Protective effect of virgin olive oil ( Olea europea L.) against oxidative damage induced by mercuric chloride in rat albinos Wistar
Автор: Necib Youcef, Bahi Ahlem, Zerizer Sakina, Abdennour Cherif, Boulakoud Mohamed Salah
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.10, 2014 года.
Бесплатный доступ
Olive oil is beneficial effects are not only related to its high content of oleic acid, but also to the antioxidant potential of its polyphenols. In this study, we assess the effects of virgin olive oil on mercuric chloride induced oxidative damage in the liver of rats. Adult male Albinos Wistar rats randomly divided into four groups, were the first was served as a control, whereas the remaining groups respectively treated with: virgin olive oil (2ml/ kg b.w; by gavage), mercuric chloride (0.5 mg/kg body weight i.p) and combination of virgin olive oil and HgCl 2. Change in liver enzyme activities, thiobarbituric acid reactive substances (TBARS) level, antioxidants and reduced glutathione (GSH) contents were determined after 2 weeks of experimental period. Exposure of rats to mercuric chloride caused a significant increase the lipid peroxidation level along with corresponding decrease in the reduced glutathione and various antioxidant enzymes in liver. And increase in serum: glucose level, APL and transaminases activities and decreased in total protein and albumin levels. Furthermore, treatment with mercuric chloride caused a marked elevation of liver weight and decreased body weight. Supplementation of virgin olive oil resulted in decreased of lipid peroxidation level and in the serum: AST, ALT and APL activities were decreased along with increase in total protein, albumin and liver GSH levels. The activities of antioxidants enzymes: glutathione peroxidase (GSH-Px) and glutathione –S-transferase (GST) were also concomitantly restored to near normal level by virgin olive oil supplementation to mercuric chloride intoxicated rats. Liver histological studies have confirmed the changes observed in biochemical parameters and proved the beneficial role of virgin olive oil. The results clearly demonstrate that virgin olive oil treatment augments the antioxidants defense mechanism in mercuric chloride induced toxicity and provides evidence that it may have a therapeutic role in free radical mediated diseases.
Antioxidant enzymes, virgin olive oil, mercury, oxidative stress, rat, liver
Короткий адрес: https://sciup.org/14323846
IDR: 14323846
Текст научной статьи Protective effect of virgin olive oil ( Olea europea L.) against oxidative damage induced by mercuric chloride in rat albinos Wistar
2002; Keys, 1995). Virgin olive oil appears to be a functional food with various components such as monounsaturated fatty acids that may have nutritional benefits. It is also a good source of phytochemicals, including polyphenolic compounds (Stark et al., 2002; Lavelli, 2002). It is known that an increased consumption of monounsaturated fatty acids ( UFA) instead of polyunsaturated fatty acids (PUFA) reduces the risk of atherosclerosis because it decreases the circulating lipoprotein’s sensitivity to peroxidation (Visioli et al., 1998). Furthermore, the dietary UFA healthy effects were attributed to decreased endothelial activation ( oreno et al., 2003), and LDL susceptibility to oxidation ( assaro et al., 2002). In recent years, scientists have focusedon the preventive effects of phenols against degenerative diseases mediated by the ROS. It has been reported that the phenolic compounds are able to interact with the biological systems and as bioactive molecules. They are particularly important inhibitors of lipid peroxidation (Bonanome et al., 1992), an are believed to be effective through their free radical scavenging and metal-chelating propertie (Salah et al., 1995; Kandaswami et al., 1994). In experimental studies, olive oil phenolic compounds showed strong antioxidant properties against lipids, DNA and LDL oxidation (Rice-Evans et al., 1996). Hydroxytyrosol (2-(3,4 dihydroxyphenyl)ethanol, DPE), one of the phenolic compounds present in extra virgin olive oil has been suggested to be a potent antioxidant, thus contributing to the beneficial properties of olive oil (Covas et al., 2006). DPE administration has been shown to reduce the consequences of passive smoking-induced oxidative stress (Deiana et al., 1996), prevent LDL oxidation (Visioli et al., 2000) and platelet aggregation and inhibit leukocyte 5-lipoxygenases (Wiseman et al., 1996). DPE has shown efficacy in preventing oxidative stress in the liver of rats intoxicated by cadmium (De la Puerta et al., 1999). In addition, when human hepatoma HepG2 cells were pre-treated with DPE for 2 or 20 h prior to submission to tert-butylhydroperoxide-induced oxidative stress, cell toxicity was completely prevented, indicating that the antioxidant-treated cells were totally protected against the oxidative insult (Goya et al., 2007). However, the liver is not only the main target for phenolic antioxidants once absorbed from the gastrointestinal tract but is the major place for phenolic metabolism. Therefore, studies dealing with the effect of antioxidant dietary phenolics on the liver should be given priority. The literature data on olive oil polyphenols is mainly concerned with purified compounds, while the antioxidant properties of the total fraction of the lipophilic or hydrophilic components have been poorly investigated. Being a complex mixture of compounds, the study of the protective effect could be more representative than of a single component. The present study investigates the effect of dietary supplementation of olive oil on oxidative stress induced by mercuric chloride in rats. In this context, we explored the hypothesis that, owing to its high content of natural antioxidants, olive oil could reduce mercuric chloride induced oxidative damage in rats.
