Protective effect of ghrelin on isoniazid-induced liver injury in rat
Автор: Kheyabany Shadi Sar Kheyr, Nabavizadeh Fatemeh, Vaezi Gholam Hassan, Alizadeh Ali Mohammad, Nahrevanian Hossein, Moslehi Azam, Azizian Saleh
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.9, 2013 года.
Бесплатный доступ
Ghrelin (GHR) is a peptide that has protective effects on many tissues injury. It has anti-inflammatory and anti-oxidant effects. Isoniazid (INH) a widely used antituberculosis drug, has hepatotoxic side effect. The aim of this study was to evaluate the protective role of ghrelin in liver toxicity due to isoniazid. Eighteen male rats were used in this study and divided in to three groups. Including: control, isoniazid, isoniazid and ghrelin groups. Nitric oxide(NO), prostaglandin E2(PGE2), and hepatic enzymes, ALT(alanine aminotransferase), AST(aspartate aminotransferase), ALK(alkaline phosphatas), were assessed and histologic study of liver were performed as indicators of liver damage following isoniazid toxicity. Ghrelin significantly increased NO metabolites and decreased PGE2 level comparison with INH group, but had no significant change compared to the control group. This study showed that ghrelin administration inhibited liver injury in rats due to isoniazid toxicity. The liver protective role of ghrelin may be mediated at least in part by its anti-inflammatory effect.
Ghrelin, isoniazid(inh), liver injury, nitric oxide(no), prostaglandin e2(pge2)
Короткий адрес: https://sciup.org/14323716
IDR: 14323716
Текст научной статьи Protective effect of ghrelin on isoniazid-induced liver injury in rat
Isoniazid or isonicotinyl hydrazine (INH) is widely used in the treatment and prophylaxis of tuberculosis throughout the world (Weber and Hein, 1979). Its toxicity is the most common cause of hepatic failure requiring liver transplantation (Nolan et al ., 1999).
Isoniazid is metabolized mainly in the liver via acetylation (Weber and Hein, 1979) and the hepatic cytochrome P450 enzyme system (CYP) (Self et al., 1999). INH is thought to be an inhibitor of CYP enzymes. The hepatotoxicity of INH is supposed to be initiated by CYP mediated metabolism of INH to acetylhydrazine and hydrazine (Sarich, 1999; Jenner and Timbrell, 1994).The acetylhydrazine can covalently bind to liver proteins (Timbrell et al., 1980) and also hydrazine (Timbrell et al, 1980; Vuilleumier et al, 2006; Whitehouse et al,1983) and cytochrome P450 (Huang et al, 2003; Shen et al, 2006; Yue et al, 2004) can cause hepatotoxicity by production of free radicals (Gonzalez, 2005). Using of isoniazid has been showed to induce hepatoxicity in some of human beings in normal doses and in experimental animals in toxic doses (Maryam et al., 2010).
Ghrelin is a newly discovered gut hormone, mainly produced in the stomach (Ariyasu et al ., 2001), but also identified in endocrine cells of the gastrointestinal tract (Date et al ., 2000). Ghrelin might also be synthesized in other organs, where it might have outocrine or paracrine effects (Leite-Moreira and Soares, 2007).
Ghrelin plays a role in a number of different physiological processes. For example, it enhances growth hormone secretion (Shiiya et al ., 2002) and increases appetite (Shiiya et al ., 2002), regulates cell proliferation (Yoshihara et al ., 2002), stimulates prolactin and adrenocorticotrophic hormone (Kojima et al ., 2004), promotes slow wave sleep (Weikel et al ., 2003) and memory retention (Carlini et al ., 2002).
In the stomach, ghrelin affects gastric acid secretion and motility, and exhibits gastroprotective effect (Brzozowski et al., 2004). In mammals, ghrelin plays an important role in the immune system (Yada et al., 2006). Previous findings showed ghrelin administration inhibited liver damage in rats due to acetaminophen toxicity (Golestan Jahromi et al., 2010). Ghrelin significantly increasing nitric oxide bioactivity (Tesauro et al., 2005) and mucosal prostaglandin E2 (PGE2) (Sibilia et al., 2003) and a decrease in cytokine production (TNF-α-NF-Kβ) (Konturek et al., 2006).The antioxidant and anti-inflammatory effects of ghrelin were previously investigated (EI Eter et al., 2007). As the main mechanism of INH toxicity is via inflammation and generation of free radicals, the present study was investigated to assessment the possible protective effect of ghrelin in liver damage secondry to INH toxicity in rats.
MATERIALS AND METHODS
Materials
Isoniazid (INH) and Ghrelin were purchased from Sigma-Aldrich (Sigma Chemical Company, St. Louis, MO, USA).
