Preparation and characterization of renewable bio-polyol from the edible seed oil
Автор: Jebisha J.L., Begila David S.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.20, 2024 года.
Бесплатный доступ
Polyol is an organic compound containing multiple hydroxyl groups. This study looked at the possibility of using an edible oil extract from Salvia hispanica seeds as a sustainable source for polyols and, eventually, biodiesel or polyurethane. For this, a combination of hydrogen peroxide and acetic acid was used to create new polyol from the aforementioned oil in one-step synthesis. Standard techniques such as physicochemical analysis, phytochemical and basic radical identification, FTIR and NMR were used to characterize the polyol derivative that was extracted from the oil. Antimicrobial activity of both oil and polyol were tested against certain bacteria and fungi. Spectral analysis demonstrated the formation of polyol and this result indicated the possible of using Salvia hispanica polyol as a raw material for the preparation of bio-polymers.
Salvia hispanica oil, polyol, ftir, nmr, antimicrobial, bio-polymers
Короткий адрес: https://sciup.org/143182811
IDR: 143182811
Текст научной статьи Preparation and characterization of renewable bio-polyol from the edible seed oil
An herbaceous plant called chia has opposing, serrated leaves that range in size from 1.5 to 3 inches long and 1 to 2 inches wide. Oval in shape, seeds are roughly 2 mm (0.08 inches) in length and 1 mm (0.04 inches) in width. he lustrous seeds contain darker irregular marks or specks on them and their coat can range in hue from cream to charcoal grey. An annual herb called chia ( Salvia hispanica L .) blooms in the summer. It is about a meter tall and has opposite, petiolate, serrated leaves that range in length from 4 to 8 cm a width of 3 to 5 cm (Ayerza et al., 2010).
herefore, this study concentrates in the obtention of new bio-based polyol from the edible oil extract from the seeds of the Salvia hispanica and also to test the phytochemicals and basic radicals present in the oil extract. In addition, we characterize the prepared polyols using various physical properties and spectroscopic methods. he application of the prepared polyol against certain microbes were also tested.
MATERIALS AND METHODS
OIL EXTRACTION:
he extraction of oil was carried out using AOAC technique Am2-93 (Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 1995). In a Soxhlet device, 5g of Salvia hispanica L. seeds were extracted with 100 ml of n-hexane as the extraction solvent. Following an 8-hour period, the n-hexane was eliminated at 40˚C and low pressure. After obtained, the Salvia hispanica L . oil (SHO) was kept in a refrigerator till additional research was conducted.
SYNTHESIS OF POLYOL:
he synthesis of the polyols was done in accordance with the approach outlined by Monteavaro et al. (2005). 5g (5.6 mmol) of oil 9.30 mL (0.162 mol) of glacial acetic acid in 20 mL of toluene, together with a few drops of sulfuric acid, were combined in a three-necked flask that was fitted with an isobaric funnel, reflux condenser, and mechanical stirrer. At room temperature, the mixture was mechanically agitated until it was completely homogenized. Subsequently, 5.30 mL of a 30% H 2 O 2 , solution was added gradually while maintaining the temperature. Following the addition of H 2 O 2 , the mixture was heated for 12 hours to 60˚C. After bringing the reaction mixture down to room temperature, surplus peroxide was removed by stirring it for 20 minutes while adding a 10% (w/v) sodium bisulfide solution. Following that, the mixture was mixed with 50 mL of ethyl ether, and the organic phase was repeatedly washed to a pH of neutral using a 10% (w/v) sodium carbonate solution. In order to extract Salvia hispanica L. (SHP), the organic phase was lastly dried over sodium sulphate and concentrated under vacuum to remove the ethyl ether. CHARACTERISATION OF OIL AND POLYOL: PHYSICO CHEMICAL ANALYSIS:
he acid value of SHP was determined by the volumetric titration method according to AS M D1980-
87. he known quantity of sample is dissolved in neutral butanol-toluene mixture and 3–4 drop of phenolphthalein indicator added, swirling gently and titrated against the 0.1 N alcoholic KOH solution. Equation (1) was used to determine the acid value of SHP.
