Protective role of sodium selenite on mercuric chloride induced oxidative and renal stress in rats
Автор: Necib Youcef, Bahi Ahlem, Zerizer Sakina
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.9, 2013 года.
Бесплатный доступ
Backgroud: Reactive oxygen species are known to play a major role in mercuric chloride induced oxidative and renal stress. Sodium selenite as an exogenous source of selenium is used for endogenous selenoprotein synthesis to scavenge the free radicals. The study was designed to investigate the possible protective role of sodium selenite in mercuric chloride induced renal stress, by using biochemical approaches. Adult male Albinos Wistar rats were randomly divided into four groups. The first group was served as the control, the second group was given sodium selenite (0.25 mg/kg b.w), while the third group was given mercuric chloride (0.25 mg/kg), finally, the fourth group was given combined treatment of sodium selenite and mercuric chloride for 3weeks. Results: The effects of sodium selenite on mercuric chloride induced oxidative and renal stress were evaluated by serum creatinine, urea, uric acid, billirubin levels and LDH activity, kidney tissue lipid peroxidation, GSH levels, GSH-Px, GST and catalase activities and hematological parameters. Administration of mercuric chloride induced significant increase in serum: creatinine, urea, uric acid and billirubin concentration showing renal stress. Mercuric chloride also induced oxidative stress, as indicate by decreased kidney tissue of GSH level, GSH-Px, GST, and catalase activities along with increase the level of lipid peroxidation. Furthermore, treatment with mercuric chloride caused a marked elevation of kidney weight and decreased body weight and erythrocytes, hemoglobin, hematocrit levels. Sodium selenite treatment markedly reduced elevated serum: creatinine, urea, uric acid and billirubin levels, and LDH activity and conteracted the deterious effects of mercuric chloride on oxidative stress markers and hematological parameters and atteneuated histopathological changes caused by HgCl 2 in kidney. Conclusion: Our results indicate that sodium selenite could have a beneficial role against mercuric chloride induced nephrotoxicity and oxidative stress in rat.
Antioxidant enzymes, lipid peroxidation, mercury, renal stress, sodium selenite
Короткий адрес: https://sciup.org/14323733
IDR: 14323733
Текст научной статьи Protective role of sodium selenite on mercuric chloride induced oxidative and renal stress in rats
Mercury is a well-known human and animalinduces extensive kidney damage nephrotoxicant. Acute oral or parenteral exposure induces extensive kidney damage (Fowler and Woods, 1977; Goyer and Rhyne, 1975; Magos et al ., 1987; Woods, 1989). Studies in vivo and in vitro have demonstrated that mercury induced lipid peroxidation, suggesting the involvement of oxidative stress in its cytotoxicity (Lund et al ., 1991; Stacey and Kappus, 1982; Yonaha and Sagai, 1983). Lund et al . (1993) reported that mercury enhances renal mitochondrial hydrogen peroxide formation in vivo and in vitro. However, cansative correlation between mercury induced lipid peroxidation and cellular toxicity remains controversial. Some authors reported that lipid peroxidation plays a critical role in cell injury induced by mercury (Lund et al ., 1991) in renal cells, whereas other investigators showed that lipid peroxidation is not directly responsible for mercury induced cell injury in hepatocytes and renal cells (Paller, 1985; Strubelt et al ., 1996). It is believed that antioxydants should one of the important components of effective treatment for mercury poisoning. Indeed, HgCl 2 induced injury can be ameliorated by superoxide dismutase (Girardi and Elias, 1995) and non-enzymatic antioxidants like vitamin C, Vitamin E, cystein and selenium (Karin and Elias Armer, 2003) have proven helpful to overcome oxidative damage.
Selenium (Se) appears in various forms such as selenite, selenate, selenomethionine, and selenocysteine and it is considered as an essential trace element for living organism, including humans, because it forms part of several enzymes such as glutathione peroxidase (GSH-Px), io-dothyronine 5’-deiosinase (Cabanero et al., 2004), and thioredoxin reductase (Rotruck et al., 1973; Xia et al., 2003; Perottoni et al., 2004a). Se plays a very important part in human body despite its slight content of about 14-20 mg. The need for Se in human and animal nutrition is well recognized. Organoselenium compounds such as selenoamino acids and derived compounds are one of the chemical forms of the selenium in foods (Block et al., 2001; Perottoni et al., 2004b). Se has been found to have detoxification effects on various heavy metals (Diplock et al., 1986). The interaction between Hg and Se in the body of mammals has been known for more than three decades, and it may be involved in a variety of toxicological and biochemical processes. Previous studies reported the co-accumulation of Se and Hg in different tissues of various biotas (Chem et al., 2006). Since Parizek and ostadalova (1967) found that the toxicity of inorganic mercury was decreased by simultaneous injection of selenite, many studies have been carried out to examine the role of Se in the detoxification of Hg, which have led to many hypotheses about the mechanism of this interaction (reviewed by Curin-aralar and Furness, 1991; Juresa et al., 2005). The purpose of this study was to evaluate the protective role of selenium on mercury chloride induced oxidative and renal stress in rats.