Animals and experimental protocols
Eighteen male Wistar rats (150-200 g) were used. All animals were kept in an animal room in a controlled temperature and 12:12 h light/dark cycle with free access to food and water. All procedures were approved by the ethical committee of Tehran University of Medical Sciences.
The animals were equally divided into three groups (n=6) including: (A) control group; animals were given saline solution as vehicle, (B) INH group; rats were given once a day INH (200 mg/kg B.W i.p) for five consecutive days (Nahrevanian etal., 2009) and (C) INH+ghrelin group; rats were treated with ghrelin (10 nmol/kg single dose i.p) 1 hour after INH injection in days 4 and 5 (Golestan Jahromi etal., 2010).
Three hours after the last injection of gherlin, rats were anesthetized with i.p injection of ketamine-zylaxine (50 and 8 mg/kg, respectively) (Golestan Jahromi et al., 2010) and the abdomen was opened medially. Then, whole blood samples (1 ml) harvested from the heart. Blood samples were centrifuged (4000 g, 15 min) and serum was collected for serological tests including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatas (ALK). Also, liver middle lob tissue was fixed in a buffered-formaldehyde solution (10%) for histological studies. Other lobs immediately frizzed kept at -70 oC for measurement of PGE2 and NO by ELISA and Griess methods, respectively.
Liver tissues homogenate preparation for determination of PGE2 and NO
Weighed samples of liver tissue (0.5 g) were placed in 1.5 ml microfuge tubes and homogenized using an electrical homogenizer (Model RS541-242, RS Components, Corby, UK) (Golestan Jahromi et al ., 2010). Then homogenates tested for PGE2 using a high sensitivity PGE2 Chemiluminescence Enzyme Immunoassay (CEI) Kit (Assay Designs Inc., MI, USA) and NO metabolites with the Griess Micro Assay method (Nahrevanian et al ., 2009).
Histology and microscopic structural examinations
The grade of histological study was semi quantitatively scored using the following parameters (Ishak et al ., 1995):
A*Piecemeal necrosis:
0-Absent,
1-Mild (focal, few portal areas),
2-Mild/moderate (focal ,most portal areas),
-
3- Moderate (continuous around<50% of tracts or septa),
-
4- Severe (continuous around>50% of tracts or septa).
B* Confluent necrosis:
0- Absent,
1-Focal confluent necrosis,
-
2- Centrolobular necrosis in some areas,
-
3- Centrolobular necrosis in most areas,
-
4- Centrolobular necrosis + occasional portal-central(P-C) bridging,
-
5- Centrolobular necrosis + multiple P-C bridging,
-
6- Panlobular or multilobular necrosis.
C*Focal (Spotty) lytic necrosis, apoptosis,and focal inflammination:
Absent,
One focus or less per 10× objective,
One to four foci per 10× objective,
Five to 10 foci per 10× objective,
More than 10 foci per 10× objective.
D*Portal inflammation:
0-None,
-
1- Mild, some or all portal areas,
-
2- Moderate, some or all portal areas,
-
3- Moderate/marked, all portal areas,
-
4- Marked, all portal areas.
**Fibrosis:
0- No fibrosis,
-
1- Fibrous expansion of some portal areas, with or without short fibrous septa,
-
2- Fibrous expansion of most portal areas, with or without short fibrous septa,
-
3- Fibrous expansion of most portal areas with occasional portal-portal (P-P) bridging,
-
4- Fibrous expansion of portal areas with marked bridging (portal-portal (P-P) as well as portal central (P-C)),
-
5- Marked bridging (P-P and/or P-C) with occasional noduls (incomplete cirrhosis),
-
6- Cirrhosis, probable or definite.
Statistical analysis
All values were expressed as Mean±S.E. Statistical analysis performed with analysis of variances (ANOVA) and post hoc Tukey tests using version SPSS 17.0. For the comparison of nonparametric data using Kruskal-Wallis test. P<0.05 was considered statistically significant.
RESULTS
Liver enzyme measurement
In INH and INH+GHR groups ALT, AST and ALK showed no significant change compared to the control group (Table 1).
NO metabolites measurement
Our finding showed that levels of NO metabolites of liver tissue in INH group (group B) had significant change compared to the control group (group A) (76.73±11.17, 141.29±24.21 μΜ/gr.Wet weight, respectively) (p<0.05) (Figure 1), it significantly increased by using ghrelin after INH administration (125.75±22.19 μΜ/gr.Wet weight) (p<0.05) (Figure 1). Also, our data showed that levels of NO of liver tissue in group that received ghrelin after INH (group C) had no significant change compared to the control group (P<0.3) (Figure 1). Therefore, ghrelin elevated it to the control group.