MATERIALS AND METHODS
The virgin olive oil used in this work originated from chetaibi (Algeria), It was extracted by a traditional method.
All chemicals used in this work were purchased from sigma chemical company. Laboratory animals, Albinos Wistar male rats, were brought from the Algiers Pasteur institute at the age of 8 weeks, with an average live weight of 200g. They were located in a room with an ambient temperature of 21±1°C and up to 12h of light daily. The rats were divided into four experimental groups; each consists of six rats. The first group was served as the control. The second group was given virgin olive oil at a dose of 2ml/kg body weight, while the third group (HgCl2) was intraperitoneally given mercuric chloride at a dose of 0.5 mg/kg body weight. Finally, the fourth group: virgin olive oil orally was given (2ml/kg body weight) 7 days before HgCl2 (0.5 mg/kg body weight) and continued up to 2weeks after mercuric chloride treatment. The treatment of all groups was lasted for 3consecutive weeks.
Twenty four hour after the last administration the blood was collected by retro- orbital sinus punction from each anesthetized rats. After centrifugation at 3000 rpm for 10min, the serum was separated immediately and stored at ̶ 20°C until determination of: glucose, albumin and total protein levels and enzymes (AST, ALT and ALP) activities. Subsequently, rats were decapitated and liver were removed.
Tissue preparation
About 500mg of liver was homogenized in 2ml of buffer solution of phosphate buffered saline (w/v: 500mg tissue with 4ml PBS, PH 7.4) homogenates were centrifuged at 10.000xg for 15min at 4°c. The resultant supernatant was used for determination of: reduced GSH, Thiobarbituric acid- reactive substance (TBARS) levels, and the activities of: GSH-PX and GST.
Determination of glucose, total protein and albumin levels and enzymes
Serum glucose, total protein and albumin levels and AST, ALT and ALP activities were determined using commercial kits (Spinreact).
Determination of lipid peroxidation (LPO)
Lipid peroxidation level in the liver was measured by the method of Buege and Aust (1978). 125µl of supernatant were homogenized by sonication with 50 µl of PBS, 125 µl of 20% TCA + BHT 1% (TCA-BHT) in order to precipitate proteins, and centrifuged (1000xg, 10min, 4°c), afterwards, 200µl of supernatant were mixed with 40µl of HCl (0,6 ), and 160µl of TBA dissolved in tris (120 m ). And the mixture was heated at 80°c for 10min; the absorbance was measured at 530nm. The amount of TBARS was calculated by using a molar extinction coeffient of 1.56x105 /Cm.
Determination of reduced glutathione (GSH)
GSH content in liver was measured spectrophotometrically by using Ellman’s reagent (DTNB) as a colouring reagent, following the method described by Weeckbekeretcory (1988).
Determination of glutathione-S-transferase (GST) (EC2.5.1.18)
The cytosolic glutathione-S-transferase activity was determined spectrophotometrically at 37°c by method of Habig et al (1974). The reaction mixture (1ml) contained 0.334ml of 100m phosphate buffer (PH 6.5), 0.033ml of 30m CDNB and 0.33ml of reduced Glutathione. After pre-incubating for 2min, the reaction was started by adding 0.01ml of diluted cytosol and the absorbance was followed for 3min at 340 nm. The specific activity of GST is expressed as µmole of GSH-CDNB conjugate formed/ min /mg protein using extinction coefficient of 9.6 m-1 cm-1
Determination of GSH-Px (E.C.1.11.1.9)
Glutathione peroxidase (EC 1.11.1.9) activity was modified from the method of Flohe and Gunzler(1984). for the enzyme reaction , 0.2ml of the supernatant was placed into a tube and mixed with 0.4ml GSH (reduced glutathione, sigma product, analytical grade), and the mixture was put into an ice bath for 30min. Then the mixture was centrifuged for 10min at 3000rpm, 0.48ml of the supernatant was placed into a cuvette, and 2.2ml of 0.32 Na2HPO4 and 0.32ml of 1m mol/l 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB, sigma) were added for color development. The absorbance at wavelength 412nm was measured with a UV spectrophotometer after 5min. The enzyme activity was calculated as a decrease in GSH within the reaction time as compared with the non-enzyme reaction.
Protein quantification
Protein was measured by the method of Bradford (1976), using bovine serum albumin as the standard.
Histopathological examination
Liver from autopsied animals were excised out and fixed in formalin (10%). five micron think section were prepared by using microtome and these section were stained with hematoxyline and eosin. For histological alterations these slides were observed under light microscope.
Statistical analysis
The data were subjected to student t test for comparison between groups. The values are expressed as mean ± SE . Significance level was set at P<0.05, P<0.01, P<0.001.
