Predictive and prognostic significance of loss of heterozygosity in ABC transporter genes in breast cancer
Автор: Tsyganov M.M., Ibragimova M.K., Garbukov E.Yu., Bragina O.D., Zdereva E.A., Usynin E.A., Litvyakov N.V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Клинические исследования
Статья в выпуске: 5 т.21, 2022 года.
Бесплатный доступ
ABC-transporter family genes have been well studied and their involvement in the development of drug resistance has been assessed. The presence of aberrant conditions in these genes can affect the treatment and prognosis of the disease. Loss of heterozygosity (LOH) is one of these conditions; it is a common event in cancer development. Therefore, the aim of this study was to investigate the relationship between LOH in ABC transporter genes in breast cancer and response to chemotherapy and disease prognosis. Material and Methods. A total of 130 breast cancer patients were included in the study. Microarray analysis was performed on Affymetrix CytoScan™ HD Array high-density DNA chips to assess LOH status. Chromosome Analysis Suite 4.1 software (Affymetrix, USA) was used to process microarray results. Results. Forty-nine ABC transporter genes were evaluated for LOH. The frequency of LOH ranged from 6.9 % to 90 %. An association analysis identified two genes: ABCG5 and ABCG8, in which the presence of LOH was associated with a lack of objective response to neoadjuvant chemotherapy. The presence of LOH in the ABCA5, ABCA6, ABCA8, ABCA9, ABCA10 and ABCC3 genes was associated with high rates of metastasis-free survival (log-rank test, p
Breast cancer, loss of heterozygosity, efficacy of neoadjuvant chemotherapy, metastasis-free survival, prognosis
Короткий адрес: https://sciup.org/140296636
IDR: 140296636 | УДК: 618.19-006.6-08-037:575.113 | DOI: 10.21294/1814-4861-2022-21-5-34-43
Текст научной статьи Predictive and prognostic significance of loss of heterozygosity in ABC transporter genes in breast cancer
ABC transporter family genes are currently well studied, and their impact on chemotherapy response and disease prognosis in various types of cancer has been evaluated [1–3]. Our previous studies have shown that ectopic expression, as well as the presence of chromosomal aberrations in the chromosomal loci, where these genes are localized, affects the response to chemotherapy in breast cancer patients [1, 4, 5].
However, one of the most effective ways to study malignant tumors at the molecular genetic level is the analysis of genomic loci imbalance, a situation in which the normal ratio of alleles changes due to the loss or increase in copies of one of them [6]. The imbalance sites, first, contain candidate genes involved in the development of the disease and, second, may themselves be molecular markers [7]. A particular case of allelic imbalance is loss of heterozygote (LOH), in which there is a loss (structural or functional) of one of the alleles of a heterozygous genotype and a decrease in detectable frequencies of heterozygous genotypes compared to genomic DNA. Loss of heterozygosity at certain chromosomal sites is a structural aberration often found in tumor cells, which can be caused by deletion of certain chromosomal regions, aberrant mitotic recombination, etc. [8]. However, the effect of LOH on treatment outcomes is highly controversial. It was demonstrated that LOH on chromosome 18p11.32 containing TYMS in metastatic colorectal cancer cells changed the genotype of the tumor cells and resulted in a different response to 5-fluorouracil-based therapy [9]. In breast cancer (BC), the main focus of research is the study of allelic imbalance in biomarkers, such as ERBB2 (HER2) [10], BRCA1 and BRCA2 [11]. In a study on allelic imbalance and LOH in breast cancer, the authors examined genes, such as EGFR, TERT, TP53, CASP8, PARP2, GATA3, and BRCA1
-
[12] . Studies on LOH of ABC transporter genes are virtually non-existent. There are only sporadic studies on this issue. The association between breast cancer risk and the frequency of the G allele 538G > A (Gl-y180Arg) and the ABCC11 gene, was studied. It was found that the frequency of the G allele was higher in breast cancer patients than in healthy individuals in the control group. The odds ratio of developing breast cancer for the genotypes (G/G+G/A) was 1.63 (p=0.026), indicating that the G allele in ABCC11 was associated with the risk of developing breast cancer [13]. A mediated effect of LOH on ABCB1 expression was also found. The presence of sites of LOH in the ABCB1 gene with amplification of this gene function is the main, if not the only, mechanism of ABCB1 overexpression in Candida albicans strains [14]. We also found that regions with significant LOH frequency included 2p25.3, 2p21, 2p15~p16.1, 2q23.3, and 16q12.1.It is worth noting that ABC genes such as ABCC11 and ABCC12 are localized in the 16q12.1 region [15].