PGE2 measurment
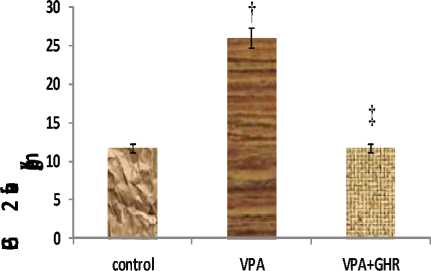
PGE2 level of liver tissue in INH group was significantly higher than control group (23.17±2.68, 11.58±1.29 Pg/gr Wet weight, respectively) (p<0.05) (Figure 2), but it significantly decreased by using ghrelin after INH administration (8.25±1.05 Pg/gr Wet weight) (p<0.01) (Figure 2). Also, our data showed that levels of PGE2 of liver tissue in group that received ghrelin after INH (group C) had no significant change compared to the control group (P<0.07) (Figure 2). Therefore, ghrelin decreased it to the control group.
Histological study
The microscopic study showed infinitesimal damage in rat liver tissue (0.83±0.3), but did not show any fibrosis in control group. Morphological changes following INH injected (5.6±0.49) and areas of confluent fibrosis (2±0.36) were significantly increased than to the control group (p<0.001, p<0.001, respectively). Treatment with ghrelin has significantly reduced liver injury and fibrosis (1.6±0.49, 0.16±0.16, respectively) compared to the INH group (p<0.001, p<0.001, respectively) (Table2, Figure 3).
Table 1: Effects of ghrelin and isoniazid on levels of hepatic enzymes.
|
Groups |
ALT (U/L) |
AST(U/L) |
ALK(U/L) |
|
Control |
65.5±3.86 |
170.8±28.23 |
565.8±44.05 |
|
INH |
58.5±3.18 |
182±28.49 |
554±43.32 |
|
INH+GHR |
53.8±3.92 |
186.6±29.15 |
548.6±42.39 |
Data were expressed as Mean±SE. n=6, ALT, AST and ALK were similar in all the groups.
group.
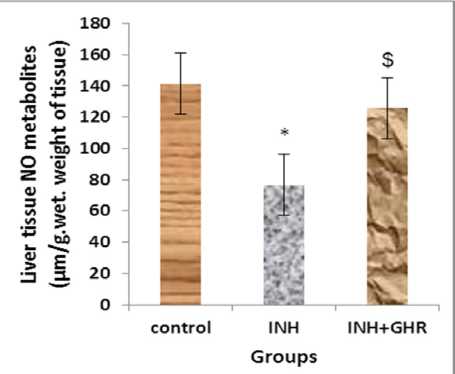
Figure 1: The level of liver tissue NO metabolites in different experimental groups (Mean±SEM, n=6). *p<0.05, liver tissue NO in INH group compared to control group, $p<0.05, liver tissue NO in INH+GHR group compared to INH group.
Groups
Figure 2: The level of liver tissue PGE2 in different experimental groups (Mean±SEM, n=6). *p<0.05, PGE2 level in INH group compared to control group, $p<0.01, PGE2 level in INH+GHR group compared to INH
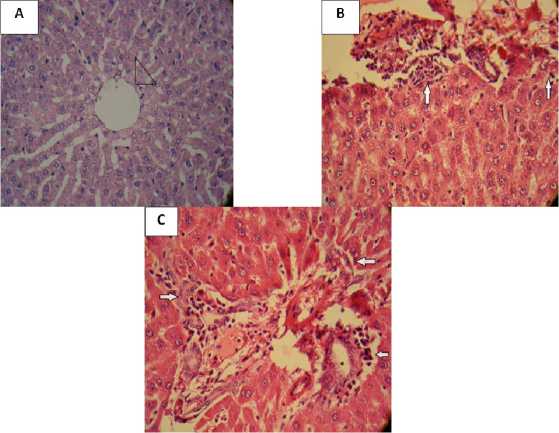
Figure 3: Liver histopathology. Representative slides from corresponding groups are shown (A: control group, Normal liver tissue. Arrowhead shows central vein (H&E ×40), B: isoniazid group, Aportal tract with moderate inflammination and piecemeal necrosis (arrows) (H&E ×40), C: ghrelin group, Aportal tract with mild inflammination and focal piecemeal necrosis (arrows) (H&E ×40).
