations, with dual role like inhibition and also the elevation of photosynthesis (Mathur et al. 2014, Morales et al. 2004, Crafts-Brandner and Law 2000, Wahid et al. 2007). Aquaporins have been proposed as influencing factors in cell wall structural changes during heat stress (Flexas et al. 2008, Flexas and Díaz-Espejo 2014). Crop yield is measured by both photosynthetic CO2 assimilation and respiration rates. Both processes are temperature sensitive. Temperature affects photosynthesis by elevating the electron transport chain of the thylakoid membrane. As leaf temperature becomes higher, the photosynthetic rate increases, until decreasing after reaching optimum temperature. It shows the effect of temperature on photosynthetic CO2 fixation, and CO2 release by photorespiration and mitochondrial respiration (Posch et al., 2019). In C3 plants, Rubisco is the principal enzyme responsible for carbon assimilation, although it can also assimilate O2, which compete with CO2 for enzyme binding sites. As substrates of Rubisco, both O2 and CO2 play competitions in regulating the response of photosynthesis to heat stress. (Law and Crafts-Brandner,1999).
Edwards 1977) and lower the Rubisco - CO 2 specificity ( Ku and Edwards 1977) . The chloroplast based enzyme Rubisco activase lowers photosynthetic activity under heat stress. Heat stress lowers the activity of Rubisco activase to maintain the activation state of Rubisco ( Yamori and von Caemmerer, 2009 ). Some studies have shown that Rubisco activity decreases when the temperature is elevated as it does not show a good balance between the activation and inactivation rates of Rubisco in Nicotiana and Gossypium plants ( Ashraf and Harris 2013) .
Elevated temperatures influences photorespiration due to greater solubility of O2 relative to CO2 (Ku and
DISABILITY IN SEED ESTABLISHMENT
LIMITED С02 DIFFUSION
CHANGES IX WATER UPTAKE
LESS COZ FIXATION
MITOCHONDRIAL RESPIRATION
LIMITED COZ RELEASE BY PHOTORESPIRATION
UPTAKE
ALTERATION OF TRANSLOCATION ASSIMILATION
ANTIOXIDANT PRODUCTION
RI BISCO ACTIVASE LOWERS - HIGH LEAF TEMPERATI RE
EVAPOTRANSPIRATION
SLOW TRANSPIRATION 4 WATER L0SS FR0M PLANT

ALTERATION OF PHOTOSYNTHESIS
ALTERATION OF GENE STRUCTURE
INDUCTION OF
PHYSIOLOGICAL PROCESSES

INCREASED PHOTOSYNTHESIS ■ UP TO A CERTAIN RANGE
CHLOROPHYLL CONTENT DECLINE ^CHANGES IN METABOLITES • DYSFUNCTION OF RUBISCO
OXIDATION IN PS II J CHANGES LN PLASTOQUINONE
POOL
INACTIVATION OF DI PROTEIN
OVERALLGROWTH DEGRADATION
Figure 1. Effect of Heat Stress on Plant Metabolism
Some researchers have also shown that the Vcmax (the maximum rate of Rubisco carboxylase activity) acts as a limiting factor on the rate of photosynthesis, having roles in temperature acclimation (Leuning, 2002), and it has been observed that, it elevates temperature rises to an optimal value and from there, it usually declines (Bernacchi et al. 2001, Yamasaki et al. 2002). This decline in Vcmax under high temperatures is probably due to the dysfunction of Rubisco activase, resulting in a reduction in Rubisco activity. The principal element of the chloroplast electron transfer chain, PSII, may be damaged by heat (Bukhov and Wiese 1999). PSII is a heat-sensitive component, changes in the D1 protein and the plastoquinone pool, the high temperature on photosynthetic components influence oxidation in PSII. This could destabilize lipid and protein interactions, which could perturb the organization and function of PSII (Mathur et al. 2014).
