Protective Effects of Wheat Grass on Histopathology of Some Organs and Biomarkers Parameters Against Lead Acetate Toxicity in Wistar Rats
Автор: Mansouri Ouarda, Zohra Hamamdia, Cherif Abdennour
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.17, 2021 года.
Бесплатный доступ
This study focuses on the search for a natural treatment to reduce lead toxicity in male Wistar rat by using the aquous extract of wheatgrass Triticum aestivum (WG). In addition to the control group (C), rats were exposed to a diet containing 600 mg Pb acetate/Kg diet (Pb), or combined with the WG at 9g/100g diet (Pb-WG) for a period of 6 weeks. Biochemical, reproductive and histological markers were evaluated. The results of haematological parameters show a very significant increase in white blood cells and lymphocytes with the group treated with the lead single. By contrast there was no difference was recorded between the treated group by Pb-WG and the control. A significant decrease in the red blood cells, the haemoglobin and haematocrit was recorded in the group treated with Pb alone. The tests of hormonal and biochemical parameters showed a decrease in the concentration of the hormone triiodothyronin (T3) and thyreostimulin (TSH) in the Pb group compared to the (control and Pb-WG). The serum testosterone concentration, urea, total cholesterol levels, the rate of TGP, TGO and creatinine were significantly increased in rats treated with (Pb) alone compared to (the control and Pb-bl group), while no change in glucose was shown. However, the (Pb-WG) group shows no change compared to the control. The level of calcium showed a significant decrease in the Pb group, and which returns to the normal state in group (Pb-WG) compared to the control. These results are confirmed by the study of histological sections. A morphological change represented by volume shrinkage of the vesicles. A return to the normal structure of follicles was observed in (Pb-WG) group. Histology study of the kidney, testis epididymis showed no change in (Pb-WG) compared to the control group. Contairement in the group exposed to lead (Pb) the parenchyma of the kidney shows dilated distal and proximal tubules causing renal tubular damage. The testes marked destruction and degeneration of germ cells and the light of some seminiferous tubules are empty. The study of the fertility parameters indicates a highly significant decrease in the concentration, the mobility of sperm counts among the treated group by Pb alone. The administration of the wheat grass has increased.
Hematological, biochemical markers, fertility, TSH, Testosterone and histological sections, Pb, wheatgrass (WG)
Короткий адрес: https://sciup.org/143173905
IDR: 143173905
Текст научной статьи Protective Effects of Wheat Grass on Histopathology of Some Organs and Biomarkers Parameters Against Lead Acetate Toxicity in Wistar Rats
The contamination of the environment by heavy metals is currently a major problem. These metals, still used in many applications, often have very negative impacts on the environment and human health (Adraoui and Aziz, 2007; Zerari, 2006). The man had used the heavy metals and continuous to use, sometimes with excess, often with unconsciousness. It should be especially noted that heavy metals are bioaccumulative indicating that their concentration in living cells (plants animals, and human beings) is increasing throughout the trophic chain (Marc and Bashir, 2006; Pracheta and ingh, 2009; Wardani et al., 2019).
Lead metal enters the body mainly through the respiratory tract, which can play an important role for the occupational exposures or for people living under the atmospheric emissions of polluting enterprises (Botta et al., 1976) and by the digestive route, mainly via the food and drinking water (Olivier et al., 1999). It has been shown to induce a wide range of behavioral, histological biochemical and physiological effects (Jackie et al. 2011; Zargar et al., 2016; Okesola et al., 2018 ; Gargouri et al., 2019 ; aritha et al., 2019). A mere dose of exposure but prolonged, acts primarily on the erythropoiesis (anemia) and the hemoglobinogenese by the inhibition of heme synthesis (Mousa et al., 2002; Adeniyi et al., 2008), on the nervous system and causes a neuropsychological deficit ( enapati et al., 2001; oltaninejad et al., 2003; Bellinger, 2008; harma et al. 2011), on the immunological system (Razani et al. 1999, Bunn et al., 2001, Rosenberg et al., 2007), on the kidney (Lockitch, 1993, Vargas et al., 2003; Rastogi 2008; Mansouri and Abdennour 2008, harma et al. 2011c), on the liver (Lockitch, 1993, Patra et al., 2001 harma et al., 2011b), on the cardiovascular system, the high blood pressure (Adeniyi et al., 2008), also on the fertility and the reproductive function (Aleksandra et al. 2007; Mansouri and Abdennour, 2011).
The exposure to moderate levels of lead causes of miscarriage, premature births in women and more retarded growth in children (Ehle and McKee, 1990; Pracheta and ingh, 2009).
The lead causes the oxidative stress by inducing the production of RO (reactive oxygen species), the reduction of the antioxidant defense system of cells by inhibition of glutathione, enzymes sulfhydrile, anti oxidizing enzymes or by increasing the sensitivity of cells to oxidative attack by modifying the integrity of the membranes and the composition of fatty acids (Xu and al., 2008; IN ERM, 1999; harma et al., 2011a, b, c). Oxidative stress leads to disruption of sperm quality reduced reproductive organ weight, and eventually male infertility (Anjum et al., 2017). Thus it induces DNA damage in spermatozoa (Li et al., 2018) and decreases its capacity to fertilize eggs (Evans, 2015).
Very few studies have focused on disruption of thyroid function, which is all the more regrettable that these alterations can have many serious consequences. Thyroid hormones play an important role in maintaining homeostasis. If the normal communication between the hormone "messenger" and cellular receptors is disrupted, the chemical message is misinterpreted and the body reacts by an abnormal response (Connor 2002, Goger et al, 2007, O '. Ormond et al, 2009). Thyroid gland is an endocrine gland, its follicular and parafollicular cell synthesize and secret important regulatory hormones such as thyroxine (T4) and triiodothyronine (T3) which are synthesized in the follicular cells, theses hormones are necessary to increase the metabolism of most cell, (stimulating growth via induction of DNA translant, which results in greater activity in cell synthesize, oxidative phosphorylation and membrane transport of electrolyte) (Dieter and Joann, 1998). Thyroid plays an important role in regulation of calcium and phosphorous metabolism ( woucki, 1995 and akare et al ., 2000).