MATERIALS AND METHODS
All chemicals used in this work were purchased from sigma chemical company. Laboratory animals, Albino Wistar male rats, were brought from the Algiers Pasteur institute at the age of 8 weeks, with an average live weight of 200g. They were located in a room with an ambient temperature of 21±1°C and up to 12h of light daily. The rats were divided into four experimental groups; each consists of eight rats. The first group was served as the control. The second group was given sodium selenite at a dose of 0.25 mg/kg body weight, while the third group (HgCl2) was intraperitoneally given mercuric chloride at a dose of 0.25 mg/kg body weight. Finally, the fourth group was given combined treatment with sodium selenite and mercuric chloride. The treatment of all groups was lasted for 3consecutive weeks.
Twenty four hours after the last administration, blood was collected by retro- orbital sinus punction into two vials, from each anesthetized rats. Blood samples were collected into no heparinized tubes, After centrifugation at 3000 rpm for 10min, the serum was separated immediately and stored at 20°C until determination of urea, creatinine, uric acid and bilirubin levels and LDH activity. And into EDTA tubes, for determination hematological parameters. Subsequently, rats were decapitated and kidneys were removed.
Tissue preparation
About 500mg of kidney was homogenized in 4mlof buffer solution of phosphate buffered saline (w/v: 500mg tissue with 4ml PBS, pH 7.4) homogenates were centrifuged at 10.000xg for 15min at 4°c. And the resultant supernatant was used for determination of: reduced glutathione (GSH), Thiobarbituric acid- reactive substance (TBARS) level, and glutathione peroxidase (GSH-PX), glutathione–S-transferase (GST) and catalase activities.
Determination of Biochemical parameters
Serum urea, creatinine, uric acid and billirubin levels and LDH activity were determined spectrophotometrically using an automated analyzer.
Determination of hematological parameters
Hematological parameters were determined spectrophotometrically using an automated analyzer.
Determination of lipid peroxidation (LPO)
Lipid peroxidation level in the liver was measured by the method of Buege and Aust (1978). 125µl of supernatant were homogenized by sonication with 50 µl of PBS, 125 µl of 20% TCA + BHT 1% (TCA-BHT) in order to precipitate proteins, and centrifuged (1000xg, 10min, 4°c), afterwards, 200µl of supernatant were mixed with 40µl of HCl (0,6M) and 160µl of TBA dissolved in tris (120 mM), and then the mixture was heated at 80°c for 10min, the absorbance of the resultant supernatant was obtained at 530nm. The amount of TBARS was calculated using a molar extinction coeffient of 1.56x105M/Cm.
Determination of reduced glutathione (GSH)
GSH content in liver was measured spectrophotometrically by using Ellman’s reagent (DTNB) as a colouring reagent, following the method described by Weeckbeker et Cory (1988). Determination of glutathione-S-transferase (GST) (EC2.5.1.18)
The cytosolic glutathione-S-transferase activity was determined spectrophotometrically at 37°c by method of Habig et al (1974). The reaction mixture (1ml) contained 0.334ml of 100mM phosphate buffer (pH 6.5), 0.033ml of 30mM CDNB and 0.33ml of reduced glutathione. After pre-incubating the reaction mixture for 2min the reaction was started by adding 0.01ml of diluted cytosol and the absorbance was followed for 3min at 340 nm. The specific activity of GST is expressed as µmole of GSH-CDNB conjugate formed/ min /mg protein using extinction coefficient of 9.6 Mm-1 cm-1
Determination of GSH-Px (E.C.1.11.1.9)
Glutathione peroxidase (EC 1.11.1.9) activity was modified from the method of Flohe and Gunzler(1984). For the enzyme reaction, 0.2ml of the supernatant was placed into a tube and mixed with 0.4ml GSH (reduced glutathione, sigma product, analytical grade), and the mixture was put into an ice bath for 30min. Then the mixture was centrifuged for 10min at 3000rpm, 0.48ml of the supernatant was placed into a cuvette, and 2.2ml of 0.32M Na2HPO4 and 0.32ml of 1m mol/l 5,5’-dithio-bis(2-nitrobenzoic acid) (DTNB, sigma) were added for color development. The absorbance at wavelength 412nm was measured with a UV spectrophotometer after 5min. The enzyme activity was calculated as a decrease in GSH within the reaction time as compared to that in the nonenzyme reaction.