However, LOH in the ABC transporter genes has hardly been studied, and the effect of heterozygosity loss in these genes on the response to chemotherapy and disease prognosis has not been assessed. Thus, the aim of this study was to investigate the relationship between LOH of ABC transporter genes in breast cancer and the response to neoadjuvant chemotherapy and metastasis-free survival.
Material and Methods
The study involved 130 patients with stage IIA–II-IB breast cancer. The median age of the patients was 48 years (range, 27–68) (Table 1). The retrospective study included 90 patients. In accordance with «Consensus Conference on Neoadjuvant Chemotherapy in Carcinoma of the Breast, April 26–28, 2003, Philadelphia, table 1/Таблица 1
clinical and pathological characteristics of breast cancer patients Клинико-патологическая характеристика больных раком молочной железы
|
Clinical and pathological parameter/Клинико-патологический параметр |
Number of patients/ Количество пациентов |
|
|
Age, years/Возраст, лет |
≤45 |
56 (43.1 %) |
|
> 45 |
74 (56.9 %) |
|
|
Menstrual status/ |
Premenopause/Пременопауза |
71 (54.6 %) |
|
Менструальный статус |
Postmenopause/Постменопауза |
59 (45.4 %) |
|
T1 |
17 (13.1 %) |
|
|
Tumor size/ |
T2 |
97 (74.6 %) |
|
Размер опухоли |
T3 |
7 (5.4 %) |
|
T4 |
9 (6.9 %) |
|
|
N0 |
53 (40.8 %) |
|
|
Lymphogenous metastasis/ |
N1 |
58 (44.6 %) |
|
Лимфогенное метастазирование |
N2 |
8 (6.2 %) |
|
N3 |
11 (8.5 %) |
|
|
Luminal В/Люминальный В |
94 (72.3 %) |
|
|
Molecular subtype/ Молекулярный подтип |
Triple negative/Трипл-негативный |
23 (17.7 %) |
|
HER2-positive/HER2-позитивный |
13 (10.0 %) |
|
|
Histological form/ |
Unicentric/Моноцентричный |
67 (51.5 %) |
|
Гистологическая форма |
Multicentric/Мультицентричный |
63 (48.5 %) |
|
CAX |
28 (21.5 %) |
|
|
AC |
45 (34.6 %) |
|
|
NAC regimens/Схема НХТ |
Taxotere |
26 (20.0 %) |
|
ACT/AT |
16 (12.3 %) |
|
|
CP |
15 (11.5 %) |
|
|
Progression/Прогрессирование |
9 (6.9 %) |
|
|
NAC response/Эффект НХТ |
Stabilization/Стабилизация |
32 (24.6 %) |
|
Partial regression/Частичная регрессия |
76 (58.5 %) |
|
|
Complete regression/Полная регрессия |
13 (10.0 %) |
|
table 2/Таблица 2 correlation of the presence loss of heterozygosity sites in aBc transporter genes at the level of expressed tendency
Связь наличия участков потери гетерозиготности в генах aBc-транспортеров на уровне выраженной тенденции
|
Effect of NAC/Эффект НХТ |
|||||
|
Genes/Гены |
Complete and partial regression/ Полная и частичная регрессия |
Stabilization and progression/ Стабилизация и прогрессирование |
p-level |
||
|
LOH |
n |
LOH |
n |
||
|
ABCA11P |
28 (31.5 %) |
61 (68.5 %) |
7 (17.1 %) |
34 (82.9 %) |
0.09 |
|
ABCB1 |
25 (28.1 %) |
64 (71.9 %) |
3 (7.3 %) |
38 (92.7 %) |
0.07 |
|
ABCB4 |
25 (28.1 %) |
64 (71.9 %) |
3 (7.3 %) |
38 (92.7 %) |
0.07 |
|
ABCB8 |
14 (15.7 %) |
75 (84.3 %) |
2 (4.9 %) |
39 (95.1 %) |
0.09 |
|
ABCF2 |
14 (15.7 %) |
75 (84.3 %) |
2 (4.9 %) |
39 (95.1 %) |
0.09 |