Lead can also cause testicular damage to varying degrees ranging from degeneration of the germinal epithelium to necrobiotic changes with complete cessation of spermatogenesis (Mabrouk, 2018). The therapeutic methods to eliminate the heavy metals from the body include the chelation, and measures of support. everal chelating compounds have been used to manage the toxicity of lead, but none is appropriate to reduce the harmful effects of lead in the case of a chronic exposure.
In this context, this study focuses mainly on finding a treatment or an effective cure against lead toxicity with no side effects. We tried to assess herbal therapy using a well known plant: The wheatgrass (Triticum eastivum): It is plant of a high bioavailability, high potential energy and essential enzymes to the life which helps to ensure the necessary daily nutritions to develop the solid foundation of a good health.
Medicinal plants are important for pharmacological research and drug development, not only when plant constituents are used directly as therapeutic agents, but also as raw material for drug synthesis (Heide, 1991; WHO, 1998).
The nutritional supplementation based on principles phytoactive (food supplements of vegetable origin), to the depurative action and detoxifying organic material anti-oxidant, immunostimulating and protective to the principal organs ( achin et al., 2013 ; areen et al. 2014; Zeng et al., 2018; Hanan 2020).
Many studies have been conducted to demonstrate the therapeutic effects of wheat grass. It has a high antioxidant activity (Boloor et al., 2000; Manish et al. 2009; ), particularly the chlorophyll and its derivatives which inhibit the proliferation of cells cancer (Lai, 1979) and have antimutagenic properties and anticytotoxiques ( mith et al., 2001; Ardelt et al., 2001; Kumar et al. 2001).
The juices of cereal grass contain a high amount of chlorophyll "green blood", which represents 70% of the chemical constituents of green herbs, their main role in participating in the regeneration of the blood (Padalia et al., 2010 ; Zeng et al., 2018).
Wheatgrass has been studied on the one hand for its property to improve growth (Lakhanpal et al., 1966) and the healing (Bowers, 1947). On the other hand, for its antibacterial property (Osborn, 1943) and regeneration of blood (Hughes and Letner, 1936; Priyabrata et al. 2012).
The objective of this study is to evaluate the therapeutic effectiveness using a natural cure by the wheatgrass plant ( Triticum aestivum ) to assure the detoxification of some organs of the body exposed to lead such as (liver, kidney, the testicles and thyroid) of rats.
MATERIALS AND METHODS
The study was conducted on 21 adult males’ albino rats were procured from the Pasteur Institute of Algiers with an average body weight (121- 175g). They were kept for three weeks for accommodation and observation before starting the experiment. The experimental animals were kept under hygienic conditions (temperature 25 C, day light 12 hr and humidity) and fed on a standards diet. The rats were divided into 3 groups. The first group was kept without any treatment and served as a control. The second group was treated with Pb (600 mg lead acetate / kg diet); the last group was treated with Pb-wheath grass (Pb-WG) (600 mg lead acetate/kg diet and 7g/ rat for a period of six weeks).
The wheatgrass is obtained from hard wheat grains drenched in water for a day. prouted grains are planted in an organic garden. Young shoots of a length of 10 to 15 cm are collected and then scrambled to mix then with the food of the 3rd group of the treatment during this study (Pb-WG).
After decapitation, blood was collected immediately blood was collected either in EDTA tubes for blood counts (Automatic Cell Counter) or in dry tubes to obtain the serum to perform the dosage of some biochemical parameters that reflect the operating status of the liver and kidney as: (calcium, cholesterol, criatinine, glucose GOT, GPT and urea).
The biochemical parameters were analyzed using spectrophotometer: clinical chemistry Analyzer Dialab DTN-410. Calcium and glucose were measured by the colorimetric method ( tern and Lewis, 1957). Cholesterol depended to enzymatic colorimetric test (CHOD-PAP) (Fasce, 1982). Dosage of GOT depended on the IFCC method (Isherwood, 1979) and urea was mesured by the UREA A-GLDH method ( ampson et al., 1980).
erum testosterone hormone was measured by the ELFA (Enzyme Linked Fluorescent Assay) method (Litwack, 1992).
erum triiodothyronin T3 and T H thyreostimuline were carried out by Test immuno-enzymatique (ELI A) according to pencer et al., 1995 (Britton et al . 1975).
Statistical analysis
The Student t- test was used by comparing each of the treated group with the control. The significance level of P < 0.05 was considered.
RESULTS AND DISCUSSION
Changes in hematological parameters
-
- The results show a significant increase in the number of white blood cells in the group treated with lead acetate compared to control. Whereas no difference was observed between the control group and the group treated by wheatgrass. However, the comparison between the treated groups in Pb alone and Pb-WG, indicates a significant difference.
-
- The results obtained indicate a significant decrease of red blood cells in the group receiving only Pb compared to control. Whereas there is no change in the number of red blood cells between the control group and the group treated by Pb-WG. A significant difference was observed between the treated group by Pb alone and Pb-WG of Wistar rats.
-
- The results show a significant decrease in the rate of hematocrit with the group treated with lead acetate alone compared to the controls. On the other side no change has been recorded as compared to the control group and the treated group by the wheatgrass (WG). A significant difference was observed between the treated group by Pb and Pb-WG.
