Production of finly despersed powder from graphite by electrolysis
Автор: I. Y. Shestakov, A. V. Kupryashov, V. D. Utenkov, I. A. Remizov
Журнал: Siberian Aerospace Journal @vestnik-sibsau-en
Рубрика: Technological processes and material science
Статья в выпуске: 4 vol.21, 2020 года.
Бесплатный доступ
Multifunctional coating is a multi-layer structure applied to the surface of an aircraft to protect it from external influences. The main tasks of the multifunctional coating are: restoration of properties, overall dimensions, mass of the surface of the product, which were violated under operating conditions; changing the initial physical, mechanical and chemical properties of the product surface to ensure the specified operating conditions. Today multifunctional coatings based on micro glass spheres with applied tungsten are widely used in aerospace engineering. However, this coating has a number of disadvantages: the coating layers heterogeneity; the composition contains a harmful and dangerous component – a fluorone dye. In this article it is suggested to replace the main component of a multifunctional coating with finely dispersed graphite powder obtained by electrolysis. For this purpose, the equipment based on the principle of a diaphragm electrolyzer was constructed. The main elements of the device are a stainless steel cathode and a graphite anode immersed in an aqueous solution. As a result of anodic processes, a finely dispersed graphite powder was obtained. The average particle size of the resulting graphite particles is 4 microns. This finely dispersed graphite powder can be used as the main component of a multifunctional coating in aircraft, since it has an even homogeneous structure, as well as higher values of the main mechanical properties of a multifunctional coating.
Multifunctional coating, fine graphite powder, electrochemical action, molecular oxygen, diaphragm electrolyzer.
Короткий адрес: https://sciup.org/148321781
IDR: 148321781 | УДК: 661.666.23 | DOI: 10.31772/2587-6066-2020-21-4-574-580
Текст научной статьи Production of finly despersed powder from graphite by electrolysis
Introduction . The main purpose of a multifunctional (functional gradient) coating in aerospace engineering is thermal protection, protection against ionizing, electromagnetic and radio radiation and laser beam reflection. The secondary purpose of a multifunctional coating in rocketry is to increase strength and rigidity of product external parts, as well as to protect them from corrosion and erosion [1].
Multifunctional coating structure and composition description. Multifunctional coating structure is rather complicated. It consists of four main components such as: a catalyst, a fluorone dye, a low-molecular polymer, and dispersed filler [2–4].
The main component of the multi-functional coating is a dispersed filler: micro-glass spheres modified with tungsten, that is, hollow particles of micro-glass spheres, on the surface of which a tungsten coating is applied. Micro glass spherical particles have various shapes: sphere, cube, parallelepiped, flake, cylinder, hexagon, various fibers, etc. [5–7]. Fig. 1 shows a schematic cross-section of the multi-functional coating layer, where you can see various shapes of microspheres and the chaotic arrangement of dispersed filler particles in the layer.
This filler is compatible with a low-molecular polymer. It is dispersed (emulsified) in the polymer to form a homogeneous composite mass [8–9]. Fig. 2 shows the outer appearance form of the dispersed filler modified with tungsten.
Multifunctional coating which consists of the described above components is actively used in aerospace engineering today [10], however there are many disadvantages: a dangerous and harmful component Rhodamine
6G [11], components high price, high labor cost for obtaining a composite mixture, heterogeneity of the coating layers (due to the variety of forms of dispersed filler, see fig. 1), which involves additional machining operations to obtain a layer of multifunctional coating of a given thickness.
Scientific research main idea . These disadvantages can be eliminated by using dispersed carbon filler [12] made on the basis of graphite with a hexagonal crystal structure.
Graphite is an allotropic form of carbon. It has a hexagonal crystal lattice. In a single crystal carbon atoms are arranged in parallel layers (basic planes). In each layer, the atoms are bound together by a strong home polar bond, due to this structure; graphite has anisotropy of physical and electrical properties [13].
Graphite powder was obtained by electrochemical method of – the anodic oxidation of high-strength fine-dispersed dense graphite of the MPG-6 brand. It is a widely used form of industrial graphite [14]. It is a dispersed modification of hexagonal polymorphic carbon. Unlike coarse-grained graphite, it is characterized by a powdery fine-dispersed structure. Basic physical and chemical characteristics are presented in table.