RESULTS
Effects of treatments on body, absolute and relative liver weights
The variations in body and relative liver weights of animals subjected to different treatments were shows in Table 1. During the course of present investigations. Et was observed that the control body weight and virgin olive oil treated group have increased progressively, contrary, in HgCl2 treated rats, results revealed significant decrease in body weight gain by -4,93% as compared to the control. Besides, a significant increase of HgCl2 treated group in absolute and relative liver weights and in virgin olive oil. HgCl2 treated group as compared to the control.
Effects of treatments on serum biochemical Parameters
Treatment with HgCl 2 caused a significant (P≤ 0.01) increase in the activities of AST, ALT and ALP as compared to the control. Only virgin olive oil treatment did not show any significant alteration. However, the combined treatment of virgin olive oil with mercuric chloride results in gradual recovery in AST, ALT and ALP activities as compared to the control (Table 2). The content of serum glucose of the HgCl 2 treated group tented to be higher compared to the control. Albumin and protein levels in HgCl 2 treated animals were decreased, but the co-administration of virgin olive oil with HgCl 2 has produced a recovery in the above mentioned biochemical variables.
Effects of treatments on hepatic oxidative stress parameters ercuric chloride exposure a highly significant depleted in reduced glutathione (GSH) level, GPx and GST activities. And a significant increase in liver lipid peroxidation level in mercury intoxicated rats was noticed. Virgin olive oil alone treatment did not show any significant decline. In combined treatment of virgin olive oil with mercuric chloride a highly significant increase in reduced glutathione (GSH) level, GPx and GST activities. And a significant depletion in lipid peroxidation level was recorded with respect to mercury intoxicated rats (Figs. 1 and 2).
Histological studies ercuric chloride induces various pathological alterations in liver of rats. These alterations were characterized by centrilobular necrosis, degranulation, destruction of membrane cells, cytoplasmic vacuolization (Fig. 3C). In combination group were virgin olive oil was administered with mercuric chloride showed reparative changes. Liver showed prominent recovery in the form of normal hepatocytes and very less centrilobular necrosis. Pronounced sinusoid with granular hepatocytoplasm were also evident (Fig. 3D). Liver of the control group had a regular histological structure with a characteristic pattern of hexagonal lobules (Fig. 3A). Furthermore, no histological alterations were observed in the liver of virgin olive oil treated group (Fig. 3B).
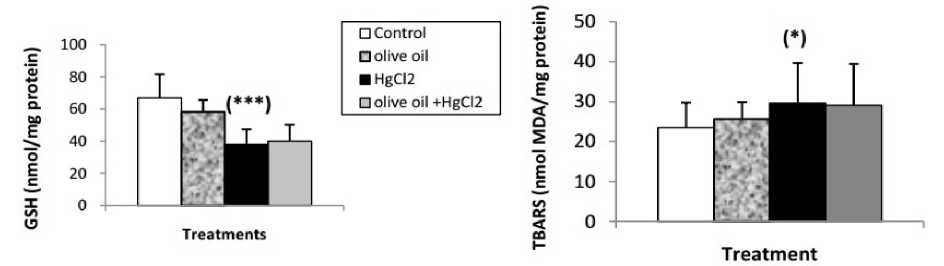
Figure 1 : Reduced glutathione (nmol/mg protein) and TBARS (nmol DA/mg protein) levels in liver of control and treated with olive oil rats, mercuric chloride and combined treatment of olive oil with mercuric chloride, after 2 weeks of treatment. Values are given as mean ± SE for group of 6 animals. (*P≤0.05; **P≤0.01; ***P≤0.001).
GPx activity (pmolGSH/тк protein]
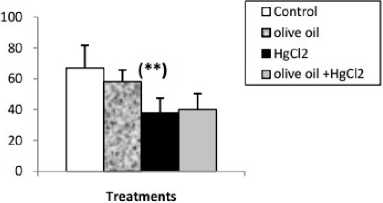
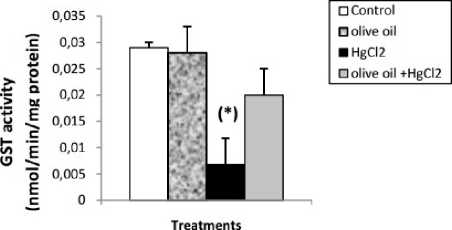
Figure 2 : Enzyme activities of GPx (µmol GSH/ mg protein) and GST (nmol /min/mg protein) in liver of control and rats treated with olive oil, mercuric chloride and combined treatment of olive oil with mercuric chloride, after 2 weeks of treatment.Values are given as mean ± SE for group of 6 animals. (*P≤0.05; **P≤0.01; ***P≤0.001).