-
- The results show a very significant decrease (P ≤ 0.01) in the treated groups by the Pb comparing to the control. No significant changes were noted between the control group and the group treated by the Pb-WG. The
comparison between the two treated groups by the Pb alone and Pb+WG indicates a significant difference (P ≤ 0.05).
-
- The results show a very significant increase in treated group Pb alone comparing to the controls. No differentiation was observed between the control group and the group treated by Pb-WG. A significant difference (P ≤ 0.05) was marked between the treated group by Pb and the group treated by Pb-WG.
Variations of Biochemical parameters
-
- erum biochemical analysis illustrated in table (2) showed a marked decrease in the levels of T3 hormone in the group treated by Pb alone compared to the controls (P≤ 0.05). The levels of T H hormone showed a significant decrease in rats of both treated groups (Pb alone and Pb-WG).
-
- The results of Transaminase glutamate Oxalate (TGO) indicate a considerable increase (P≤ 0.01) and (P≤ 0.05) in the group treated by Pb alone compared to the controls. Whereas, the comparison between the control group and the one receiving the Pb-WG shows no difference.Concerning the groups treated by Pb and Pb-WG, we have recorded a significant modification (P ≤ 0.01 and P ≤ 0.05).
-
- A significant increase (P ≤ 0.05) in concentration glutamate pyruvate transaminase (GPT) was recorded in the treated group compared to controls Pb. No difference between the control group and that of Pb- WG firstly, and secondly between the two treated groups (Pb and Pb-WG).
-
- The comparison between the group treated only by Pb and the control showed a significant increase in the rate of urea. There is no considerable difference not only between the control group and the one which received Pb-WG but also between this group and that of the Pb.
-
- Results of calcium showed a significant reduction in the rate of calcium in the group treated by Pb alone compared to the control. No change has been marked between the group treated by Pb-WG and the control. A significant improvement was marked between the group treated by Pb and the group treated by Pb-WG.
-
- The results of cholesterol showed a significant increase in the treated group by AcPb alone compared
to the control. Whereas, no change was recorded for the group treated by the Pb-WG. Therefore, there is a significant difference by comparing the two groups treated by Pb and Pb-WG.
A significant change was observed in the cholesterol levels between treated groups and the control group.
No significant change was observed in the glucose levels between treated groups and the control group.
Table 1: The level of some hematological parameters(mean± D ) of wistar rats exposed to lead and wheatgrass during
Histological sections
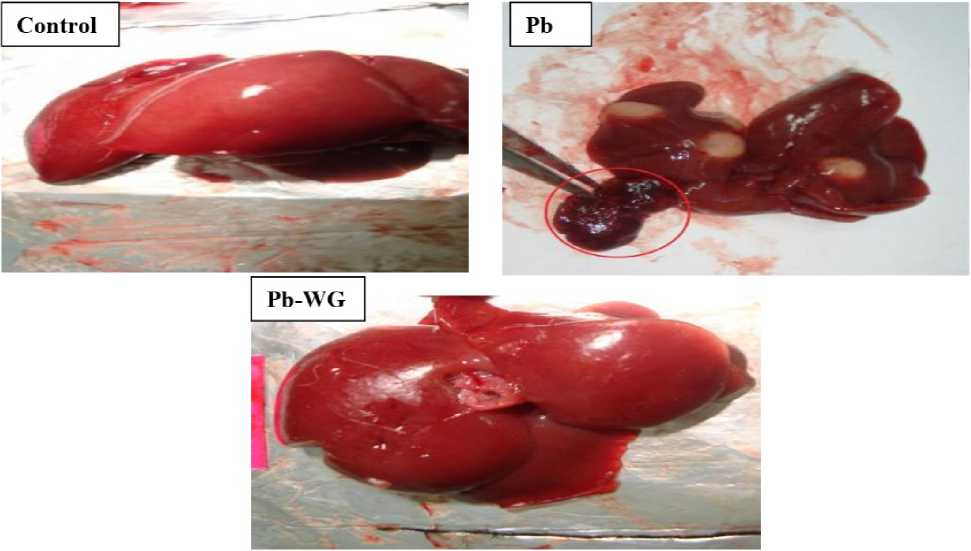
Figure 1. The macroscopic picture of liver showing in the Pb treated rats the presence of multiple cysts (3-7/liver) with a diameter ranges from 0.5 cm to 1.5cm. The other two groups look normal.
(6) weeks
|
Control |
Pb |
Pb-WG |
||
|
White bolod cells |
106 |
6.39±0.59 |
14.73±0.61 (***) |
7.0.41±0.89 (**) |
|
Red blood cells |
(1012) |
8.96±0.71 |
6.53±0.91 (**) |
7.90±0.50 (*) |
|
Hematocrit % |
37.2±1.21 |
27.1±1.13 (***) |
29.2±0.95 |
|
|
Hemoglobin g/l |
13.88±4.46 |
11.4±3.27 (**) |
12.2±1.58 |
|
|
Lymphocytes |
(109) |
2.72±0.66 |
8.45±0.97 (***) |
3.95±1.33 |
Table 2: The level of some biochimical paramètres (mean± D) of wistar rats exposed to lead and wheatgrass during (6) weeks
Thyroid gland
Control group
col
Pb group
Pb -WG group
Figure 2. A histomicrograph of thyroid gland exposed to Pb and WG (A : x100 ; B : x600).
Control: The histological structure of the normal thyroid parenchyma was observed. Vesicles are surrounded by a single layer of cubic cells. (cart.: cartilage ; V. :vesicles, col. : colloïd).
Pb group : hrinkage and reduction of volume of 95% of the vesicles was observed causing hypothyroidism.
Pb-WG group: light changes were observed in the histarchitecture of the thyroid gland.