Table 2. Histological activity index (HAI) was assessed based on the degree of microscopic lesions (Piecerneal necrosis, Confluent necrosis, Focal (Spotty litic necrosis, apoptosis, focal inflamination), Portal inflamination, Fibrosis). Data of each group were reported as Means±SEM based on sum of the scores histological criteria (overall score) using Kruskal-Wallis test (n=6).
|
Groups |
Piecerneal necrosis |
Confluent necrosis |
Focal (Spotty litic necrosis, apoptosis, focal inflamination) |
Portal inflamination |
Overall score |
Fibrosis |
|
Control |
0 |
0 |
0.66±0.21 |
0.16±0.16 |
0.83±0.3 |
0 |
|
INH |
1.5±0.34 |
0.16±0.16 |
2.3±0.21 |
1.6±0.21 |
5.6±0.49 $ |
2±0.36 ^ |
|
INH+GHR |
0.33±0.21 |
0 |
0.16±0.16 |
0.5±0.22 |
1.6±0.49 + |
0.16±0.16 × |
$ P<0.001, compared to control group,+ P<0.001, compared to INH group, ^ P<0.001, compared to control group, × p<0.001, compared to INH group.
DISCUSSION
In the present study, hepatotoxicity by acute administration of isoniazid was investigated; it could indeed be ameliorated by treatment with ghrelin.
Gut hormones play a major role in the regulation of fibrogenic processes and inflammatory in a variety of tissues. Ghrelin is a gut hormone that is also produed by extraintestinal tissue and it has protective effects in different organs including the liver, pancreas, heart and gastrointestinal tract (Golestan Jahromi et al ., 2010; Moreno et al ., 2010).
In the previous study, INH-induced hepatotoxicity reported that serum ALT and AST, the diagnostic marker of liver damage, were elevated by isoniazid after 21 days of treatment (Yue et al ., 2004; Yue et al ., 2009). Also Golestan Jahromi et al . have shown that treatmen of rats with ghrelin attenuated liver toxicity and reduced the level of these enzyme (Golestan Jahromi et al ., 2010). But in this study, we showed that in INH and INH+ghrelin groups ALT, AST and ALK no significant changes compared to the control group, this discrepaney of finding may be due to duration and/or dosage of drug (INH).
Kuppfer cells are the phagocytic macrophages of the liver. When activated, kuppfer cells release numerous signaling molecules, including hydrolytic enzymes, eicosanoids, NO and superoxide (Jaeschke et al ., 2002; James et al ., 2003). They may also release a number of inflammatory cytokines, including TNF-α, interleukins, prostaglandins and oxygen radicalse are released in liver toxicity (Martinez et al ., 1992).
The present study demonstrates isoniazid significantly decreases NO level in the liver tissue, but ghrelin elevated it to the normal level. In addition to in our study, indicate INH significantly increase PGE2 level in the liver tissue, but ghrelin reduced it even lower than control group.
But we did not show any research about INH-induced hepatotoxicity that measurement NO and PGE2 in the liver tissue. Therefore, it seems in our study ghrelin can improve hepatotoxicity by increasing of NO and decreasing of PGE2 level.
Ghrelin also improves endothelial function by inhibiting basal and tumour necrosis factor (TNF-α)-induced production of chemotactic cytokines, increasing nitric oxide (NO) bioactivity (Li et al ., 2004; Tesauro et al ., 2005).
Recently, Golestan Jahromi et al. showed that ghrelin reduced pro-inflammatory cytokines (e.g. TNF-α) in liver toxicity (Golestan Jahromi et al., 2010). Also, it is probable, in present study, ghrelin decrease PGE2 and improve liver damage.
INH-induced hepatotoxicity and the pathogenesis of hepatocellular disintegration and vacuolation in the centrilobular region (Young et al ., 2008) and kupffer cell hyperplasia with congestion of hepatocytes and microvesicular fatty in fillteration with special stains have been observed by Khedun et al (Khedun et al ., 1993). We also observed piecerneal necrosis, conflauent necrosis, spotty lytic necrosis, apoptosis, focal inflamination, portal inflamination and fibrosis in the liver tissue due to isoniazid administration. Treatment with ghrelin improved these histological changes in the liver.
In conclusion, this study showed that ghrelin administration inhibited liver injury in rats due to isoniazid toxicity. The liver protective role of ghrelin may be mediated at least in part by its antiinflammatory effect.
REFRENCES
Brzozowski, T., Konturek, P., Konturek, S., Kwiecie, S., Drozdowicz, D., Bielanski, W., Pajdo, R., Ptak, A., Nikiforuk, A., Pawlik, W.W., Hahn, E.G.
-
(2004) . Exogenous and endogenous ghrelin in
gastroprotection against stress-induced gastric damage. Regulatory Peptides ., 120 , 39-51.
Carlini, V., Monzon, M., Varas, M., Cragnolini, A., Schioth, H., Scimonelli, T., de Barioglio, S.R. (2002). Ghrelin increases anxiety-like behavior and memory retention in rats. Biochemical and Biophysical Research Communications ., 299 , 739-743.