Acid value= B Х N Х 56.1 (1)
W where B = Burette reading (ml), N = Normality of alcoholic KOH solution, W = weight of sample (g).
he hydroxyl value of SHP was determined by acetic anhydride-pyridine method according to AS M D4274-16. he calculation of hydroxyl value was done by using Equation (2).
Hydroxyl value = (B - S) × N × 56.1 (2)
W where B = Burette reading for blank (ml), S = Burette reading for sample (ml), N = Normality of alcoholic KOH solution, W = weight of sample (g).
PHYTOCHEMICAL AND BASIC RADICAL IDENTIFICATION:
Phytochemical screening and basic radical identification were performed to asses the qualitative chemical composition of different samples of crude extracts using commonly employed precipitation and colouration reactions to identify the major and secondary metabolites.
FTIR ANALYSIS:
he chemical structure of the SHO and SHP were identified by F IR on a Bruker A R spectrophotometer.
he spectra were observed in the 600 – 4000 cm-1 wavelength range.
1H NMR SPECTROSCOPY:
he supportive confirmation of chemical structure was given by 1H nuclear magnetic resonance (NMR)spectroscopy. 1H spectra of the product were analyzed using Bruker DPX 400 MHz spectrophotometer with CDCl 3 as solvent.
ANTIMICROBIAL ACTIVITY:
Antimicrobial activity was examined using “ he Kirby-Bauer Method” against the number of pathogens including both gram-positive and gram-negative bacteria and also certain fungi. he zone of inhibition of both SHO and SHP were examined against certain pathogens.
RESULTS AND DISCUSSION
PHYSICO CHEMICAL ANALYSIS:
he acid number is expressed as the number of milligrams of KOH required to neutralize the acidity of sample. he OH number is the amount of available reactive hydroxyl groups on polyol molecules. he viscosity was measured using Brookfield viscometer and the density of the polyol were also analysed. he SHP is well dissolved in Chloroform . he results of the certain physico chemical analysis were mentioned in the able 1.
PHYTOCHEMICAL SCREENING:
he preliminary phytochemical study reveals the presence of glycosides, reducing sugars, phenolic compounds and saponins ( able 2) in the SHO. he color change in the extract were also listed in the table. BASIC RADICAL IDENTIFICATION:
Basic radical test shows the presence of bismuth, barium calcium and magnesium in the SHO were listed in the able 3.
FTIR:
he successful conversion of SHO to SHP was confirmed qualitatively by F -IR spectroscopy. Fig. 1 and Fig. 2 shows the F IR spectra of SHO and SHP respectively. he SHP spectra shows a broad band at 3562 cm-1 which were assigned to the presence of a hydroxyl (–OH) stretching vibration. A strong band at 3181 cm-1 was attributed to the presence of aromatic -CH-stretching and bands at 1644 cm-1 and 1400 cm-1 were assigned to the presence of amide band from protein carbonyl stretches and C-O-H bending vibrations. he appearance of new peak at 3562 cm-1 in SHP spectra confirms the formation of polyol.