Kidneys
Control
Pb
group
Hfr1
VW 4Hv'
■1
v^ttfl
Pb-WG group
Figure 3. A histomicrograph of kidney exposed to Pb and WG (A : x100 ; B : x600).
Control : the normal renal parenchyma has a structure on the cortical area (A) and spinal cord (B) Gr x100. Pb group : dilation of the distal and proximal tubules and interstitial fibrosis. Gr.x100 ; (C et D) : Gr.x600
Pb-WG group: the return of the structure of almost to normal kidney tubes at the cortex
Gr (A et B): x100
The epididymis
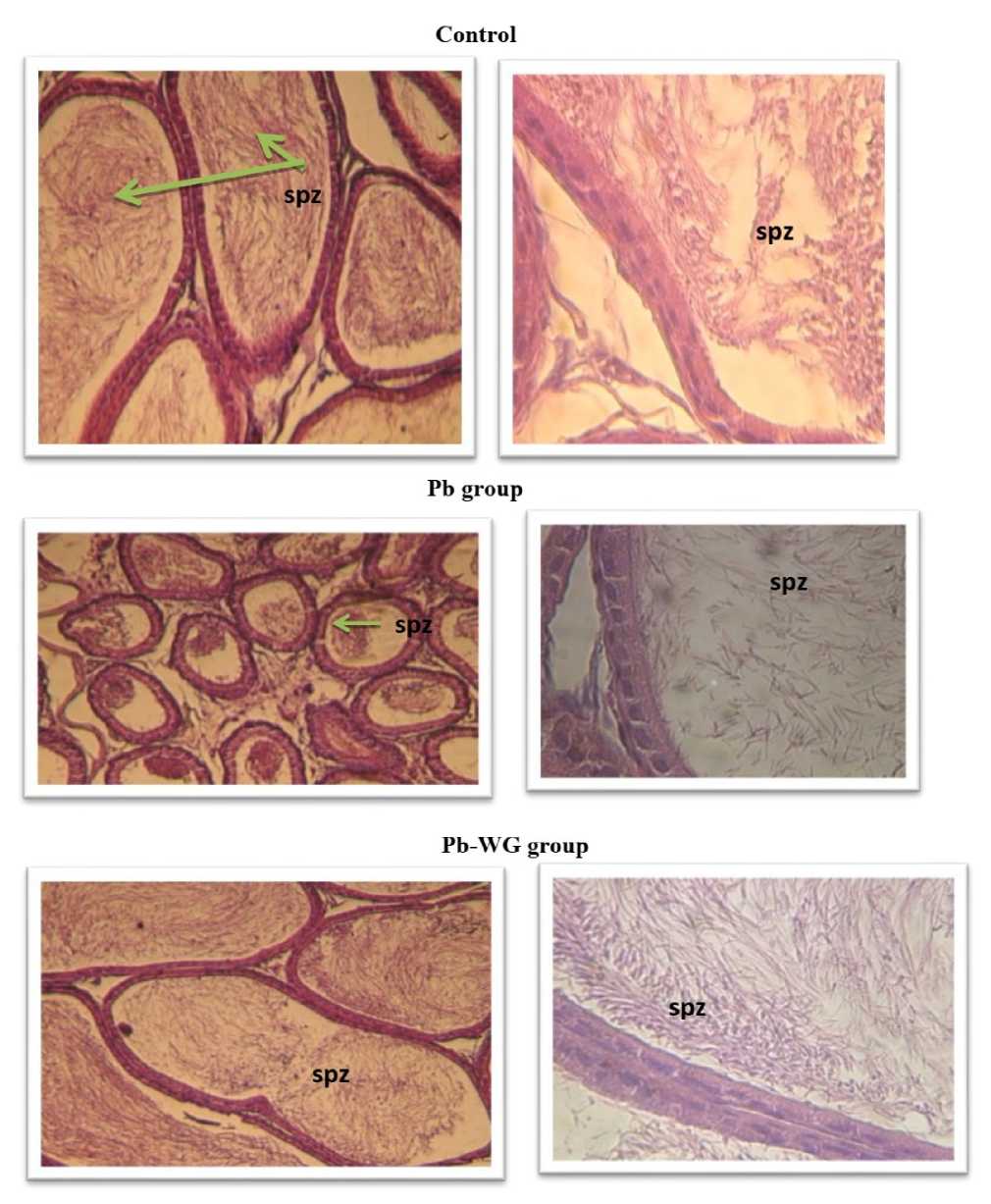
Figure . A histomicrograph of The epididymis exposed to Pb and WG (A : x100 ; B : x600).