Date, Y., Kojima, M., Hosoda, H., Sawaguchi, A., Mondal, M., Suganuma, T., Matsukura, S., Kangawa, K., Nakazato, M. (2000). Ghrelin a novel growth hormone-releasing acylated peptide is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology ., 141 , 4255-4261.
EI Eter, E., Al Tuwaijiri, A., Hagar, H., Arafa, M. (2007). In vivo and in vitro antioxidant activity of ghrelin: attenuation of gastric ischemic injury in the rat. Journal of Gastroenterology and Hepatology. , 22 , 1791-1799.
Golestan Jahromi, M., Nabavizadeh, F., Vahedian, J., Nahrevanian, H., Dehpour, A.R., Mehrjardi, A.Z. (2010). Protective effect of ghrelin on acetaminophen-induced liver injury in rat. Peptides Journal. , 31 , 2114-2117.
Gonzalez, F.J. (2005). Role of cytochromes P450 in chemical toxicity and oxidative studies with CYP2E1. Mutat. Res. , 569 : 101-110.
Huang, Y.S., Chern, H.D., Chang, S.C., Chiang, C.H., Chang, F.Y., Lee, S.D. (2003). Cytochrome P4502E1 genotype and the susceptibility to antituberculsis drug-induced hepatitis. Hepatology ., 37 , 924-930.
Ishak, K., Baptista, A., Bianchi, L., Callea, F., Gudat, F., Denk, H., Desmet, V., Korb, B., Macsween, R., Philips, M.J., Portmann, B.G., Poulsen, H., Scheuer, P.J., Schmid, M., Thaler, H. (1995). Histological grading and staging of chronic hepatits. J. Hepatology., 22, 696-699.
Jaeschke, H., Gores, G., Cederbaum, A., Hinnson, J., Pessayre, D., Lemasters, J. (2002). Mechanisms of hepatotoxicity. Toxicological Sciences . 65 , 166-176.
James, L., Mayeux, P., Hinson, J. (2003). Acetaminophen-induced hepatotoxicity. Drug Metabolism and Disposition ., 31 : 1499-1506.
Jenner, A.M., Timbrell, J.A. (1994). Influence of inducers and inhibitors of cytochrome P450 on the hepatotoxicity of hydrazine in vivo. Arch Toxicol. , 68 , 349-357.
Khedun, S., Lear, W., Maharai, B., Naicker, T. (1993). Effects of supra-therapeutic doses of isoniazid on liver function in the erfused rat liver. Isr. J. Med. , 29 , 791–794.
Kojima, M., Hosoda, H., Kangawa, K. (2004). Ghrelin a novel growth-hormone-releasing and appetite-stimulating peptide from stomach. Best Practice & Research Clinical Endocrinology & Metabolism. , 18 , 517-30.
Konturek, P., Brzozowski, T., Walter, B., Burnat, G., Hess, T., Hahn, E., Konturek, S.J. (2006). Ghrelin-induced gastroprotection against ischemiareperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. European Journal of Pharmacology ., 536 , 171-181.
Leite-Moreira, A.F., Soares, J.B. (2007).
Physiological, pathological and potential therapeutic roles of ghrelin. Drug Discovery Today. , 12 , 276-288.
Li, W.G., Gavrila, D., Liu, X., Wang, L., Gunnlaugsson, S., Stoll, L.L., McCormick, M.L., Sigmund, C.D., Tang, C., Weintraub, N.L. (2004). Ghrelin inhibits proinflammatory responses and nuclear factorkappa B activation in human endothelial cells. Circulation ., 109 , 2221-2226.
Martinez, F., Abril, E.R., Earnest, D.L., Watson, R.R. (1992). Ethanol and cytokine secretion. Alcohol ., 9 , 455-458.
Maryam, S., Bhatti, A.S.A., Shahzad, A.W. (2010). Protective Effects of Silymarin in Isoniazid Induced Hepatotoxicity in Rabbits. Annals ., 16 , 43-47.
Moreno, M., Chaves, J., Sancho-Bru, P., Ramalho, F., Ramalho, L., Mansego, M., Ivorra, C., Dominguez, M., Conde, L., Millán, C., Marí, M., Colmenero, J., Lozano, J.J., Jares, P., Vidal, J., Forns, X., Arroyo, V., Caballería, J., Ginès, P., Bataller, R. (2010). Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology ., 51, 974-85.
Nahrevanian, H., Hajihosseini, R., Arjmand, M., Farahmand, M., Ghasemi, F. (2009). Evaluation of anti-leishmanial activity by induction of nitric oxide and inhibition of prostaglandin in Balb/c mice infected with leishmania major. Southeast Asian Journal of Tropical Medicine and Public Health ., 40(6) , 1188-1198.