Determination of catalase (CAT) (EC 1.11.1.6)
It was estimated in the liver homogenate in a UV-VIS spectrophotometer as describe by Aebi (1984). The reaction mixture (1ml, vol) contained 0.02ml of suitably diluted cytosol in phosphate buffer. The specific activity of catalase has been expressed as µmol of H 2 O 2 consumed/min/ mg protein. The difference in absorbance at 240nm per unit time is a measure of catalase activity.
Histopathological examination
Kidney from autopsied animals were excised out and fixed in formalin (10%). Five micron think section were prepared by using microtome and these section were stained with hematoxyline and eosin. For histological alterations these slides were observed under light microscope.
Protein quantification
Protein was measured by the method of Bradford (1976) using bovine serum albumin as the standard.
Statistical analysis
The data were subjected to student t test for comparison between groups. The values are expressed as mean ± SEM. Significance level was set at P<0.05, P<0.01, P<0.001.
RESULTS
Effects of treatments on body, absolute and relative kidney weights
Table 1 shows the effect of mercuric chloride, sodium selenite and combined treatment with mercuric chloride and sodium selenite. The marked decreased in rats body weight was observed in mercuric chloride treated rats and mercuric chloride + sodium selenite group, but the result was not significant as compared to control. Along sodium selenite showed increased body weight but result was not significant. The kidneys of rats treated with mercuric chloride were enlarged. Mercuric chloride treated rats showed a highly significant increased kidney weight and relative kidney weight (P≤0.001) as compared to control. Combined treatment with sodium selenite showed significant increased relative kidney weight, while alone sodium selenite treatment had showed no significant effect.
Effects of treatment on serum biochemical parameters
A highly significant (P≤0.001) elevation in serum urea, creatinine, uric acid, total bilirubin and direct bilirubin levels was observed in mercuric chloride intoxicated rats. Only sodium selenite treatment did not show any significant alteration. However, the combined treatment of sodium selenite with mercuric chloride show a highly significant decline in serum urea, creatinine, uric acid, total and direct billirubin was noticed respect to mercuric chloride treated animals (table 2). A significant (P≤0.05) elevation in serum lactate dehydrogenase activity was observed in mercuric chloride intoxicated rats. Only sodium selenite treatment did not show any significant alteration. However, the combined treatment of sodium selenite with mercuric chloride results a decline in serum lactate dehydrogenase activity was noticed respect to mercuric chloride treated animals (table 2).
Effects of treatments on hematological parameters
Mercuric chloride exposure induced a highly significant depleted in red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), platelet (PLT) contents and a significant increase in white blood cells (WBC), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), lymphocyte (LYM) levels in mercury intoxicated rats was noticed. Sodium selenite alone treatment did not show any significant decline. In combined treatment of mercuric chloride with sodium selenite, a significant increase in RBC, HGB, HCT, MCV, PLT contents and no significant depletion in WBC, MCH, MCHC, LYM levels was recorded with respect to mercury intoxicated rats (table 3).
Effects of treatments on renal oxidative stress parameters
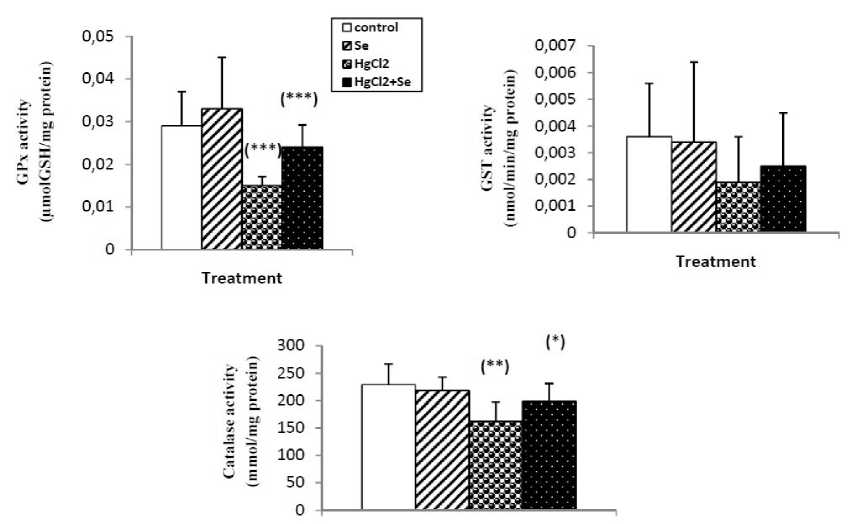
Mercuric chloride exposure a significant depleted in reduced glutathione level, GSH-Px, GST and catalase activities. And highly significant increase in kidney lipid peroxidation level in mercury intoxicated rats was noticed. Sodium selenite alone treatment did not show any significant decline. In combined treatment of mercuric chloride with sodium selenite highly significant increase in reduced glutathione level, GSH-Px, GST and catalase activities. And significant depletion in lipid peroxidation level was recorded with respect to mercury intoxicated rats (Fig.1 and 2).