Pennsylvania» [16] all patients received 2–8 courses of neoadjuvant chemotherapy: AC (doxorubicin (60 mg/m2 intravenously on the 1st day), cyclophosphamide (600 mg/m2 intravenously on the 1st day)), CAX (cyclophosphamide (100 mg/m2 intravenously on the 1st-14th days), doxorubicin (30 mg/m2 intravenously on the 1st and 8th days), xeloda (2000 mg/m2 intravenously on the 1st–14th days, by mouth)), ACT/ АТ (doxorubicin (60 mg/m2 intravenously on the 1st day), cyclophosphamide (600 mg/m2 intravenously on the 1st day), (100 mg/m2 hourly infusion per day)), CP (cyclophosphamide (600mg/m2 1st day), cisplatin (100 mg/m2 – 1st day)).
We analyzed biopsy tumor samples before treatment (~10 mm3 volume) and 3–5 weeks after the last course of neoadjuvant chemotherapy (~60–70 mm3 volume). Tumor samples were placed in an RNAl-ater solution (Ambion, USA) and stored at –80°C (after a 24-hour incubation at +4° C) for further DNA isolation.
DNA extraction. DNA was isolated from 130 samples of tumor tissue using the QIAamp DNA mini Kit (Qiagen, Germany). DNA concentration and purity of isolation were evaluated on a NanoDrop-2000 spectrophotometer (Thermo Scientific, USA) (from 50 to 190 ng/ц!, A260/A 2 8 =2.05-2.20; A 260 / A =1.95-2.20). DNA integrity was assessed by capillary electrophoresis on a TapeStation instrument (Agilent Technologies, USA); DNA fragments had a mass of more than 60 kbp.
Microarray analysis. Microarray analysis was performed on high density microarrays (DNA chips) of Affymetrix (USA) CytoScanTM HD Array to determine the loss of heterozygosity. Sample preparation, hybridization, and scanning procedures were performed according to the manufacturer’s protocol on an Affymetrix GeneChip® Scanner 3000 7G system (Affymetrix, USA). To process the results of microchipping, we used the Chromosome Analysis Suite 4.0 program (Affymetrix, USA) for detection of loss of heterozygosity.
Statistical methods. Statistical analysis was performed using the Statistica 8.0 software package (StatSoft Inc., USA). .The survival probability was calculated using the Kaplan-Meier method. The log-rank test was used to compare the significance of differences between groups in terms of survival. Frequency comparisons were based on two-tailed Fisher’s test and/or Chi-square test.
Results
The frequency of LOH in the ABC transporter genes was estimated (Fig. 1, Supplement 1 Table 1). It was found that the highest frequency of LOH (over 50 %) was observed in genes: ABCC11 (90.0 %), ABCC12 (90.0 %), ABCB7 (69.2 %), ABCD1 (67.7 %), and ABCD2 (53.8 %), (Supplement 1, Table 1). The genes: ABCC9 (9.2 %), ABCD3 (9.2 %), ABCB5 (8.5 %), ABCA7 (7.7 %), ABCG1 (7.7 %), and ABCA13 (6.9 %) had the lowest frequency of LOH.
Analysis of the association of the presence of LOH in 49 ABC-transporter genes with the response to neoadjuvant chemotherapy showed that the frequency of LOH in ABCG5 and ABCG8 genes was significantly higher in patients with tumor stabilization and progression (26.8 %, 11/41 cases) compared to that in patients with objective response to treatment (5.6 %, 5/89 cases), p=0.001 (Fig. 2).