Nolan, C.M., Goldberg, S.V., Buskin, S.E. (1999). Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis Clinic. JAMA ., 281 , 1014-1018.
Samuel, M.P., Michael, A.T., Danielle, I.P., Robert, B.R., Wen, X., Reginald, F.F., Michael, A.Z. (2004). The effect of isoniazid on CYP2E1- and CYP4-Mediated hydroxylation of Arachidonic acid in the rat liver and kidney. Drug Metabolism and Disposition ., 32 , 727-733.
Sarich, T.C., Adams, S.P., Petricca, G, Wright, J.M. (1999). Inhibition of isoniazid induced hepatotoxicity in rabbits by pretreatment with an amidase inhibitor. J Pharmacol Exp Ther. ,
289 , 695-702.
Self, T.H., Chrisman, C.R., Baciewicz, A.M., Bronze, M.S. (1999). Isoniazid drug and food interactions. AM. J. Med Sci. , 317 , 304-311.
Shen, C., Zhang, H., Zhang, G., Meng, Q. (2006). Isoniazid-induced hepatotoxicity in rat hepatocytes of gel entrapment culture. Toxicol. Lett. , 167 , 66-74.
Shiiya, T., Nakazato, M., Mizuta, M., Date, Y., Mondal, M., Tanaka, M., Nozoe, S., Hosoda, H., Kangawa, K., Matsukura, S. (2002). Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. Journal of Clinical Endocrinology & Metabolism. , 87 , 240244.
Sibilia, V., Rindi, G., Pagani, F., Rapetti, D., Locatelli, V., Torsello, A., Campanini, N., Deghenghi, R., Netti, C. (2003). Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanism of action. Endocrinology ., 144 , 353-359.
Tesauro, M., Schinzari, F., Iantomo, M., Rizza, S., Melina, D., Lauro, D., Cardillo, C. (2005). Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation ., 112 , 29862992.
Timbrell, J.A., Mitchell, J.R., Snodgrass, W.R., Nelson, S.D. (1980). Isoniazid hepatoxicity: the relationship between covalent binding and metabolism in vivo. J. Pharmacol. Exp. Theor. , 213 , 364-369.
Vuilleumier, N., Rossier, M.F., Chiappe, A., Degoumois, F., Dayer, P., Mermillod, B., Nicod, L., Desmeules, J., Hochstrasser, D. (2006). CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur. J. Clin. Pharmacol. , 62 , 423429.
Weber, W.W., Hein, D.W. (1979). Clinical pharmacokinetics of isoniazid. Clin Pharmacokinet., 4 , 401-422.
Weikel, J., Wichniak, A., Ising, M., Brunner, H., Friess, E., Held, K., Mathias, S., Schmid, D.A., Uhr, M., Steiger, A. (2003). Ghrelin promotes slow-wave sleep in humans. American Journal of Physiology-Endocrinology and Metabolism. , 284 , 407-415.
Whitehouse, L.W., Tryphonas, L., Paul, C.J., Solomonraj, G., Thomas, B.H., Wong, L.T. (1983). Isoniazid-induced hepatic steatosis in rabbits: an explanation for susceptibility and its antagonism by pyridoxine hydrochloride. Can. J. Physiol . Pharmacol ., 61 , 478-487.
Yada, T., Kaiya, H., Mutoh, K., Azuma, T., Hyodo, S., Kangawa, K. (2006). Ghrelin stimulates phagocytosis and superoxide production in fish leukocytes. Journal of Endocrinology ., 189 , 5765.
Yoshihara, F., Kojima, M., Hosoda, H., Nakazato, M., Kangawa, K. (2002). Ghrelin: a novel peptide for growth hormone release and feeding regulation. Current Opinion in Clinical Nutrition & Metabolic Care ., 5 , 391-395.
Young, T.H., Tang, H.S., Chao, Y.C., Lee, H.S., Hsiong, C.H., Pao, L.H., Hu, O.Y.P. (2008). Quantitative rat liver function test by galactose single point method. Laboratory Animals ., 42 , 495-504.
Yue, J., Peng, R.X., Yang, J., Kong, R., Liu, J. (2004). CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol. Sin. , 25 , 699-704.
Yue, J., Dong, G., He, C., Chen, J., Liu, Y., Peng, R. (2009). Protective effects of thiopronin against isoniazid-induced hepatotoxicity in rats. Toxicology ., 264 , 185-191.
Список литературы Protective effect of ghrelin on isoniazid-induced liver injury in rat
- Ariyasu, H., Takaya, K., Tagami, T., Ogawa, Y., Hosoda, K., Akamizu, T., et al. (2001). Stomach is a Major Source of Circulating Ghrelin and Feeding State Determines Plasma Ghrelin-Like Immunoreactivity Levels in Humans. Journal of Clinical Endocrinology& Metabolism., 86, 4753-8.