Control: The tubes of epididymis are made of a single layer of cuboidal epithelial cells. Light is occupied by a high density of spermatozoa ( PZ). A : Gr x100 et B : x600
Pb group: A decrease of the size of the tubes epididymal and the sperm density is low level
Pb-WG group: The light tube is full of sperm compared to Pb group
The testicles
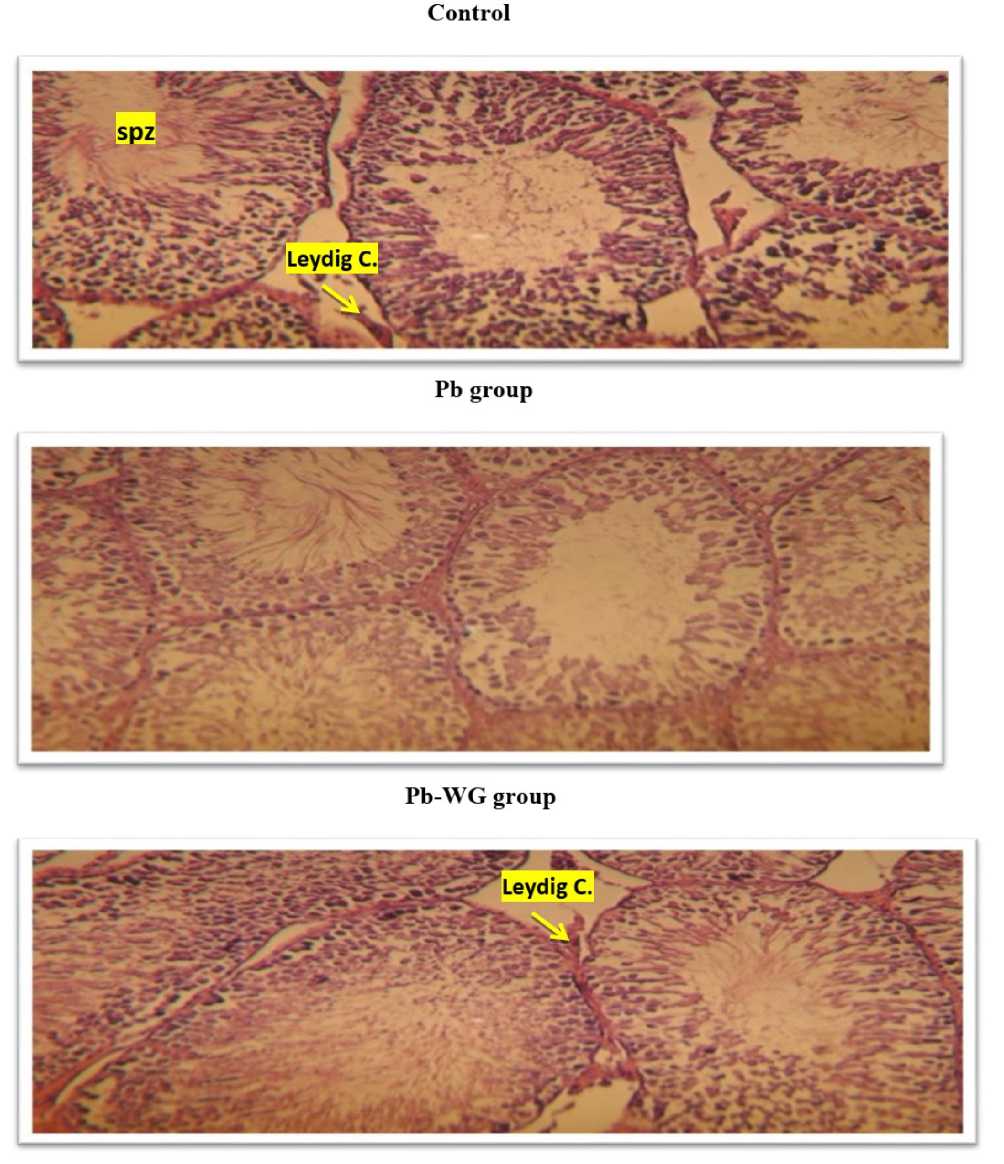
Figure 5. A histomicrograph of The epididymis exposed to Pb and WG (A : x100 ; B : x600).
Control: The various stages of the germ line are observed, and the light is employed by the sperm. Gr x100
Pb group: There is a destruction and degeneration of the cells except the germline spermatogonial cells. The joining of the seminiferous tubules and interstitial tissue which locates the Leydig cells.
Pb-WG group: The return of spermatogenesis normally.
Reproductive parameters
-
- According to the results mentioned in Figure 1, showed a marked a very highly significant decrease in the concentration of sperm (P ≤ 0.001) between the control and Pb only. No difference between the control group and Pb+WG was recorded. The statistical analysis shows a highly significant increase (P ≤ 0.001) between the two treated groups (Pb and Pb + WG).
-
- Motility of sperm indicates the presence of a very significant decrease (P ≤ 0.001) in the group treated only by Pb compared to the control. No changes have been observed between the control group and that of the Pb. Whereas, the comparison between the two treated groups (second and third groups) shows a very highly significant increase (P ≤ 0.001).
DISCUSSION
The Lead toxicity is the most common form of heavy metal intoxication (Flora et al., 2006, El- ayed and El-Neweshy 2009, Ashry et al., 2010).
The use of chelating agents and antioxidants such as vitamin C and E (Mehta and Flora, 2001) may increase the excretion of lead. The natural therapies for the chelation, detoxification and the protection of the body have popularity because of their minimal side effects and have a beneficial role in the reduction of lead poisoning.
The medicinal properties of plants have also been studied during the recent scientific development throughout the world, and this because of their powerful pharmacological activities and the economic viability (Janmeda et al., 2011).
The disruption of the immune response is translated by the very significant increase in white blood cells and lymphocytes in the group treated by AcPb. The studies confirm that the exposure to lead causes a drop in the number of neutrophils and an increase or decrease in lymphocytes (Queiroz et al., 1993).
The scavenging activity of free radicals weakens the immune system, causes a genetic mutation and reduces its vitality. The wheatgrass is a powerful antioxidant, this plant is considered a good source of antioxidants such as beta-carotene, vitamin C and vitamin E. tudies suggest that the supplement of wheatgrass could reduce the oxidative stress ( hyam et al., 2007, Priyabrata et al., 2012).
In the present study, the treatment by the acetate of lead causes anemia which is characterized by a very significant decrease of red blood cells, haemoglobin and haematocrit.
The anemia results from the decrease of the lifetime of the erythrocytes and the decrease in heme synthesis enzyme inhibition (Amdur et al., 1996; Garnier, 2011). By inhibiting the deshydratase of delta-aminolevulinic acid (ALAD) which catalyzes the transformation of the delta-aminolevulinic acid (ALA) in porphobilinogen (PBG) and the ferrochelatase (or heme-synthetase) which controls the last step of the synthesis of heme in which four molecules of protoporphyrin are associated with an iron atom.