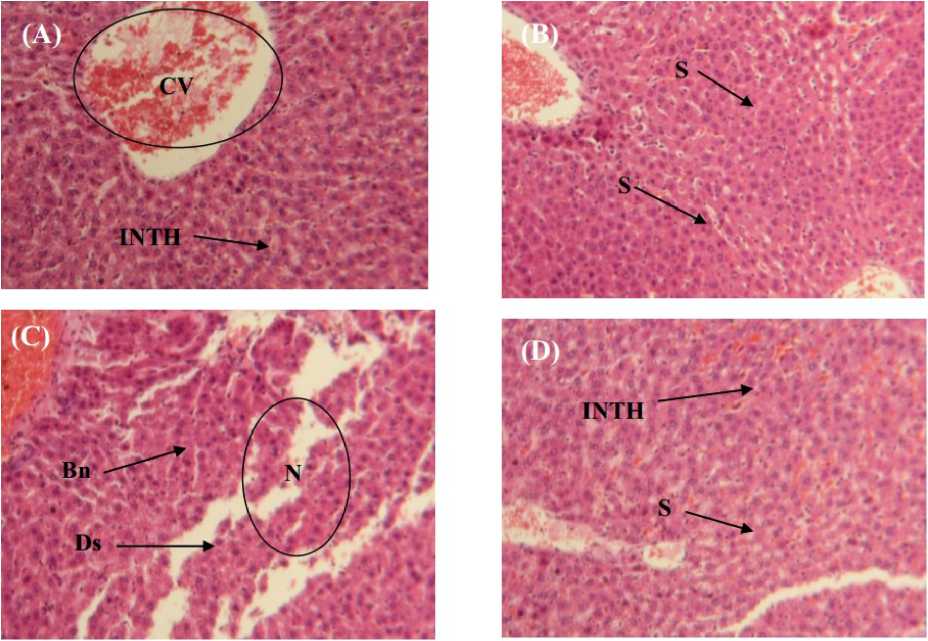
Figure 3 : icroscop evaluation of hepatic tissue from (A) control, (B) treated with olive oil, (C) mercuric chloride and (D) olive oil pre-and post-treated with mercuric chloride after 2 weeks of treatment, section were stained using the hematoxylin-eosin method (100X). Bn: Bright nuclei, Ds: destruction of membrane cells, N: Necrosis, INTH: Intact hypatocyte cells, CV: central vein, S: Sinusoid. Olive oil coadministrated with mercuric chloride shows granular cytoplasm and normal hepatocytes.
Table 1 : Changes in body and absolute and relative liver weights of control and rats treated with olive oil, mercuric chloride and combined treatment of mercuric chloride with olive oil after 2 weeks of treatment.
|
Parameters |
Treatment groups |
|||
|
Control |
Olive oil |
HgCl 2 |
Olive oil + HgCl 2 |
|
|
Initial body weight (g) |
172±46.1 |
176.66±45.1 |
171.33±52.3 |
177.49±50.3 |
|
Final body weight (g) |
175.49±75.2 |
179.16±32.2 |
176.16±42.21 |
173.61±41.1 |
|
Absolute Liver weight (g) |
4.17±0.14 |
4.50±0.13 |
4.40±0.17 |
4.90±0.41 |
|
Relative Liver weight (g/100g b.w) |
2.42±0.3 |
2.54±0.28 |
2.56±0.32 |
2.76±0.81 |
Table 2 : Changes in biochemical parameters of control and rats treated with olive oil, mercuric chloride and combined treatment of mercuric chloride with olive oil after 2 weeks of treatment.
|
Parameters |
Treatment groups |
|||
|
Control |
Olive oil |
HgCl 2 |
Olive oil + HgCl 2 |
|
|
Glucose (mg/dl) |
67.28± 11.28 |
67.45± 8.1 |
88.06± 43.42 |
75.9± 23.17 |
|
Total protein (g/dl) |
6.51± 0.58 |
6.57± 0.28 |
5.27± 0.31*** |
6.2± 0.13 |
|
Albumin (g/dl) |
3.29± 0.47 |
3.28± 0.55 |
2.46± 0.3 |
3.2± 0.7 |
|
AST (UI/l) |
128,33± 23.34 |
152.5± 15.7 |
167.66± 33.6 * |
162.33± 40.4 |
|
ALT (UI/l) |
33.83± 10.38 |
44.16± 6.43 |
58.66± 11.11*** |
57± 7.18 |
|
ALP (UI/l) |
257.7± 11.04 |
252.06± 6.47 |
271.35± 13.65 |
26.913± 12.95 |
Values are given as mean В± SE for group of 6 animals each. controls. ***P≤0.001, compared to controls.