- Brzozowski, T., Konturek, P., Konturek, S., Kwiecie, S., Drozdowicz, D., Bielanski, W., et al. (2004). Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regulatory Peptides., 120, 39-51.
- Carlini, V., Monzon, M., Varas, M., Cragnolini, A., Schioth, H., Scimonelli, T., et al. (2002). Ghrelin increases anxiety-like behavior and memory retention in rats. Biochemical and Biophysical Research Communications., 299, 739-743.
- Date, Y., Kojima, M., Hosoda, H., Sawaguchi, A., Mondal, M., Suganuma, T., et al. (2000). Ghrelin a novel growth hormone-releasing acylated peptide is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology., 141, 4255-61.
- EI Eter, E., Al Tuwaijiri, A., Hagar, H., Arafa, M. (2007). In vivo and in vitro antioxidant activity of ghrelin: attenuation of gastric ischemic injury in the rat. Journal of Gastroenterology and Hepatology., 22, 1791-1799.
- Golestan Jahromi, M., Nabavizadeh, F., Vahedian, J., Nahrevanian, H., Dehpour, A.R., Mehrjardi, A.Z. (2010). Protective effect of ghrelin on acetaminophen-induced liver injury in rat. Peptides Journal., 31, 2114-2117.
- Gonzalez, F.J. (2005). Role of cytochromes P450 in chemical toxicity and oxidative studies with CYP2E1. Mutat. Res., 569: 101-110.
- Huang, Y.S., Chern, H.D., Chang, S.C., Chiang, C.H., Chang, F.Y., Lee, S.D. (2003). Cytochrome P4502E1 genotype and the susceptibility to antituberculsis drug-induced hepatitis. Hepatology., 37, 924-930.
- Ishak, K., Baptista, A., Bianchi, L., Callea, F., Gudat, F., Denk, H., Desmet, V., Korb, B., Macsween, R., Philips, M.J., Portmann, B.G., Poulsen, H., Scheuer, P.J., Schmid, M., Thaler, H. (1995). Histological grading and staging of chronic hepatits. J. Hepatology., 22, 696-699.
- Jaeschke, H., Gores, G., Cederbaum, A., Hinnson, J., Pessayre, D., Lemasters, J. (2002). Mechanisms of hepatotoxicity. Toxicological Sciences. 65, 166-176.
- James, L., Mayeux, P., Hinson, J. (2003). Acetaminophen-induced hepatotoxicity. Drug Metabolism and Disposition., 31: 1499-1506.
- Jenner, A.M., Timbrell, J.A. (1994). Influence of inducers and inhibitors of cytochrome P450 on the hepatotoxicity of hydrazine in vivo. Arch Toxicol., 68, 349-357.
- Khedun, S., Lear, W., Maharai, B., Naicker, T. (1993). Effects of supra-therapeutic doses of isoniazid on liver function in the erfused rat liver. Isr. J. Med., 29, 791-794.
- Kojima, M., Hosoda, H., Kangawa, K. (2004). Ghrelin a novel growth-hormone-releasing and appetite-stimulating peptide from stomach. Best Practice & Research Clinical Endocrinology & Metabolism., 18, 517-30.
- Konturek, P., Brzozowski, T., Walter, B., Burnat, G., Hess, T., Hahn, E., et al. (2006). Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. European Journal of Pharmacology., 536, 171-181.
- Leite-Moreira, A.F., Soares, J.B. (2007). Physiological, pathological and potential therapeutic roles of ghrelin. Drug Discovery Today., 12, 276-288.
- Li, W.G., Gavrila, D., Liu, X., Wang, L., Gunnlaugsson, S., Stoll, L.L., McCormick, M.L., Sigmund, C.D., Tang, C., Weintraub, N.L. (2004). Ghrelin inhibits proinflammatory responses and nuclear factor-kappa B activation in human endothelial cells. Circulation., 109, 2221-2226.
- Martinez, F., Abril, E.R., Earnest, D.L., Watson, R.R. (1992). Ethanol and cytokine secretion. Alcohol., 9, 455-458.
- Maryam, S., Bhatti, A.S.A., Shahzad, A.W. (2010). Protective Effects of Silymarin in Isoniazid Induced Hepatotoxicity in Rabbits. Annals., 16, 43-47.
- Moreno, M., Chaves, J., Sancho-Bru, P., Ramalho, F., Ramalho, L., Mansego, M., Ivorra, C., Dominguez, M., Conde, L., Millán, C., Marí, M., Colmenero, J., Lozano, J.J., Jares, P., Vidal, J., Forns, X., Arroyo, V., Caballería, J., Ginès, P., Bataller, R. (2010). Ghrelin attenuates hepatocellular injury and liver fibrogenesis in rodents and influences fibrosis progression in humans. Hepatology., 51, 974-85.