The coproporphyrinogene- decarboxylase is also inhibited, but to a lesser degree (AT DR, 1999; IPC 1995). This metal causes a hyperstimulation of the erythropoiesis of erythroblasts with nuclear abnormalities and a abnormal hemoglobin, which results in an increased production of abnormal erythrocytes (Venugopal and Luckey, 1974). The lead induces oxidative damage in the membranes of red blood cells which leads to the inhibition of the heme and the synthesis of hemoglobin ( kerfving et al., 1998, Warren et al., 1998).
It reacts strongly with thiol group ( SH ) in the enzymes and proteins containing biological molecules (Flora et al., 2006).
On the contrary the group treated by Pb -WG was able to return the rate of haematological parameters (white, red blood cells, hematocrit, hemoglobin and lymphocytes) and the maintaining to their normal level.
The effectiveness of the wheatgrass results in the synergy between its many components. The high content of chlorophyll which promotes the renewal of blood, blood circulation and general detoxification of the body.
Indeed, the chemical structure of chlorophyll is close to that of blood hemoglobin. The only difference: the chlorophyll contains a magnesium atom and a hemoglobin iron atom (Marawaha et al., 2004). Thus the chlorophyll will quickly pass into the blood and also by providing the iron, promotes regeneration and then allows the production of new blood (Marawaha et al. 2004; Priyabrata et al., 2012).
The co-administration of this plant can activate the synthesis of a heme by the maintenance of mitochondrial enzymes involved in this process ( ingh et al., 2010; Marawaha et al., 2004).
The thalassemia is a hereditary blood disease characterized by an excessive destruction of red blood cells. The blood transfusion at regular intervals is the only option to increase the lifetime of patients suffering from major thalassemia.
The clinical trials suggest that the wheat grass improves the quality of life in patients suffering from thalassemia, by reducing the requirement of blood transfusion. The tablets of wheatgrass will contribute to increase the haemoglobin rate and decrease the amount of blood required for a transfusion ( ingh et al., 2010; Priyabrata et al., 2012).
The thyroid is an endocrine gland whose follicular cells and parafollicular cells (C cells), synthesize and secrete important regulatory hormones, including triiodothyronine (T3), tetraiodothyronine (T4) and calcitonin (CT). Iodothyronines (T3, T4), synthesized in follicular cells, are necessary for normal growth and development (Zoeller et al. 2002) Parafollicular cells, via CT secretion, play an important role in the regulation of calcium and phosphate metabolism ( akai et al. , 2000 awicki , 1995). The present study was aimed to evaluate the effect of Pb exposure on the function and structure of the thyroid follicular cells. Yousif and Asma (2009) recorded inhibition in the production of thyroid hormones in the presence of Pb. This indicates that animals exposed to cadmium may be at risk of thyroid damage (primary and secondry hypothyrodism). In our present study that T H was reached to zero in rats treated with Pb after 6 week and this result has been suggested that Pb interference in synthesis or secretion of T H by the pituitary gland or TRH by the hypothalamus gland, thyroid releasing hormone (TRH) is responsible for regulation the secretion of T H from the pituitary gland.
The rate of TGO and GPT presents a significant increase. This result is confirmed by the works done by (Cobot, 2006, Hesham et al. 2020) where this increase causes damage to the membrane of hepatocytes and the appearance of inflammation of the liver. imilar effects were also presented by (Georing, 1993; andhir and Gill 1995; halan et al., 2005, harma et al., 2011B; Hesham et al. 2020) in rats exposed to lead.
The lead absorbed by the liver will be transmitted to the kidney, where a small amount is excreted in the urine and the rest accumulates in various organs leading to morphological alterations that persist even after the decrease in the level of this metal.
It also causes many biological activities at the molecular level, cellular and intercellular (Jarrar, 2003; idhu and Nehru, 2004; Taib et al., 2004; Flora et al., 2006). The works of ( aka et al., 2011) show the role of the system of glutathione in the detoxification of toxic metabolites of lead acetate in rats treated by three doses (25, 50 and 100 mg/kg) of body weight, thus ensuring the protection of the living cell.
According to the results obtained in this study, the rate of urea is high in the group treated by the Pb alone. (Muhammad uleman et al., 2011) suggests that an increase in the level of urea in the blood means the inability of the kidney to excrete in a definitive way.
The lead provokes an interstitial tubular nephropathy, at the level of renal proximal tubes but also glomerular. This alteration is reflected by a weak or no proteinuria, but especially a glycosuria, a aminoacidurie and the disturbances of ion transports (Bonnard et al., 2006).
The rate of calcium shows very significant decrease of calcium level for the treated group by the Pb only. The fall in calcium concentration is induced when the ions Ca2+ competes with lead (Goldstein, 1993; imons 1993; Finkelstein et al. , 1998), as well as the calcium channels blockade, inhibits the Na+ /K+ -ATPase membrane (IN ERM, 1999).
Whereas the wheatgrass could improve the rate of calcium. According to Dweik 2003, this plant contains a high quantity of calcium ten times more than that found in the milk.
A very significant increase in the level of cholesterol in the group treated by the Pb alone is marked. The level of serum cholesterol is affected by thyroid activity and varies inversely with the degree of its activity. In initial screen, increased in cholesterol is often the first clue to hypothyrodism. Also, Mary (2003) mentioned that hypothyroidism is the most common secondary cause of high cholesterol level in blood.
The wheat grass also contributes to reduce the rate of total cholesterol and increases the high-density lipoprotein (HDL) or the level of healthy cholesterol in the blood, by increasing the levels of antioxidant enzymes in the body ( ethi et al., 2010; Hala et al. 2014, Hesham et al. 2020).