DISCUSSION
P≤0.05, compared to controls. **P≤0.01, compared to
The present investigation revealed that mercuric chloride intoxication causes significant increase in lipid peroxidation and glucose levels, AST, ALT and ALP activities, and significant decrease in the serum albumin and total protein also in reduced glutathione, glutathione peroxidase and glutathione-s-transferase in liver. The principal toxic effects of mercury involve interaction with a large number of cellular processes, including the formation of complexes with free thiols and protein thiol groups, which may lead to oxidative stress (Stacey et al., 1982). Due to its sulfhydryl group binding capability, HgCl2 can also inhibit the activities of many enzymes, especially those involved in the cellular glucose uptake, gluconeogenesis, fatty acid. Oxidation and production of glutathione. In the present study, a significant decrease in serum protein and albumin levels was recorded. The decreased in the protein concentration of Hg treated rats might be due to changes in protein synthesis and/or metabolism. Once absorbed in the cell, both Hg+2 and eHg from covalent bonds with GSH and the cysteine residue of proteins. GSH, the primary intracellular antioxidant and the conjugating agent, was shown to be depleted and to have impaired function in Hg toxicity. A single Hg ion can bind to and cause irreversible excretion of up to two GSH molecules (QUIG, 1998). In fact, GSH serves as a primary line of cellular defense against Hg compounds. Released Hg ions form complexes with GSH and cysteine results in greater activity of the free Hg ions, disturbing GSH metabolism and damaging cells
(Hultberg et al ., 2001). As a result of binding of mercury to glutathione, levels of GSH are lowered in the cell and decrease the anti-oxidant potential of the cell. Antioxidant enzymes such as glutathione peroxidase and glutathione–S-transferase play a major role in the intracellular defense against oxygen radical damage to aerobic cells. Chung et al (1982) demonstrated that 10mg/kg of mercury caused time dependent decreases in the activities of the enzyme of the glutathione metabolism pathway in the rat kidney. Girardi and Elias (1995) reported that mercury inhibits the activities of redox cycles enzymes. Recent finding of Bando et al (2005) and Jadhav et al (2006) proved our point that this antioxidant enzymes show decreased level following mercury intoxication. Because of the low activity of antioxidant enzymes in the liver and decreased content of GSH, the liver is hypothesized to be highly susceptible to oxidative stress. ercury induced oxidative stress turn to severe; the inbuilt mechanism of body fails to alleviate the damage. It has been demonstrated that mercury decreases the anti-oxidative systems and produces oxidative damages via H 2 O 2 generation therby leading to lipid peroxidation (Cheng et al ., 2006; Jadhav et al ., 2007). All these possible mechanisms of mercuric chloride toxicity may lead to the formation of reactive oxygen species (ROS), as found in the present investigation. Therefore, an increase in the formation of ROS by mercuric chloride may induce membrane biochemical and functional alterations and thus induced liver cell damage.
Further, mercury intoxication also induces a significant elevation in serum and: AST, ALT and ALP activities. This increase may be due to cellular necrosis of hepatocytes, which causes increase in the permeability of cell resulting release of transaminases and ALP in blood stream
(Vandenberghe, 1995; Rana et al ., 1996; Kumar et al ., 2005). This confirms our earlier reports on histopathological alterations in liver induced by mercury intoxication (Sharma et al ., 2000; Ketterer, 1998).
It was observed that virgin olive oil when given in combination with mercuric chloride significantly increases liver GSH level, GSH-Px and GST activities as antioxidant potential and thereby declines the level of lipid peroxidation, which in turn reduces the transaminases and ALP activities and glucose, total protein and albumin levels in serum. In present investigation, the elevated level of GSH protects cellular proteins against oxidation through glutathione redox cycle and directly detoxifies reactive species (Kromidas et al ., 1990). Glutathione, as both a carrier of mercury and an antioxidant, has specific roles in protecting the body from mercury toxicity. Glutathione, specifically bind with methylmercury, forms a complex that prevents mercury from binding to cellular proteins and causing damage to both enzymes and tissue (Velioglu et al ., 1998). Glutathione-mercury complexes also reduce intracellular damage by preventing mercury from entering tissue and cells, and becoming an intracellular toxin. The elevated level of GSH-Px and GST by virgin olive oil as compared to the HgCl 2 may have facilited the conjugation reaction of xenobiotics metabolism and may have increased the availability of non-critical nucleophile for inactivation of electrophiles and therefore might be playing a major role in metalloprotection. Several studies have demonstrated the ability of olive oil to inhibit oxidative stress in the liver through various mechanisms (Kyle et al ., 1987; De La Cruz et al ., 2000).