1-H NMR spectra:
Fig. 3 and Fig. 4 gives the 1-H NMR spectra of the SHO and SHP respectively. he methylene protons of the aliphatic chains may be responsible for the signal seen as a triplet between 1.63 to 1.67 ppm (3H) in Fig. 4. he proton of the hydroxyl group is represented by the sharp singlet peak at 2.731 ppm (6H); the electronegative influence of the oxygen atom is responsible for the absorption shift towards the downfield. he multiplet signal at 3.8 ppm (2H) is indicative of the protons next to the acid’s carbonyl
|
group. he peak shift downwards as a result of the present in the range of 5.3 to 5.3 ppm (Fig. 3) in SHO carbonyl group’s electronegative action, which de-shield vanished, indicating that the SHP structures were the area (Dai et al., 2005). he signals corresponding to essentially unsaturated. Lastly, the hydroxylation the toluene structure were found at 7.5-7.6ppm as reaction was verified by the lack of signals in the range multiplet (Fig. 4). his evidence confirmed that the of 2.8 to 3.3ppm in relation to the epoxide groups (elimination of toluene was ineffective. Furthermore, the CH(O)CH-). olefinic hydrogen (-CH=CH-) signal that had been Table 1 : Physico chemical analysis of polyol |
|
|
Parameters |
Chia based polyol |
|
Acid number (mg KOH/g) |
5.386 |
|
OH number (mg KOH/g) |
166.54 |
|
Density (g/cm3) |
0.868 |
|
Viscosity (cps) |
464 |
|
Solubility |
Chloroform |
Table 2: Phytochemical screening of SHO
|
EXPERIMENT |
OBSERVATION |
INFERENCE |
|
NaOH test Extract + 2ml of NaOH |
No blue green color obtained |
Absence of anthocyanin |
|
Bromine water test: Extract + bromine water |
Pale yellow color obtained |
Presence of glycosides |
|
1ml of + lead acetate solution |
No precipitate obtained |
Absence of phenol |
|