The results of (Apostoli et al., 1998; Moorman and et al., 1998; Mansouri and Abdennour, 2011; Hesham et al. 2020) indicate a very significant decrease in the concentration and mobility of the sperm is in the group treated by the Pb alone or combined with the garlic that is similar to the results obtained in this study.
It is suggested that this metal may act on the function of mitochondria to the intermediate part and inhibits the needed energy to power the sperms movement and to make them infertile (Gardan and Lausanne, 2010).
The gross organ pictures of liver has recorded a number of 3 to 7 cysts/liver with a diameter ranges from 0.5 to 1.5 cm in rats intoxicated with Pb. uch cysts are completely absent in the control and even in the Pb-WG group. Moreover, liver histological study of the Pb group is marked with vacuolization and cystic structure, but it has a remarkable improvement in its parenchyma with the presence only of few vacuoles in the Pb-WG treated group; this is undoubtedly due to the beneficial effect of this herb.
The results obtained in the group Pb-WG are totally opposed to those of lead alone. It is supposed the richness of this plant chlorophyll which contains a significant amount of the ion Zn2+ and magnesium which greatly favor the fertility rate (Gardan and Lausanne, 2010) .
CONCLUSION
The consumption of wheatgrass is an effective natural cure and with no side effects. This plant increases fertility, purified, regenerates the blood. In addition, it ensures the detoxifying of liver, kidneys and thyroid. Wheatgrass is a biogenic plant, provides the body with energy and materials that needs to regain and maintain a vibrant good health.
ACKNOWLEDGMENTS
Список литературы Protective Effects of Wheat Grass on Histopathology of Some Organs and Biomarkers Parameters Against Lead Acetate Toxicity in Wistar Rats
- Adeniyi T.T, Ajayi G.O, Akinloye O.A. (2008). Effect of Ascorbic acid and Allium sativum on tissue lead in female Rattus navigicus, J. Health Biomed. Sci. 7(2): 38-41.
- Adraoui I., Aziz A. (2007). Détermination des métaux lourds par voie électrochimique. J. les technologies de laboratoire. 3: 16.
- Aleksandra K., Sławomir K., Stanisław H., Alina O., Ewa G.M, Ewa R., Anita O. et Ewa B. (2007). Assessment of semen function and lipid peroxidation among lead exposed men, Toxicology and Applied Pharmacology. 228(3): 378-384.
- Amdur M.O, Doull J., Klaassen C.D. (1996). Lead.In Casarrett and Doull’s Toxicology, 5eéd. New York. McGraw-hill.
- Anjum M.R., Madhu P., Reddy K.P., Reddy P.S., (2017). The protective effects of zinc in leadinduced testicular and epididymal toxicity in Wistar rats. Toxicology and Industrial Health, 33 (3), 265-276.
- Ann Wigmore, (2001). L'herbe de blé : Source de santé et de vitalité, Editions Jouvence: 160.
- Apostoli P., Kiss P., Porru S.J.P. et Vanhourne M. (1998). Occup Environ, Med. 55: 364-374.
- Ardelt B., et al., (2001). Chlorophyllin protects cells from the cytostatic and cytotoxic effects of quinacrine mustard but not of nitrogen mustard, Int.J.Oncol. 18: 849-53.
- Ashry K.M, El-Sayed Y.S, Khamiss R.M, El-Ashmawy I.M. (2010). Oxidative stress and immunotoxic effects of and their amelioration with myrrh (Commiphoramolmol) emulsion, Food ChemToxicol. 48(1): 236–241.
- ATSDR (Agency for Toxic Substances and Disease Registry), (1999). Toxicological profile for lead, US Department of Health and Human Services: 640.
- Bellinger D.C. (2008). Very low lead exposures and children’s neurodevelopment current opinion in Pediat. 20: 172-177.
- Bonnard N., Falcy M., Hesbert A., Jargot D., Pillière F., Schneider O., Serre P. (2006). Plomb et composés minéraux Fiche établie par les services techniques et médicaux de l’INRS, FT. 59: 7-8.
- Botta A., Poyen D., Signouret M. et Mathias A. (1976). Les différents tests biologiques de dépistage d'une imprégnation saturnine applicables en médecine du travail, Arch. Mal Prof. 37: 4-5, 437-443.
- Boloor K.K et al., (2000). Chlorophyllin as a protector of mitochondrial membranes against gamma-radiation and photosensitization, J.Toxicology. 155: 63-71.
- Bowers W. F. (1947). Chlorophyll in Wound Healing and Suppurative Disease, Am. J. Surg. 73: 37–50.
- Bunn T.L, Ladics G.S, Holsapple M.P. (2001). Developmental immunotoxicology assessment in the rat.Age.Gender and strain comparisons after exposure to Pb, Toxicol.Met. 11: 41-58.
- Cobot S. (2006). The liver tests, http://www./Weighcontrodoctor.Com/ healthtopics/healthyliverbowelbook: 98.
- Dieter, D. and Joann, E. (1998):"Textbook of Veterinary Histology. "5th ed., Waverlg Company, Paris, Hong Kong,Sydney, Tokyo.
- Ehle A.L, McKee D.C. (1990). Neuropsychological effect of lead in occupational exposed workers, Crit. Rev. Toxico. 20: 237-255.
- El-Sayed Y.S., El-Neweshy M.S. (2009). Impact of lead toxicity on male rat reproduction at “hormonal and histopathological levels”, Toxicol Lett. 189 (Suppl.1): 219–20.
- Evans T.J., (2015). Reproductive toxicity and endocrine disruption of potential chemical warfare agents. Handbook of Toxicology of Chemical Warfare Agents, Elsevier. pp. 599-613.