The presence of LOH in ABCA11P, ABCB1, ABCB4, ABCB8, and ABCF2 genes demonstrated a tendency towards increase in the frequency of tumor response to neoadjuvant chemotherapy (Table 2).
The frequency of LOH in ABCA11P, ABCB1, ABCB4, ABCB8, and ABCF2 genes was 3–4 times higher in patients with complete and partial regression.
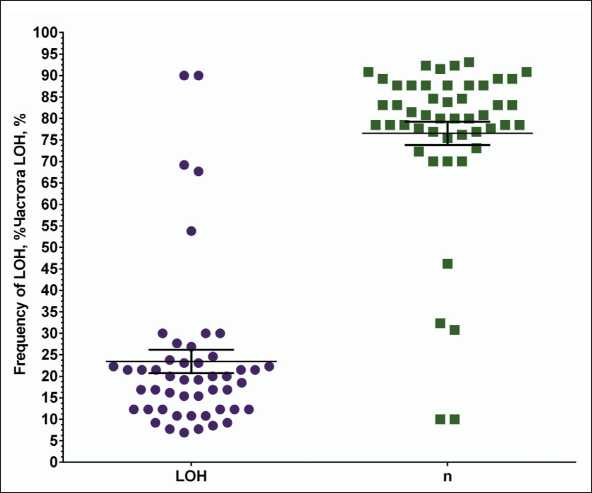
Fig. 1. Variation in the frequency of loss of heterozygosity sites in ABC transporter genes Рис. 1. Разброс частоты встречаемости участков потери гетерозиготности в генах ABC-транспортеров
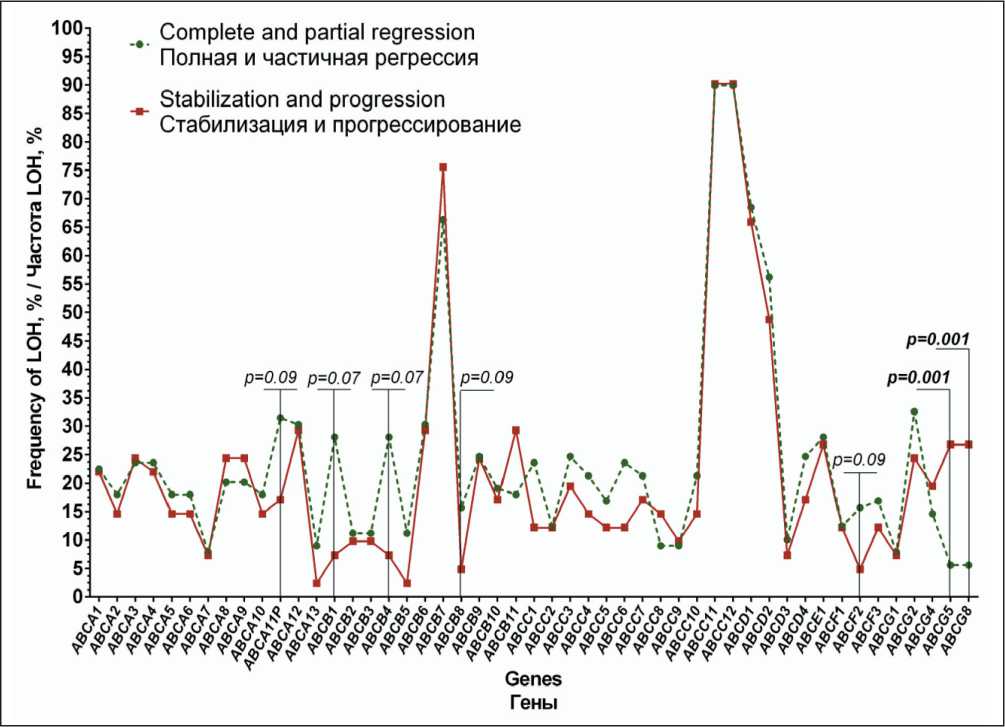
Fig. 2. Frequency of LOH in ABC-transporter genes with respect to the response to neoadjuvant chemotherapy
Рис. 2. Частота встречаемости участков потери гетерозиготности генов ABC-транспортеров в зависимости от эффекта неоадъювантной химиотерапии
Analysis of metastasis-free survival rates with respect to the presence of LOH is shown in Figure 3. In the total group of 130 patients examined, distant metastases developed in 37 (29 %) patients within 4-130 days after diagnosis.