5 ml of extract + 2ml of CHCl 3 + 3 ml of conc. H 2 SO 4 |
No reddish-brown precipitate obtained |
Absence of terpenoids |
|
LEAD ACETATE TEST: Extract + 1ml of lead acetate |
No red precipitate |
Absence of tannins |
|
Extract + CHCl 3 + conc. H 2 SO 4 |
No purple color obtained |
Absence of steroids |
|
Extract + Molisch’s reagent |
Purple color is obtained |
Presence of reducing sugars |
|
Extract + 2N HCl and remove the aqueous layer and add Mayer’s reagent |
No white precipitate obtained |
Absence of alkaloids |
|
Alcohol + extract + ferric chloride |
Intense color |
Presence of phenolic compounds |
|
Extract + water + shake well |
Foamy lather is obtained |
Presence of saponins |
|
Alcohol + extract + Mg ribbon + Conc. HCl |
No color change |
Absence of flavonoids |
|
Alcohol + Conc. HNO 3 + NH 3 |
No reddish orange color is obtained |
Absence of xanthoproteins |
Table 3: Basic radicals’ identification
|
EXPERIMENT |
OBSERVATIONS |
INFERENCE |
|
Extract + KI |
No golden spangles |
Absence of lead |
|
Extract + NH 4 OH |
white precipitate |
Presence of bismuth |
|
Extract + cupron reagent + NaOH |
No green color obtained |
Absence of copper |
|
Extract + potassium ferrocyanide |
No white precipitate obtained |
Absence of zinc |
|
Extract + dil. HCl + water + H2S |
No yellow precipitate |
Absence of cadmium |
|
Extract + potassium thiocyanate |
No red or blue color |
Absence of ferric and cobalt |
|
Extract + dil. HCl + aluminon reagent + (NH 4 ) 2 CO 3 |
No bright red precipitate is obtained |
Absence of aluminum |
|
Extract + conc. HNO 3 + sodium bismuthate + water |
No pink color |
Absence of manganese |
|
Extract + dimethyl glyoxime + NH 4 OH |
No scarlet red precipitate |
Absence of nickel |
|
Extract + potassium chromate |
Pale yellow precipitate |
Presence of barium |
|