- Fasce C.F. (1982). Serum Cholesterol determined colorimetrically with enzyme. Clin. Chem, 18: 901
- Finkelstein Y., Markowitz M.E., and Rosen J.F. (1998). Low-level lead-induced neurotoxicity in children: an update on central nervous system effects, Brain Res Rev. 27(2): 168-176.
- Flora S., Flora G. et Saxena G. (2006). Environmental occurrence, health effects and management of lead poisoning, In: J.S. Casas and J. Sordo, Editors lead chemistry, analytical aspects, environmental impact and health effects, Elsevier Science, Amsterdam, Netherlands: 158–228.
- Gardan M.J, Lausanne E. (2010). Comment renforcer les défenses immunitaires par la nutrithérapie ? 2: 6-74.
- Gargouri M., Soussi A., Akrouti A., Magné C., El Feki A., (2019). Potential protective effects of the edible alga Arthrospira platensis against lead-induced oxidative stress, anemia, kidney injury, and histopathological changes in adult rats. Appl. Physiol. Nutr. Metab. 44, 271-281.
- Garnier R. (2011). Toxicité du plomb et de ses dérivés inorganiques, Diplôme Interuniversitaire (DIU) de Toxicologie Médicale.
- Georing PL. (1993). Lead- protein interaction as a basis for toxicity, Neurotoxicol. 14: 45-60.
- Goldstein GW. (1993). Evidence that lead acts as a calcium substitute in second messenger metabolism, Neurotoxicology. 14(3): 97-103.
- Hanan Salah Eldeen Eldamaty (2020). Protective effects of barley and wheat grasses on nephrotoxicity in rats and some biochemical parameters induced by tramadol. Egypt. J. of Nutrition and Health Vol. 15 No. 1
- Hala MFED, Mohamed SS, El-Messery TM (2014) Role of the functional food (pomegranate-yoghourt) as hepatoprotective effect on liver injured rats. Int J Curr Microbiol App Sci 3(8):185–196.
- Heide L. (1991). Traditionelle Arzneipflanzen in der Gesundheitsversorgung der dritten Welt-Möglichkeiten und Grenzen. Zeitschrift für Phytotherapie, 12: 1-8.
- Hughes et Letner (1936). Chlorophyll and Hemoglobin Regeneration, American Journal of Medical Science: 188-206.
- INSERM (1999). Plomb dans l’environnement. Quels risques pour la santé?, Expertise collective. Paris: 461.
- IPCS (1995). Environmental health criteria 165, Inorganic lead. Geneva. WHO: 300.
- IFCC (International Federation of Clinical Chemistry), (1980). Recommandation of IFCC Methods of the measurement of catalytic concentrations of Enzymes, Clin. Chem. Acta, 105: 147-157.
- Isherwood D. (1979). Med. Lab. Sci. 36: 211-235.
- Jackie T., Haleagrahara N., Chakravarthi S., (2011). Antioxidant effects of Etlingeraelatior flower extract against lead acetate-induced perturbations in free radical scavenging enzymes and lipid peroxidation in rats. BMC Res. 4, 67-75.
- Janmeda P., Sharma V., Singh L., Paliwal R., Sharma S., Yadav S. (2011). Chemopreventive Effect of Hydro-Ethanolic Extract of Euphorbia neriifolia Leaves against DENA-Induced Renal carcinogenesis in Mice, Asian Pacific J. Cancer Prev.12: 677-683
- Jarrar B.M. (2003). Histological and histochemical alterations in the kidney induced, by Ann Saudi Med. 23 (1–2): 10–15.
- Kumar S.S et al., (2001). Scavenging of reactive oxygen species by chloropyllin: an ESR study, Free Rad Res. 35: 563-74.
- Lai C.N. (1979). Chlorophyll: The active factor in wheat sprout extract inhibiting the metabolic activation of carcinogens in vitro, Nutrition and Cancer.1 (3): 19-21.
- Lakhanpal R., Davis J., Typpo J., Briggs G. (1966). Evidence for an unidentified growth factor from alfalfa and other plant sources for young guinea pigs, J. Nutr. 89: 341-346.
- Lockitch G., (1993). Perspective on lead toxicity, Cline. Biochem. 26: 371-381.
- Mabrouk A., (2018). Therapeutic effect of thymoquinone against lead-induced testicular histological damage in male Wistarrats, Andrologia. 50, 13014.
- Manish V., Vasudha S., Somnath S. (2009). Evaluation of antioxidant profile and activity of amalaki (Emblicaofficinalis), spirulina and wheat grass, Indian Journal of Clinical Biochemistry. Feb. 24(1): 70-75.
- Mansouri O., Abdennour C. (2011). Evaluation of the therapeutic efficiency of raw garlic on reproduction of domestic rabbits under lead induced toxicity, Scholars Research Library Annals of Biological Research. 2(3): 389-393.
- Mansouri O., Abdennour C. (2008). Influence of Sudden Cystine Supplementation and Suppression on Adrenal and Ovary of Lead Exposed Rat. EuroJournals Publishing, Inc. 23(4): 548-558.
- Marawaha R.K, Bansal D., Kaur S., Trehan A. (2004). Wheatgrass Juice Reduces Transfusion Requirement in Patients with Thalassemia Major: A Pilot Study, Indian Pediatric. 41: 716-720.
- Marc S. and Béchir S. (2006). Guide technique de l’assainissement, 3é édition. le moniteur. Paris: 30.
- Mary, Y. S. (2003). "Competinghigh cholesterol with hypothyrodism. "www about hypothyrodism.Com.
- Mehta A., Flora S.J. (2001). Possible role of metal redistribution, hepatotoxicity and oxidative stress in chelating agents induced hepatic and renal metallothionein in rats, Food Chem. Toxicol. 39: 1029-1038.