- Nahrevanian, H., Hajihosseini, R., Arjmand, M., Farahmand, M., Ghasemi, F. (2009). Evaluation of anti-leishmanial activity by induction of nitric oxide and inhibition of prostaglandin in Balb/c mice infected with leishmania major. Southeast Asian Journal of Tropical Medicine and Public Health., 40(6), 1188-1198.
- Nolan, C.M., Goldberg, S.V., Buskin, S.E. (1999). Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis Clinic. JAMA., 281, 1014-1018.
- Samuel, M.P., Michael, A.T., Danielle, I.P., Robert, B.R., Wen, X., Reginald, F.F., Michael, A.Z. (2004). The effect of isoniazid on CYP2E1-and CYP4-Mediated hydroxylation of Arachidonic acid in the rat liver and kidney. Drug Metabolism and Disposition., 32, 727-733.
- Sarich, T.C., Adams, S.P., Petricca, G, Wright, J.M. (1999). Inhibition of isoniazid induced hepatotoxicity in rabbits by pretreatment with an amidase inhibitor. J Pharmacol Exp Ther., 289, 695-702.
- Self, T.H., Chrisman, C.R., Baciewicz, A.M., Bronze, M.S. (1999). Isoniazid drug and food interactions. AM. J. Med Sci., 317, 304-311.
- Shen, C., Zhang, H., Zhang, G., Meng, Q. (2006). Isoniazid-induced hepatotoxicity in rat hepatocytes of gel entrapment culture. Toxicol. Lett., 167, 66-74.
- Shiiya, T., Nakazato, M., Mizuta, M., Date, Y., Mondal, M., Tanaka, M., Nozoe, S., Hosoda, H., Kangawa, K., Matsukura, S. (2002). Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. Journal of Clinical Endocrinology & Metabolism., 87, 240-4.
- Sibilia, V., Rindi, G., Pagani, F., Rapetti, D., Locatelli, V., Torsello, A., Campanini, N., Deghenghi, R., Netti, C. (2003). Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanism of action. Endocrinology., 144, 353-359.
- Tesauro, M., Schinzari, F., Iantomo, M., Rizza, S., Melina, D., Lauro, D., Cardillo, C. (2005). Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation., 112, 2986-2992.
- Timbrell, J.A., Mitchell, J.R., Snodgrass, W.R., Nelson, S.D. (1980). Isoniazid hepatoxicity: the relationship between covalent binding and metabolism in vivo. J. Pharmacol. Exp. Theor., 213, 364-369.
- Vuilleumier, N., Rossier, M.F., Chiappe, A., Degoumois, F., Dayer, P., Mermillod, B., Nicod, L., Desmeules, J., Hochstrasser, D. (2006). CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur. J. Clin. Pharmacol., 62, 423-429.
- Weber, W.W., Hein, D.W. (1979). Clinical pharmacokinetics of isoniazid. Clin Pharmacokinet., 4, 401-422.
- Weikel, J., Wichniak, A., Ising, M., Brunner, H., Friess, E., Held, K., Mathias, S., Schmid, D.A., Uhr, M., Steiger, A. (2003). Ghrelin promotes slow-wave sleep in humans. American Journal of Physiology-Endocrinology and Metabolism., 284, 407-15.
- Whitehouse, L.W., Tryphonas, L., Paul, C.J., Solomonraj, G., Thomas, B.H., Wong, L.T. (1983). Isoniazid-induced hepatic steatosis in rabbits: an explanation for susceptibility and its antagonism by pyridoxine hydrochloride. Can. J. Physiol. Pharmacol., 61, 478-487.
- Yada, T., Kaiya, H., Mutoh, K., Azuma, T., Hyodo, S., Kangawa, K. (2006). Ghrelin stimulates phagocytosis and superoxide production in fish leukocytes. Journal of Endocrinology., 189, 57-65.
- Yoshihara, F., Kojima, M., Hosoda, H., Nakazato, M., Kangawa, K. (2002). Ghrelin: a novel peptide for growth hormone release and feeding regulation. Current Opinion in Clinical Nutrition & Metabolic Care., 5, 391-5.
- Young, T.H., Tang, H.S., Chao, Y.C., Lee, H.S., Hsiong, C.H., Pao, L.H., Hu, O.Y.P. (2008). Quantitative rat liver function test by galactose single point method. Laboratory Animals., 42, 495-504.
- Yue, J., Dong, G., He, C., Chen, J., Liu, Y., Peng, R. (2009). Protective effects of thiopronin against isoniazid-induced hepatotoxicity in rats. Toxicology., 264, 185-191.