Extract + NH 4 OH + ammonium oxalate |
White precipitate |
Presence of calcium |
|
Extract + Magneson reagent + NaOH |
Blue precipitate |
Presence of magnesium |
|
Extract +NaOH + Nesseler’s reagent |
No reddish-brown precipitate |
Absence of ammonium |
Table 4: Shows the zone of inhibition against certain microbes
|
Bacteria |
Zone of inhibition |
||
|
SHO |
SHP |
Control (Amikacin) |
|
|
Proteus mirabilis |
12 mm |
11 mm |
18 mm |
|
Salmonella typi |
13 mm |
14 mm |
19 mm |
|
Fungi |
Zone of inhibition |
||
|
SHO |
SHP |
Control (nystatin) |
|
|
Aspergillus niger |
10 mm |
8 mm |
15 mm |
|
C. tropicalis |
15 mm |
14 mm |
13 mm |
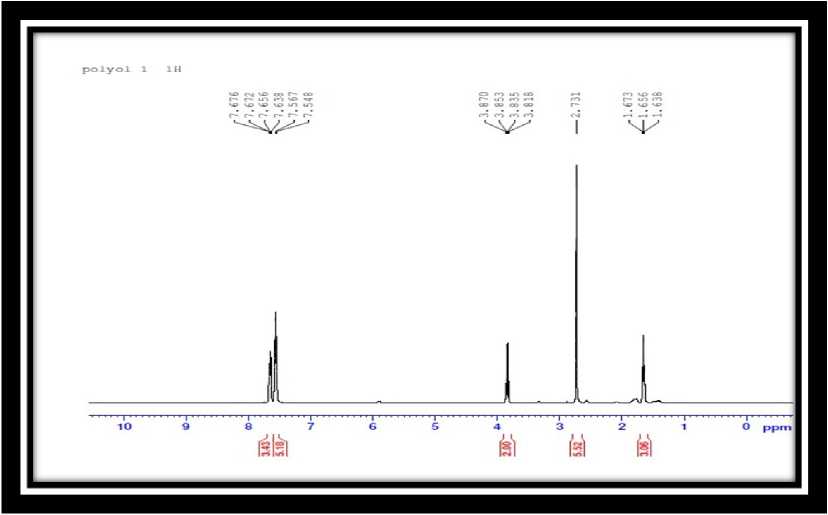
Figure 4. 1-H NMR spectra of SHP
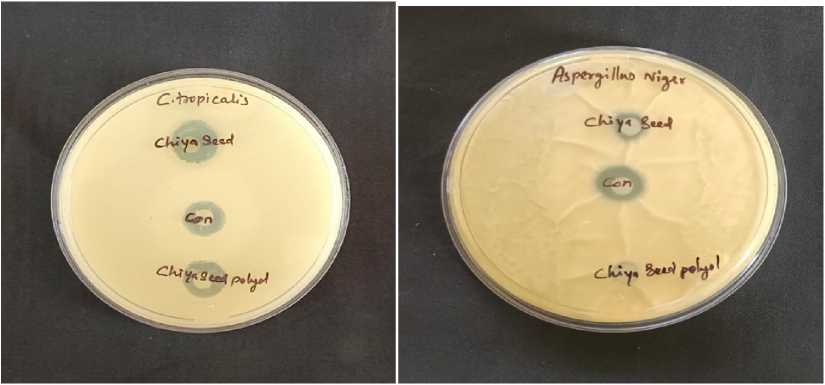
Figure 5. Zone of Inhibition against fungi
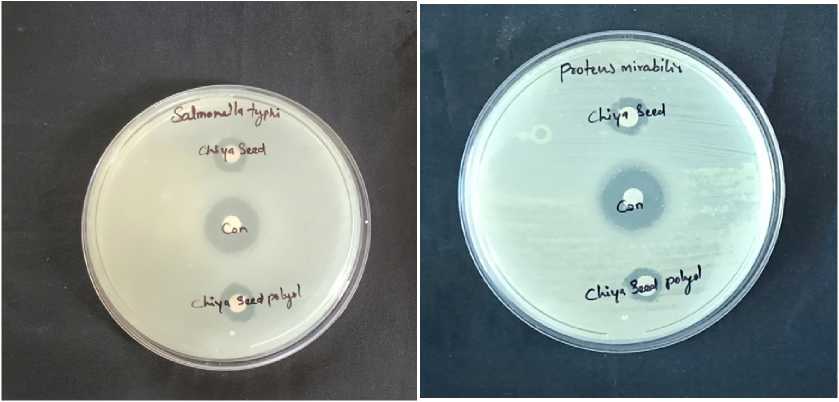
Figure 6. Zone of Inhibition against bacteria
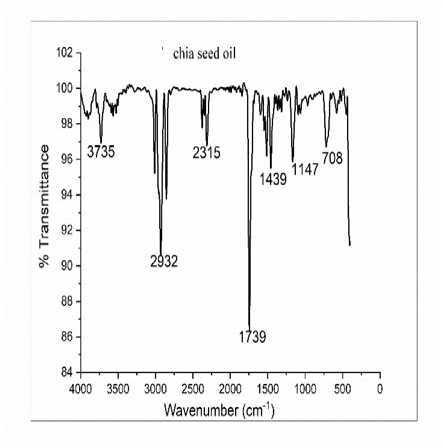
Figure 1. F IR spectra of SHO
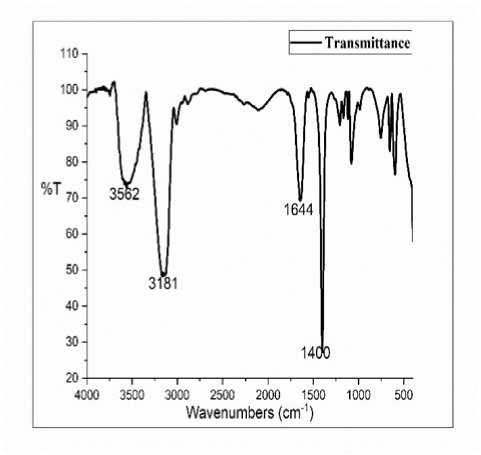
Figure 2. F IR spectra of SHP
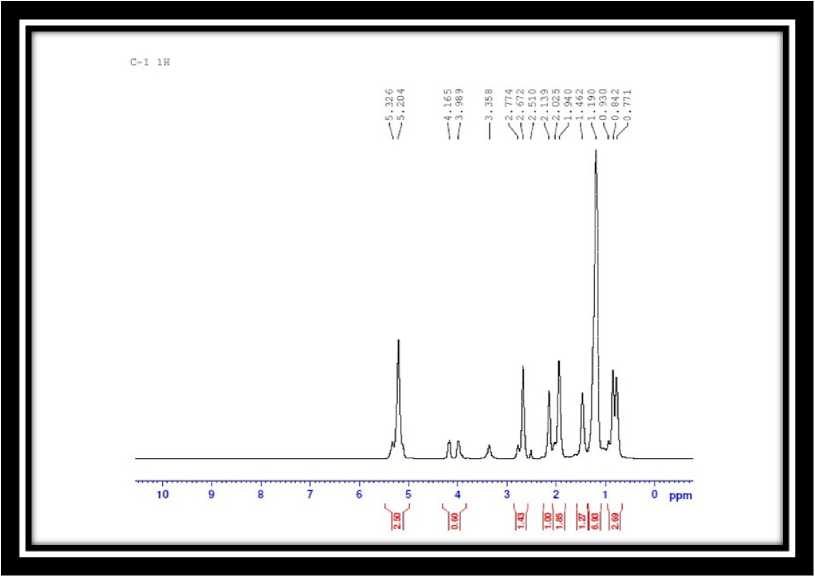
Figure 3. 1-H NMR spectra of SHO
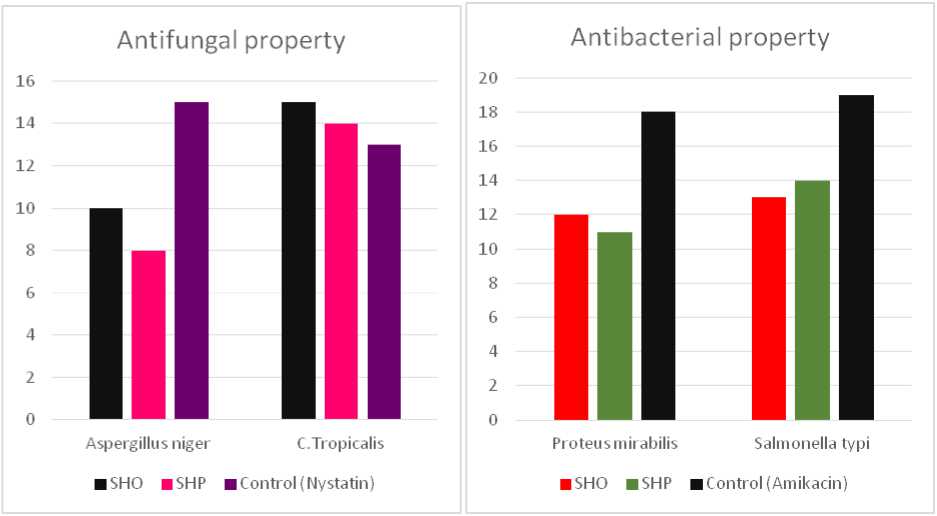
Figure 7. Comparative study
APPLICATION:
ANTIMICROBIAL ACTIVITY:
wo bacterial cultures (Fig. 6) including Proteus mirabilis (gram positive), Salmonella typi (gram negative) and two fungal cultures (Fig. 5) including Aspergillus niger and C. tropicalis were used to check the antimicrobial potential of both SHO and SHP. Amikacin was used as a control against bacterial cultures while Nystatin was used as a control against fungal cultures. he zone of inhibition against both bacteria and fungi were listed in the able 4. he bar graph shows the comparative study of SHO and SHP against certain microbes (Fig. 7).
CONCLUSION