It can be seen from table that in comparison with the micro-glass sphere with deposited tungsten (≈0.62 g/cm3) the density of MPG-6 graphite is much higher. It means that the main mechanical properties (breaking stress at separation, ultimate strength, hardness) of the multifunctional graphite-based coating will be of higher values in comparison with the coatings used today in aerospace engineering.
Fig. 1. Schematic section of one layer of a multifunctional coating with micro glass spheres in the composition
Рис. 1. Схематичный срез слоя многофункционального покрытия с микростеклосферами

Fig. 2. The appearance of the dispersed filler modified with tungsten
Рис. 2. Внешний вид дисперсного наполнителя, модифицированного вольфрамом
Basic physical and chemical characteristics and properties of grade MPG-6 graphite
|
№ п/п |
Параметр |
Значение |
|
1 |
Mass fraction of ash, not more than % |
0.02 |
|
2 |
Density, g / cm 3 |
1.65 |
|
3 |
Thermal conductivity coefficient W / m×K |
95 |
|
4 |
Tensile strength at compression, MPa, not less |
73 |
|
5 |
Tensile strength at bending, MPa, not less |
34.3 |
|
6 |
Hazard class according to GOST 12.1.007 |
4 (low risk) |
Scientific research description. For the graphite powder production a special device was created, fig. 3 shows its schematic diagram The unit consists of a cylindrical body ( 1 ), inside which the electrodes: cathode ( 2 ), anode ( 3 ) are placed. Between the electrodes there is a cylindrical perforated container ( 4 ), with a canvas cloth inside ( 5 ), which acts as a diaphragm. A graphite electrode of the MPG-6 brand is used as the anode. Current leads ( 7 ) and the anode ( 3 ) are installed on the cover. The cold tap water which has been kept for 8–10 hours in an open glass container at room temperature is used a working medium.
A perforated container with a diaphragm is coaxially installed in a container with a cathode. After that some water is poured into the near-cathode and near-anode space up to the same level. Then a cover with the anode is installed and voltage is applied to the electrodes. The process of electric current transfer starts by ions moving to the electrodes in the electrolyte and electrons in the external circuit. Positively charged ions migrate to the cathode, and negatively charged ions migrate to the anode under the influence of the electric field. The electron transition takes place on the electrodes. The cathode releases electrons into the solution and reduction processes occur in the near-electrode space. The processes of elec- tron transfer from reacting particles to the electrode – oxidation takes place in the near-anode space [15–18].
Anodic oxidation and cathodic reduction form the basis of the electrolysis process that occurs in the device. In the first six minutes when the electric current is passing through the anode, the C4+ ion is formed from carbon atoms [19; 20]:
C 0 - 4 e ^ C 4 + (1)
A hydrate shell is formed around C4+ ions. The hydrated ions formed stay in the water:
C 4 ++ H 2 O ^ C ( H 2 O ) n (2)
Immediately after the unit is switched off, the cover with the anode is removed and the end of the anode is placed on the film. The obtained water solution is dried; as a result, graphite particles (a fine powder) remain on the film.
Scientific research results . The graphite powder was examined with a digital microscope. The photo of the graphite powder obtained using a digital microscope is shown in fig. 4.
As we see in fig. 4, the particles of the obtained powder reach the desired size of 0.004 mm.