oreover, we have shown that the oral supplementation of olive oil to rats administered ethanol chronically restored damage caused to the liver by inhibiting lipid peroxidation and improving enzymatic activities (Kasdallah-Grissa et al., 2008). The mechanism proposed to explain the positive effects of olive oil may be attributed to its richness in UFA, mainly oleic acid which has different effects on lipid profiles and peroxidation in rabbit hepatic mitochondria (Ochoa-Herrera et al., 2001). Indeed, EVOO contains a considerable amount of oleuropein, hydroxytyrosol, tyrosol and caffeic acid which all have potent inhibition effects against ROS (Owen et al., 2000; Feng et al., 2008). Hydroxytyrosol is highly effective against DNA damage by peroxynitrite in vitro (Covas et al., 2006). Caffeic acid phenethyl ester and its related compounds limit the functional alterations of the isolated mouse brain and liver mitochondria submitted to in vitro anoxia–reoxygenation (Gutteridge et al., 2000). Lipid peroxidation is the process of oxidative degradation of PUFA and its incidence in biological membranes resulting in impaired membrane function, structural integrity, decreased membrane fluidity and the inactivation of several membrane-bound enzymes(Gutteridge et al., 2000). Therefore, some particular attention was given to the liver’s fatty acid composition in rats used in the current experiment. In healthy humans, the short-term consumption of olive oil decreased serum oxidative stress (Weinbrenner et al., 2004) and their isolated lipoprotein fractions; LDL and HDL were shown to be enriched with oleic acid and resistant to oxidation (Aviram et al., 1993; Sola et al., 1997). oreover, PUFAs are more susceptible to peroxidation resulting in DA formation in mammalian tissues (Esterbauer et al., 1991). In fact, because of their peculiar structure -that is the presence of one or more double bonds-UFA are more susceptible to free radical damage and thus could increase the susceptibility of LDL particles to oxidation. ost of the studies comparing the effects of a UFA-rich diet with PUFA-rich diet on LDL oxidation parameters have found a higher resistance of LDL particles to oxidation after the consumption of UFA-rich diet (Aguilera et al., 2004; Kratz et al., 2002). Finally, the healthy effects of dietary UFA, including lower endothelial activation ( oreno et al., 2003) and susceptibility of LDL to oxidation (Aguilera et al., 2004; Kratz et al., 2002) are indeed to be considered. Nevertheless, it is also remarkable to establish the amount and quality of phenolic compounds in virgin olive oil.
It may be concluded that combined treatment of virgin olive oil has a preventive and protective effect on mercuric chloride induced oxidative stress.
ore-over, it protects from HgCl 2 induced hepatic dysfunction and executes its modulatory role in mercury induced free radical production.
Список литературы Protective effect of virgin olive oil ( Olea europea L.) against oxidative damage induced by mercuric chloride in rat albinos Wistar
- Aguilera, C.M., Mesa, M.D., Ramirez-Tortosa, M.C., Nestares,M.T., Ros, E., Gil, A. (2004). Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clin. Nutr. 23, 673-681
- Aviram, M., Eias, K. (1993). Dietary olive oil reduces low-density lipoprotein uptake by macrophages and decreases the susceptibility of the lipoprotein to undergo lipid peroxidation. Ann. Nutr. Metab. 37, 75-84
- Bando, I., Rens, M.I., Andres, D., Cascales, M. (2005). Endogenous antioxidant defence system in rat liver following mercuric chloride oral intoxication. J. Biochem. Mol. and Toxicol. 19(3), 154-161
- Bonanome, A., Pagnan, A., Biffanti,S., Opportuno, A., Sorgato, F., Dorella, M., Maiorino, M., Ursini, F. (1992). Effect of dietary monounsaturated and polyunsaturated fatty acids on the susceptibility of plasma low density lipoproteins to oxidative modification. Arterioscler. Thromb. 12, 529-533
- Boukhobza, M., Pichon, P. (1988). L’arganier, resource économique et médicinale pour le maroc, Phytotherapie, 27, 21-26
- Buege, J.A., Aust, S.D. (1984). Microsomal lipid peroxidation, Methods. Enzymology. 105, 302-10
- Bradford, M.A. (1976). Rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principale of protein-dye binding. Anal. Biochemical. 72, 248-54
- Cheng, J.P., Hu,W.X., Lin, X.J.M., Shi, w., Wang, W.H. (2006). Expression of C-fos and oxidative stress on brain of rats reared on food from mercury-selenium coexisting mining area. J. Envir. Scie. (china). 18(4), 788-792
- Chung, A.S., Maines, M.D., Reynolds, W.A. (1982). Inhibition of the enzymes of glutathione metabolism by mercuric chloride in the rat kidney: reversal by selenium. Biochem. Pharmacol. 31, 3093-3100