Several studies have focused on biochemical reactions happened in heat stress, related to hormones, primary and secondary metabolites (Gulen and Eris, 2004). At the molecular level, an alteration in the expression of genes leads to the synthesis of stress-related proteins, like heat shock proteins activation, which are essential under this heat stress (Wahid et al. 2007). All plants are bound to change their metabolism to prevent damage for stress adaptation. So, researchers are looking for the heat tolerance through the development of heat-tolerant transgenics via molecular breeding and bioengineering tools (Driedonks et al, 2016, He et al. 2019). In ( ig 1), we have described role of heat stress mediated alterations in plant system. Heat stress damage several physiological and genetic processes as displayed in the below mentioned figure.
CONCLUSION
Global warming creates temperature elevation in environment. CO 2 gas is the major reason for global warming. High CO 2 enhances plant net photosynthesis up to a certain range. But extreme level of high temperature more than optimum level has detrimental effects on plant metabolisms. It harms plant anabolic processes by limiting photosynthesis, stomatal CO 2 diffusion, antioxidant production, transcription of genes.
High temperature alters photosynthesis process by changing chloroplast proteins and “Z – scheme” members. So, if temperature increases in this way, it will result a future warmer world. Plant survival rate will decline in the future world. Cold loving plants and plants enlisted in IUCN red data list will not survive in the warmer world, that will result in scarcity of food. Development of transgenic crops and novel plant breeding techniques are required to control this condition.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Prediction of Future World on The Basis of High Heat conditions on Plant Metabolism
- Alsajri, F.A., Singh, B., Wijewardana, C., Irby, J.T., Gao, W., & Reddy, K.R. (2019). Evaluating Soybean Cultivars for Low- and High-Temperature Tolerance During the Seedling Growth Stage Agronomy, 9: 13.
- Apel, K., & Hirt, H. (2004). Annual review of plant biology, 55: 373
- Ashraf, M., & Harris, P.J. (2013). Photosynthetica (51): 163.
- Asseng, S., Ewert, F., Rosenzweig, C., Jones, J.W., Hatfield, J.L., Ruane, A.C., Boote, K.J., Thorburn, P.J., Rötter, R.P., Cammarano, D., Brisson, N., Basso, B., Martre, P., Aggarwal, P.K., Angulo, C., Bertuzzi, P., Biernath, C., Challinor, A.J., Doltra, J., Gayler, S., Goldberg, R., Grant, R.F., Heng, L., Hooker, J., Hunt, L.A., Ingwersen, J., Izaurralde, R.C., Kersebaum, K.C., Müller, C., Kumar, S.N., Nendel, C., O'Leary, G., Olesen, J.E., Osborne, T.M., Palosuo, T., Priesack, E., Ripoche, D., Semenov, M.A., Shcherbak, I., Steduto, P., Stöckle, C.O., Stratonovitch, P., Streck, T., Supit, I., Tao, F., Travasso, M., Waha, K., Wallach, D., White, J.W., Williams, J.R., & Wolf, J. (2013). Nature Climate Change, (3): 827
- Bernacchi, C. J., Portis, A. R., Nakano, H., von Caemmerer, S., & Long, S. P. (2002). Plant physiology, 130(4): 1992
- Bernacchi, Carl & Singsaas, E. & Pimentel, Carlos & Portis, Archie & Long, S.. (2001). Plant, Cell and Environment. 24: 253
- Berry, J.A., & Björkman, O. (1980). Annual Review of Plant Biology, 31: 491
- Blokhina, O., Virolainen, E., & Fagerstedt, K. V. (2003). Annals of botany, 91(2): 179.
- Brestic, M., Cornic, G., Freyer, M., & Baker, N.R. (2004). Planta, 196: 450
- Brooks, A., & Farquhar, G. D. (1985). Planta, 165(3): 397
- Bukhov, N.G., Wiese, C., Neimanis, S., & Heber, U. (2004). Photosynthesis Research, 59: 81.
- Chaves, M. M., Pereira, J. S., Maroco, J., Rodrigues, M. L., Ricardo, C. P., Osorio, M. L., Carvalho, I., Faria, T., & Pinheiro, C. (2002). Annals of botany, 89 (7): 907
- Crafts-Brandner, S.J., & Law, R.D. (2000). Planta, (212): 67.