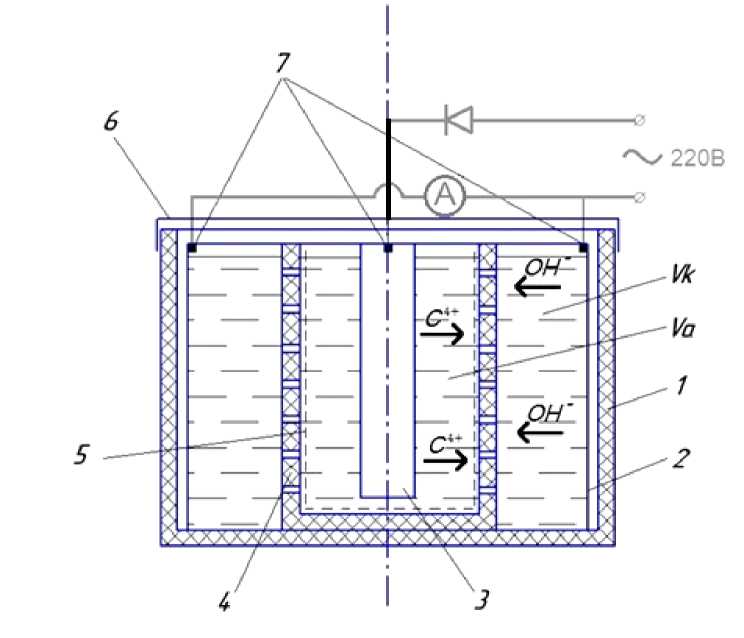
Fig. 3. Schematic diagram of the device:
1 – fluoroplastic device body; 2 – stainless steel cathode; 3 – graphite anode; 4 – cylindrical perforated container; 5 – tarpaulin-based fabric; 6 – plastic cover;
7 – current leads; V k – cathode space volume; V a – anode space volume
Рис. 3. Принципиальная схема устройства:
-
1 – корпус из фторопласта; 2 – катод из нержавеющей стали; 3 – анод из графита; 4 – цилиндрическая перфорированная ёмкость; 5 – брезентовая ткань; 6 – крышка;
-
7 – токоподводы; V k – объём прикатодного пространства; V a – объём прианодного пространства
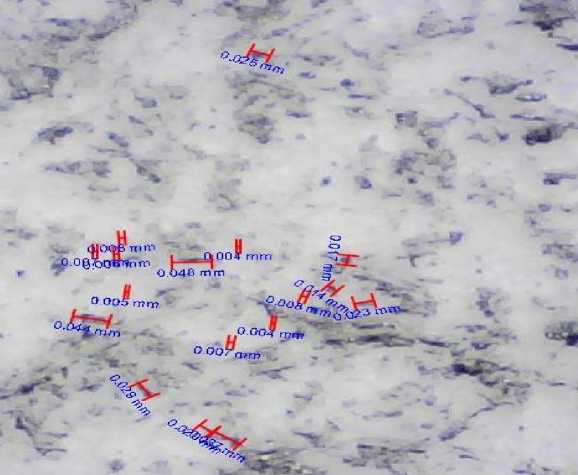
Fig. 4. A photograph of the graphite powder obtained during the experiment
Рис. 4. Графитовый порошок, полученный в ходе эксперимента
Fig. 5. A graphite powder image, magnified up to 0.001 mm
Рис. 5. Снимок графитового порошка, с увеличением до 0,001 мм
Fig. 5 shows the image obtained with a video measuring device of the TESA-VISIO 300GL laser principle with a magnification of up to 0.001 mm.
In fig. 5 the granular microstructure of the powder can be seen, which means that the particles have a suitable hexagonal shape. Due to such form of the graphite powder particles the multifunctional coating will have a smooth uniform structure, since the particles are tightly bound to each other.
The smooth, uniform surface structure of the multifunctional coating layers eliminates the necessity of using the extraction-photometric quality determination method with the use of the xanthene (fluorone) dye – Rhodamine 6G, a toxic and dangerous component.
Conclusion. In fig. 5, there is a granular structure of the powder, which means that the particles have an almost regular octagonal shape. The multifunctional coating will have a smooth uniform structure, due to the graphite powder particles form as the particles are tightly bound to each other.
The smooth, uniform surface structure of the multifunctional coating layers eliminates the necessity of using the extraction-photometric quality determination method with the use of the xanthene (fluorone) dye – Rhodamine 6G, a toxic and dangerous component.
Thus, the dispersed carbon filler (graphite powder obtained by electrolysis from MPG-6 graphite with a particle size of approximately 0.004 mm) provides the following advantages:
-
1. Elimination of the 2nd hazard class toxic substance – Rhodamine 6G from the multifunctional coating composition.
-
2. Higher values of the main multifunctional coating mechanical properties, due to the high particle density of the main component (1.65 g/cm3 for graphite powder, in comparison with micro-glass spheres modified with tungsten ≈0.62 g/cm3).
-
3. The main component lower cost of (≈9500 ru-bles/kg for graphite powder, compared to 103000 ru-bles/kg for micro glass spheres modified with tungsten).
-
4. Labor cost reduction in the production the multifunctional coating by eliminating mechanical processing (saving up to 10 % of the total labor intensity of the multifunctional coating production), due to the smooth homogeneous structure of the main component.
Список литературы Production of finly despersed powder from graphite by electrolysis
- Kupryashov A. V., Shestakov I. Ya. [Multifunctional coating in modern rocket and space technology]. Materialy XIX Mezhdunar. nauch. konf. [Volume of the XIX-th International Scientific Conference of bachelor students, master students, post-graduate students and young scientists]. Krasnoyarsk, SibSU, 2020, P. 310–311.