- Covas, M.I., Ruiz-Gutiérrez, V., De la Torre, R., Kafatos, A., Lamuela-Raventos, R. M., Osada JOwen, R. W., Visioli, F. (2006). Minor components of olive Oil: evidence to date of health benefits in humans. Nutr. Rev. 64(9), 20-30
- Deiana, M., Aruoma, O.I., Bianchi, M.L.P., Spencer, J.P., Kaur, H., Halliwell, B., Aeschbach, R., Banni, S., Dessi, M.A., Corongiu, F.P. (1999). Inhibition of peroxynitrite dependent DNA basemodification and tyrosine nitration by the extra virgin olive oilderived antioxidant hydroxytyrosol. Free. Radic. Bio. Med. 26(5-6), 762-769
- De La Cruz, Quintero, J.P., Auxiliadora Villalobos, L., M., Sánchez de la Cuesta, F. (2000). Lipid peroxidation and glutathione system in hyperlipemic rabbits: influence of olive oil administration. Biochim. Biophys. Acta. 1485, 36-44
- De la Puerta, R., Ruiz Gutierrez, V., Hoult, J.R.S. (1999). Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 57(4), 445-449
- Esterbauer, H., Schaur, R.J., Zollner, H. (1991). Chemistry and biochemistry of of 4-hydroxynonenal, malondialdehyde and related aldehydes. Free. Radic. Biol. Med. 11, 81-88
- Farima, M., Soares, F.A., Zeni, G., Souza, D.O., Rocha, J.B. (2004). Additive prooxidative effect of methylmercury and ebselen in liver from suckling rat pups. Toxicol. let. 146(3), 227-235
- Feng, Y., Lu, Y.W., Xu, P.H., Long, Y., Wu, W.M., Li, W., Wang, R. (2008). Caffeic acid phenethyl ester and its related compounds limit the functional alterations of the isolated mousebrain and liver mitochondria submitted to in vitro anoxia-reoxygenation: relationship to their antioxidant activities. Biochim. Biophys. Acta. 1780(4), 659-672
- Flohe, L., Gunzler, W.A. (1984). analysis of glutathione peroxidase, Methods. Enzymol. 105, 114-21
- Garg, M.C., Chaudhary, D.P., Bansal, D.D. (2005). Effect of vitamin E supplementation on diabetes induced oxidative stress in experimental diabet in rats. India. J. Exp. Biol. 43, 177-180
- Girardi, G., Elias, M.M. (1995). Mercury chloride effects on rat renal redox enzymes activities: SOD protection. Free. Rad. Biol. Med. 18(1), 61-66
- Goya, L., Mateos, R., Bravo, L. (2007). Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells. Eur. J. Nutr. 46(2): 70-78
- Gutteridge, J.M., Halliwell, B. (2000). Free radicals and antioxidants in the year 2000: a historical look to the future. Ann. Acad. Sci. 899, 136-147
- Habig, W.H., Pabst Jakoby, W.B. (1974). Glutathione-S-transferase the first step in mercapturic acid formation. J. Biol. Chemical. 249, 7130-9
- Hultberg, B., Andersson, A., Isaksson, A. (2001). Interaction of metals and thiols in cell damage and glutathione distribution: potentiation of mercury toxicity by dithiothreitol. Toxicol. 156, 93-100
- Hussain, S., Atkinson, A., Thompson, S.J., Khan, A.T. (1999). Accumulation of mercury and its effect on antioxidant enzymes in brain, liver and kidneys of mice. J. Enviro. Scie. and Health, Part-B. 34(4), 645-660
- Jadhav, S.H., Sarkar, S.N., Tripathi, H.C. (2006). Cytogenetic effects of a mixture of selected metals following subchronic exposure through drinking water in male rats. Ind. J. Exp. Biol. 44(12), 997-1005
- Jadhav, S.H., Sarkar, S.N., Aggarwal, M., Tripathi, H.C. (2007). Induction of oxidative stress in erythrocytes of male rats subchronically exposed to a mixture of eight metals found as ground water contaminants in different parts of india, Arc. Environ. Contam. Toxicol. 52(1), 145-151
- Kandaswami, C., Middleton, E (1994). Free radical scavenging and antioxidant activity of plant flavonoids. Adv. Exp. Med. Biol. 366, 351-376
- Kasdallah-Grissa, A., Nakbi, A., Koubâa, N., El-Fazaâ, S., Gharbi, N., Kamoun, A. (2008). Hammami M: Dietary virgin olive oil protects against lipid peroxidation and improves antioxidant status in the liver of rats chronically exposed to ethanol. Nutr. Res. 28, 472-479
- Ketterer, B. (1998). Glutathione-stransferase and prevention of cellular free radical damage. Free. Rad. Res. 28, 647-658
- Keys, A. (1995). Mediterranean diet and public health: personal reflections. Am. J. Clin. Nutr. 61, 1321S-1323S
- Kim, S.H., Sharma, R.P. (2005). Mercury alters endotoxin induced inflammatory cytokine expression in liver: differential role of P38 and extra cellular signal-regulated mitogen activated protein kinases. Immunopharmacol and Immunotoxicol. 27(1), 123-135
- Kratz, M., Cullen, P., Kannenberg, F., Kassner, A., Fobker, M., Abuja, P.M., Assmann, G., Wahrburg, U. (2002). Effects of dietary fatty acids on the composition and oxidizability of low-density lipoprotein. Eur. J. Clin. Nutr. 56(1), 72-81
- Kromidas, L., Trombetta, L.D., Jamall, I.S. (1990). The protective effects of glutathione against methylmercury cytotoxicity. Toxicol. Let. 51, 67-80