The 5-year MFS rate in patients with the presence of LOH in the ABCA5, ABCA6, and ABCA10 genes was 95 % versus 67.5 % in the group of patients without the presence of LOH (log-rank test p=0.007). For the ABCA8 and ABCA9 genes, this rate was 96 % versus 66 % (log-rank test p=0.008). For the ABCC3 gene, we also showed statistically significant differences in survival rates between patients with and without LOH (log-rank test p=0.04).
No statistically significant association with treatment efficacy and disease prognosis was found for the remaining ABC-transporter genes.
Discussion
The phenomenon of LOH occurs when a tumor cell initially heterozygous for a certain locus loses one of its two alleles at that locus, either by simple deletion of one allele or by deletion of one allele accompanied by duplication of the remaining allele [17].
There are no available data on the effect of LOH in ABC-transporter genes on the functional component, as well as on the response to chemotherapy and disease prognosis. Nevertheless, it has been shown that the presence of polymorphisms in the ABCG2 gene correlates with severe myelosuppression induced by the irinotecan, while the presence of polymorphisms in the ABCG1 and ABCC5 genes correlate with gastrointestinal toxicity [18].
In our study, we observed the highest frequency of LOH in genes, such as: ABCC11, ABCC12, ABCB7, ABCD1, and ABCD2. However, the presence of LOH in the ABCG5 and ABCG8 genes is associated with the effect of LOH. According to the literature data, genes ABCG5 and ABCG8 function as semitransporters and form a heterodimeric complex, which is reflected in the functioning of these genes [19].
ABCB1 is the most extensively investigated gene in terms of mutations. The missense variant (rs2032582) and synonymous substitution (rs1045642) have been shown to be associated with the risk of adverse reactions during fluoropyrimidine therapy [20], as well as the high toxicity of taxanes [21] and anthracyclines [22]. The results of the meta-analysis indicate associations between the ABCB1 G2677T/A polymorphism and the risk of breast cancer and the effect on treatment, with an overall comparison across four genetic models (heterozygous model: OR=1. 01, 95 % CI=0.92–1.09, p=0.90; homozygous model: OR=1.01, 95 % CI=0.65–1.55, p=0.97; recessive model: OR=1.06, 95 % CI=0.75–1.50, p=0.76; dominant model: OR=0.98, 95 % CI=0.77–1.24, p=0.85) [23]. The ABCB1 G1199 T/A (rs2229109) polymorphism was found to have no effect on overall and recurrence-free survival in breast cancer patients [24]. Despite the fact that the effect of LOH in the ABCF2 gene on the response to NAC is shown only at the level of a marked trend, the authors report that ABCF2 plays a role in mediating drug resistance induced by cisplatin [25]. In addition, Seborova K. et al. showed that the best effectiveness of chemotherapy and high rates of progression free survival occurred in patients with ovarian cancer with loss of function of ABCF1, ABCF2 and ABCF3 genes [26].