We can infer from the results above that SHP was successfully prepared from the hexane extract of Salvia Hispanica and it was characterized by using F IR and 1H NMR spectroscopy. he physico-chemical analysis of the SHP and phytochemical screening and basic radical identification of SHO were examined. he antimicrobial potential of the prepared polyol and the oil were tested against certain bacteria and fungi. hus, the prepared bio-polyol can be used as an alternative for organic polyols which can be effectively use for the preparation of certain bio- polymers.
CONFLICTS OF INTEREST he authors declare that they have no potential conflicts of interest.
Список литературы Preparation and characterization of renewable bio-polyol from the edible seed oil
- AOAC. (1995) Official Method Cd 3d-63, Official Methods and Recommended Practices of the American Oil Chemists' Society; AOCS Press: Champaign, IL, USA,.
- Ayerza, R. (2010). Effects of seed color and growing locations on fatty acid content and composition of two chia (Salvia hispanica L.) genotypes. Journal of the American Oil Chemists' Society, 87, 11611165.
- Biermann, U., Friedt, W., Lang, S., Lühs, W., Machmüller, G., Metzger, J. O.....& Schneider, M. P. (2000). New syntheses with oils and fats as renewable raw materials for the chemical industry. Angewandte Chemie International Edition, 39(13), 2206-2224.
- Chaudhari, A., Kulkarni, R., Mahulikar, P., Sohn, D., & Gite, V. (2015). Development of PU coatings from neem oil based alkyds prepared by the monoglyceride route. Journal of the American Oil Chemists' Society, 92, 733-741.
- Dai, J., Ma, S., Wu, Y., Han, L., Zhang, L., Zhu, J., & Liu, X. (2015). Polyesters derived from itaconic acid for the properties and bio-based content enhancement of soybean oil-based thermosets. Green chemistry, 17(4), 2383-2392.