- Kernozhitskiy V. A., Kolychev A. V., Okhochinskiy D. M. Termoemissionnyi sposob teplovoi zashchity chastei letatel'nykh apparatov pri ikh aerodinamicheskom nagreve [Thermal emission method of thermal protection of aircraft parts during their aerodynamic heating]. Patent RF, No. 2404087, 2010.
- Orlov V. G., Savvateeva O. A., Shumov A. E. Teplozashchitnoe pokrytie [Heat protective coating]. Patent RF, No. 2631302, 2017.
- Zhukov A. V., Mushenko V. D., Baratova T. N. Kataliticheskaya smes' dlya otverzhdeniya siloksanovykh kauchukov [Catalyst mixture for curing silicone rubbers]. Patent RF, No. 2424260, 2011.
- Kolosova A. S., Sokol'skaya M. K., Vitkalova I. A. [Fillers for modification of modern polymer composite materials]. Fundamental'nye issledovaniya, 2017, No. 10, P. 459–465 (In Russ.).
- Babaevskiy P. G. Napolniteli dlya polimernykh kompozitsionnykh materialov [Fillers for polymer composite materials]. Moscow, Khimiya Publ., 1981, 736 p.
- Bondaletova L. I., Bondaletov V. G. Polimernye kompozitsionnye materialy (chast' 1) [Polymer composite materials (part 1)]. Tomsk, Tomsk State Polytechnic University Publ., 2013, 118 p.
- Zaponov A. E. [Theoretical and experimental study of the processes of chemical decomposition of a modified low-molecular-weight stirosil polymer in a continuous field of laser radiation]. Humanitarian Bulletin of the Military Academy of Strategic Rocket Troops. 2018, No. 11 (43), P. 295–302 (In Russ.).
- Ustinov A. S. [Method of applying fire-retardant composite material “liquid glass-graphite microparticles” on the surface of the fence]. Scientific and Technical Journal of Information Technologies, Mechanics and Optics. 2018, No. 6 (18), P. 1001–1007 (In Russ.).
- Shaidurova G. I., Vasil'ev I. L., Karmanova L. I. [Development and confirmation of the working capacity of the repair composition for the external heat-protective coating]. PNRPU Aerospace Engineering Bulletin. 2014, No. 36, P. 49–63 (In Russ.).
- Mittsel' Yu. A., Levshchin L. V., Golovina A. P. [Spectroscopic study of the state of rhodamine 6G molecules in non-polar solvents]. Vestnik Moskovskogo Universiteta. 1968, No. 1, P. 74–79 (In Russ.).
- Leonov D. V. Razrabotka poliamida-6 funktsional'nogo naznacheniya, modifitsirovannogo okislennym grafitom. Kand. Diss. [Development of polyamide-6 functional purpose modified with oxidized graphite. Cand. Diss.]. Saratov, 2018. 163 p.
- Koritskiy Yu. V., Pasynkova V. V., Tareeva B. M. Spravochnik po elektrotekhnicheskim materialam [Electrical Materials Handbook]. Leningrad, Energoatomizdat ubl., 1988, Vol. 3, 728 p. (In Russ.).
- Pitukhin E. A., Ustinov A. S. [Investigation of the fire resistance limit of a composite material liquid glassgraphite micro particle]. Scientific and Technical Journal f Information Technologies, Mechanics and Optics. 2016, Vol. 16, No. 2, P. 277–283 (In Russ.).
- Tarasevich M. R. Elektrokhimiya uglerodnykh materialov [Electrochemistry of carbon materials]. Moscow, Nauka Publ., 1984, 253 p.
- Stankovic V. Electrochemical Engineering – its appearance, evolution and present status. Approaching an anniversary. Journal of Electrochemical Science and Engineering. 2012, No. 2, P. 1–14.
- Chen G. Electrochemical technologies in wastewater treatment. Separation and Purification Technology. 2004, No. 38, P. 11–41.
- Wright H. Electrochemical Engineering: Emerging Technologies and Applications. USA, Willford Press, 2016, 254 p.
- Stolten D., Emonts B. Fuel Cell Science and Engineering: Materials, Processes, Systems and Technology. New York, John Wiley & Sons, 2012, 1268 p.
- Yakimenko L. M. Elektrodnye materialy v prikladnoi elektrokhimii [Electrode materials in applied electrochemistry]. Moscow, Khimiya Publ., 1977, 264 p.