- Kumar, M., Sharma, M.K., Kumar, A. (2005). Spirulina fusioformis: a food supplement against mercury induced hepatic injury. J. health. Scie. 51 (4), 424-430
- Kyle, M.E., Miccadei, S., Nakae, D., Farber, J.L. (1987). Supeoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem. Bioph. Res. Co. 149(3), 889-896
- Lavelli, V. (2002). Comparison of the antioxidant activities of extra virgin olive oils. J. Agric. Food. Chem. 50(26), 7704-7708
- Massaro, M., De Caterina, R. (2002). Vasculoprotective effects of oleic acid: epidemiologicalbackground and direct vascular antiatherogenic properties. Nutr. Metab. Cardiovasc. Dis. 12(1), 42-51
- Miller, O.M., Lund, B.O., Woods J.S. (1991). Reactivity of Hg (II) with superoxide: evidence for catalytic dismutation of superoxide by Hg (II). J. Biochem. Toxicol. 6, 293
- Moreno, J.J., Mitjavilab, M.T. (2003). The degree of unsaturation of dietary fatty acids and the development of atherosclerosis (Review). J. Nutr. Biochem. 14(4), 182-195
- Ochoa-Herrera, J.J., Huertas, J.R., Quiles, J.L., Mataix, J. (2001). Dietary oils high in oleic acid, but with different non-glyceride contents, have different effects on lipid profiles and peroxidation in rabbit hepatic mitochondria. J. Nutr. Biochem. 12(6), 357-364
- Owen, R.W., Giacosa, A., Hull, W.E., Haubner, R., Wurtele, G., Spegelhalder, B., Bartsch, H. (2000). Olive-oil consumption and health: the possible role of antioxidant. Lancet. Oncol. 1, 107-112
- Quig, D. (1998). Cysteine metabolism and metal toxicity. Alt. Medical. Rev. 3, 262-270
- Rana, S.V.S., Singh, R., Verma, S. (1996). Protective effects of few antioxidants on liver function in rats treated with cadmium and mercury, Ind. J. Exp. Biol. 34, 177-179
- Rice-Evans, C.A., Miller, N.J., Paganga, G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Bio. Med. 20(7), 933-956
- Risher-John, F., Amler-Sherlita, N. (2005). Mercury exposure: evaluation and intervention, the inappropriate use of chelating agents in diagnosis and treatment of putative mercury poisoning. Neurotoxicol. 26(4), 691-699
- Salah, N., Miller, N.J., Paganga, G., Tijburg, L., Bolwell, G.P., Rice-Evans, C. (1995). Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 322(2), 339-346
- Sharma, M.K., Kumar, M., Kumar, A. (2000). (study of mercury induced toxicity in liver and its modulation by spirulinafusiformis and ocimum sanctum). In: proceeding of third word congress on cellular and molecular biology held at jena, Germany. Cell. Mol. Biol. 46, 227
- Sola, R., LaVille, A.E., Richard, J.L., Motta, C., Bargallo, M.T., Girona, J., Masana, L., Jacotot, B. (1997). Oleic acid rich diet protects against the oxidative modification of high density lipoprotein. Free. Radic. Biol. Med. 22(6), 1037-1045
- Stacey, N.H., Kappus, H. (1982). Cellular toxicity and lipid peroxidation in response to mercury. Toxicol. App. Pharmacol. 63(1), 29-35
- Stark, A.H., Mader, Z. (2002). Olive oil as a functional food: epidemiology and nutritional approaches. Nutr. Rev. 60(6), 170-176
- Vandenberghe, J. (1995). Hepatotoxicology: mechanisms of liver toxicity and methodological aspects. In: Niesink JM, Vries JD, CRC press, Bocaroton, P.718
- Velioglu, Y.S., Mazza, G., Gao, L., Oomah, B.D. (1998). Antioxidant activity and total phenolies in selected fruits,vegetables and grain products. J. Agr. Food. Chem. 46, 4113-4117
- Visioli, F., Galli, C. (1998). The effect of minor constituents of olive oil on cardiovascular disease: new findings. Nutr. Rev. 56(5 Pt1), 142-147
- Visioli, C., Galli, E., Plasmati, S., Viappiani, A., Hernandez, C., Colombo, A. (2000). Olive phenol hydroxytyrosol prevents passive smoking-induced oxidative stress. Circulation. 102, 2169-2171
- Visioli, F., Galli, C. (2002). Biological properties of olive oil phytochemicals. Crit. Rev. Food. Sci. Nutr. 42(3), 209-221
- Weckbercker, G., Cory, J.G. (1988). Ribonucleotide reductase activity and growth of glutathione-depended mouse leukaemia L 1210 cells in vitro, Cancer. Letters. 40, 257-264
- Weinbrenner, T., Fito, M., de la Torre, R., Saez, G.T., P., Rijken, C., Tormos, S., Coolen, M.F., Albaladejo, S., Abanades, H., Schroder, J., Marrugat, M.I. (2004). Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. J. Nutr. 134(9), 2314-2321
- WHO (1991). Environmental health criteria 118: inorganic mercury-environmental aspects. World healthorganization, geneva, Switzerland. P. 115-119
- Wiseman, S.A., J.N., Mathot, N.J., de Fouw, L.B., Tijburg, (1996). Dietary non-tocopherol antioxidants present in extra virgin olive oil increase the resistance of low density lipoproteins to oxidation in rabbits. Atherosclerosis. 120(1-2), 15-23