- de Espinosa, L. M., & Meier, M. A. (2011). Plant oils: The perfect renewable resource for polymer science?!. European Polymer Journal, 47(5), 837852.
- Eissen, M., Metzger, J. O., Schmidt, E., & Schneidewind, U. (2002). 10 years after Rio— concepts on the contribution of chemistry to a sustainable development. Angewandte Chemie International Edition, 41(3), 414-436.
- Fernandes, F. C., Kirwan, K., Lehane, D., & Coles, S. R. (2017). Epoxy resin blends and composites from waste vegetable oil. European Polymer Journal, 89, 449-460.
- Guner, F. S., Yagci, Y., & Erciyes, A. T. (2006). Polymers from triglyceride oils. Progress in polymer science, 31(7), 633-670.
- Ji, D., Fang, Z., He, W., Luo, Z., Jiang, X., Wang, T., & Guo, K. (2015). Polyurethane rigid foams formed from different soy-based polyols by the ring opening of epoxidised soybean oil with methanol, phenol, and cyclohexanol. Industrial crops and products, 74, 76-82.
- Kosari, A., Moayed, M. H., Davoodi, A., Parvizi, R., Momeni, M., Eshghi, H., & Moradi, H. (2014). Electrochemical and quantum chemical assessment of two organic compounds from pyridine derivatives as corrosion inhibitors for mild steel in HCl solution under stagnant condition and hydrodynamic flow. Corrosion Science, 78, 138150.
- Kuranska, M., Prociak, A., Kirpluks, M., & Cabulis, U. (2015). Polyurethane-polyisocyanurate foams modified with hydroxyl derivatives of rapeseed oil. Industrial Crops and Products, 74, 849-857.
- Monteavaro, L. L., Da Silva, E. O., Costa, A. P. O., Samios, D., Gerbase, A. E., & Petzhold, C. L. (2005). Polyurethane networks from formiated soy polyols: synthesis and mechanical
- characterization. Journal of the American Oil Chemists' Society, 82(5), 365-371.
- Saalah, S., Abdullah, L. C., Aung, M. M., Salleh, M. Z., Biak, D. R. A., Basri, M., & Jusoh, E. R. (2015). Waterborne polyurethane dispersions synthesized from jatropha oil. Industrial Crops and Products, 64, 194-200.
- Septevani, A. A., Evans, D. A., Chaleat, C., Martin, D. J., & Annamalai, P. K. (2015). A systematic study substituting polyether polyol with palm kernel oil based polyester polyol in rigid polyurethane foam. Industrial Crops and Products, 66, 16-26.
- Stan, R., Chira, N., Ott, C., Todasca, C., & Perez, E. (2008). Catanionic organogelators derived from D-sorbitol and natural fatty acids. Rev. Chim, 59(3), 273-276.
- Tian, H., Tang, Z., Zhuang, X., Chen, X., & Jing, X. (2012). Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Progress in Polymer Science, 37(2), 237-280.
- Ugarte, L., Saralegi, A., Fernández, R., Martín, L., Corcuera, M. A., & Eceiza, A. (2014). Flexible polyurethane foams based on 100% renewably sourced polyols. Industrial Crops and Products, 62, 545-551.
- Yoo, S. H., Kim, Y. W., Chung, K., Baik, S. Y., & Kim, J. S. (2012). Synthesis and corrosion inhibition behavior of imidazoline derivatives based on vegetable oil. Corrosion Science, 59, 42-54.
- Zhang, C., Madbouly, S. A., & Kessler, M. R. (2015). Biobased polyurethanes prepared from different vegetable oils. ACS applied materials & interfaces, 7(2), 1226-1233.
- Zieleniewska, M., Leszczynski, M. K., Kuranska, M., Prociak, A., Szczepkowski, L., Krzyzowska, M., & Ryszkowska, J. (2015). Preparation and characterisation of rigid polyurethane foams using a rapeseed oil-based polyol. Industrial Crops and Products, 74, 887-897.