According to our data, the presence of LOH predominantly in the ABCA family is associated with metastasis-free survival rates. This is consistent with the literature data: the presence of a deletion
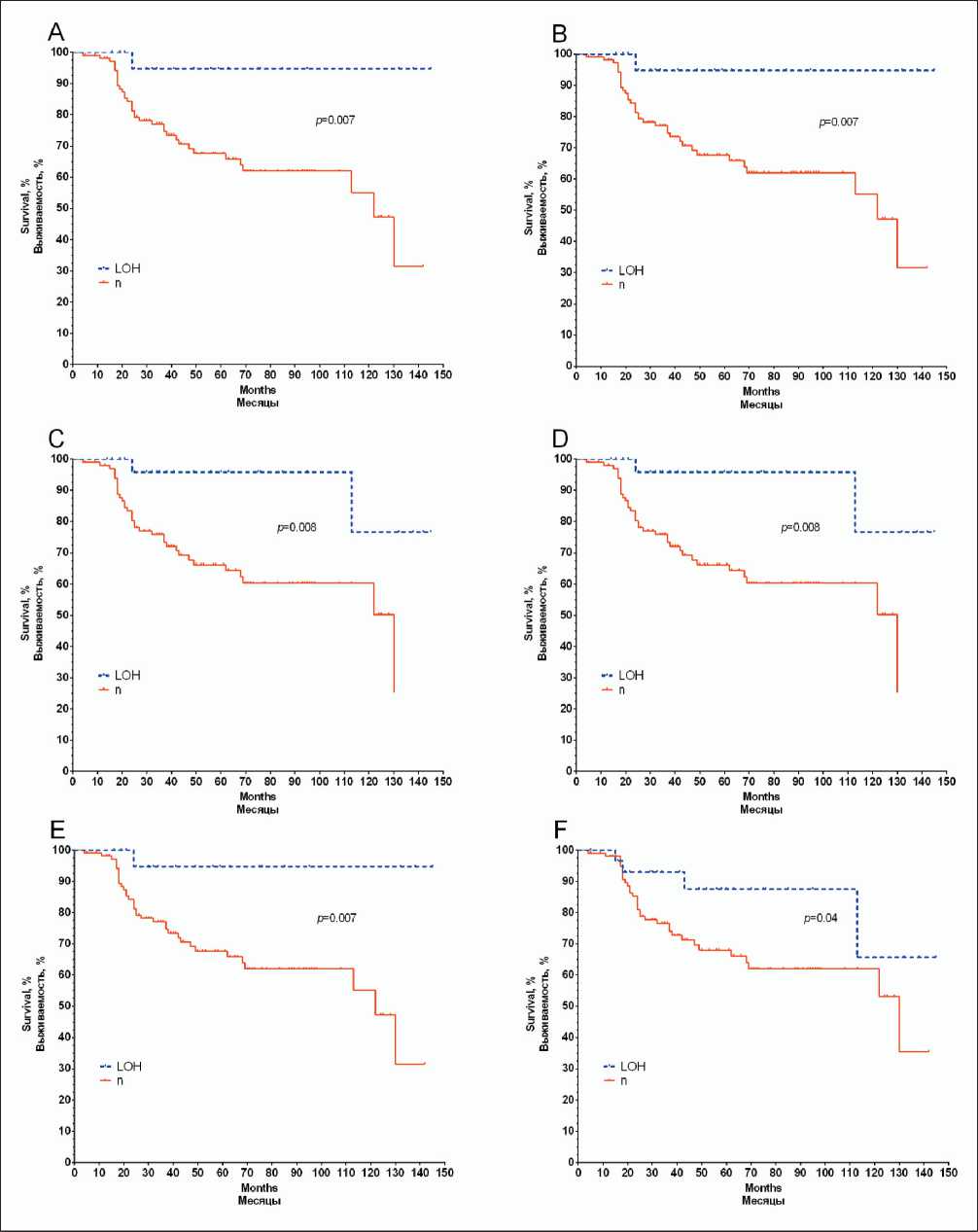
Fig. 3. Metastatic-free survival curves of breast cancer patients as a function of the presence of loss of heterozygosity sites in the ABCA5 (A), ABCA6 (B), ABCA8 (C), ABCA9 (D), ABCA10 (E), ABCC3 (F) genes
Рис. 3. Показатели безметастатической выживаемости больных раком молочной железы в зависимости от наличия участков потери гетерозиготности в генах ABCA5 (A), ABCA6 (B), ABCA8 (C), ABCA9 (D), ABCA10 (E), ABCC3 (F)
supplement 1. table 1/Таблица 1
Frequency of loss of heterozygosity in aBc transporter genes in breast tumorsЧастота потери гетерозиготности в генах транспортера aBc в опухолях молочной железы