- Djanaguiraman, M., Boyle, D., Welti, R., Jagadish, S.V., & Prasad, P.V. (2018). BMC Plant Biology, 18 the development of heat-tolerant transgenics via molecular breeding and bioengineering tools (Driedonks Plant reproduction, 29(1-2): 67.
- J., & Medrano, H. (2008). Plant, cell & environment, 31(5): 602. Flexas, Jaume & Diaz-Espejo, Antonio. (2014). Plant, cell & environment. 38.
- Plant, Cell & Environment. 25: 1373 Gulen, H.; Eris, A. (2004). Plant Sci., 166: 739. Guo, P., Baum, M., Grando, S., Ceccarelli, S., Bai, G., Li, R., von Korff, M., Varshney, R. K., Graner, A., & Valkoun, J. (2009). Journal of experimental botany, 60(12): 3531
- X., Mo, B., Chen, X., & Liu, L. (2019). Plant physiology, 181(2): 609.
- Hu, Y., & Schmidhalter, U., (2005). J. Plant Nutr. Soil Sci., 168: 541
- Ismail, A.M., & Hall, A. (1999). Reproductive-Stage Heat Tolerance, Leaf Membrane Thermostability and Plant Morphology in Cowpea. Crop Science, 39, 1762-1768.
- Kangasjärvi, J., Jaspers, P., & Kollist, H. (2005). Plant, Cell & Environment. 28: 1021
- Ku, S. B., & Edwards, G. E. (1977). Plant physiology, 59(5): 986.
- Law, R. D., & Crafts-Brandner, S. J. (1999). Plant physiology, 120(1): 173.
- Leuning, R., (2002). Plant, Cell & Environment. 25: 1205
- Matsui, T., & Omasa, K. (2002). Annals of botany, 89(6): 683.
- Morales, D., Rodríguez, P., Dell'Amico, J.M., Nicolas, E., Torrecillas, A., & Sánchez-blanco, M.J. (2004). Biología Plantarum, (47): 203.
- Posch, B. C., Kariyawasam, B. C., Bramley, H., Coast, O., Richards, R. A., Reynolds, M. P., Trethowan, R., & Atkin, O. K. (2019). Journal of experimental botany, 70(19): 5051.
- Rivelli, A., R., & James, R., & Munns, R., & Condon, A., G., (2002). Functional Plant Biology, 29: 1065
- Sánchez-Rodríguez, E., Cuauhtémoc, María del Mar Rubio-Wilhelmi, Luis Miguel Cervilla, Begoña Blasco, Juan José Ríos, Rocío Leyva, Luis Romero and Juan Manuel Pastor Ruíz. (2010). Plant and Soil, 335: 339
- Sharkey, T.D., Bernacchi, C.J., Farquhar, G.D., & Singsaas, E.L. (2007). Plant, cell & environment, 30(9): 1035
- Wahid, A., Gelani, S., Ashraf, M., & Foolad, M.R. (2007). Environmental and Experimental Botany. (61): 199.
- Warren C. R. (2008). Journal of experimental botany, 59(7): 1475.
- Weis, E., & Berry, J.A. (1988). Symposia of the Society for Experimental Biology, 42: 329
- Yamakawa, H., Hirose, T., Kuroda, M., & Yamaguchi, T. (2007). Plant physiology, 144(1): 258
- Yamasaki, T., Yamakawa, T., Yamane, Y., Koike, H., Satoh, K., & Katoh, S. (2002). Plant physiology, 128(3): 1087.
- Yamori, W., & von Caemmerer, S. (2009). Plant physiology, 151(4): 2073.
- Driedonks, N., Rieu, I., & Vriezen, W. H. (2016)..
- Flexas, J., Ribas-Carbó, M., Díaz-Espejo, A., Galmés, Gao, Q., Zhao, P., Xp, Z., Cai, X., & Shen, W. (2002).
- He, J., Jiang, Z., Gao, L., You, C., Ma, X., Wang, X., Xu, KumarTewari, A., & CharanTripathy, B. (1998).
- Lobell, D. B., & Gourdji, S. M., (2012).
- Mathur, S., Agrawal, D., & Jajoo, A. (2014).