Histological studies
The histological change in kidney are presented in Fig. 3. Mercuric chloride induced various pathological alterations in kidney of rats. This alteration were characterized by renal tubular damage, indicating by degeneration of tubular cells (Fig 3.C). In combination group were sodium selenite was administration with mercuric chloride showed reparative changes. Kidney showed prominent recovery in the form of normal renal tubular (Fig 3D). Kidney of the control group had a regular histological structure (Fig. 3A). Furthermore, no histological alteration were observed in the kidney of sodium selenite treated group (Fig.3B).
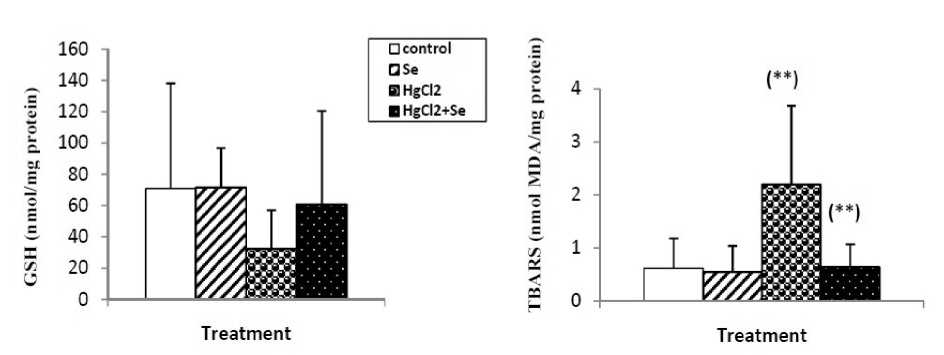
Figure 1: Reduced glutathione (nmol/ mg protein) and TBARS ( nmol MDA /mg protein) levels in kidney of control and rats treated with selenium, mercuric chloride, and combined treatment of mercuric chloride with selenium after 3weeks of treatment. Values are given as mean ± SEM for group of 8animals each significant difference: * compared to controls (*P,0.05; **P,0.01; ***P,0.001).
Treatment
Figure 2: Enzyme activities of GPx (4mol GSH/ mg protein), GST (nmol /min/mg protein) and Catalase (mmol/mg protein) in kidney of control and rats treated with selenium, mercuric chloride, and combined treatment of mercuric chloride with selenium after 3weeks of treatment. Values are given as mean ± SEM for group of 8animals each significant difference: * compared to controls (*P,0.05; **P,0.01; ***P,0.001).
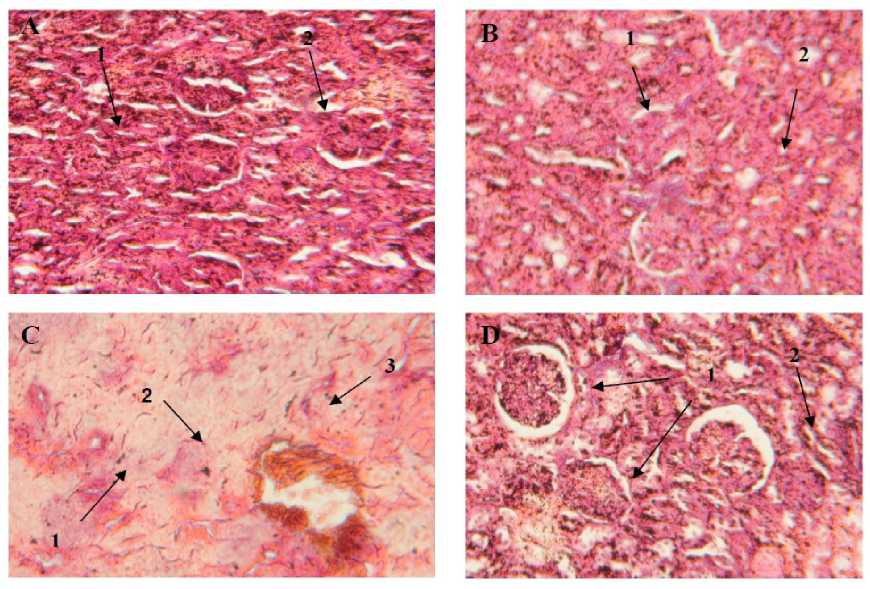
Figure 3: T.S. of kidney of male rat treated with mercuric chloride (Hg) alone, and in combination with sodium selenite . (A) control (H&E100X): showing well develop glomerulus (1), with normal tubular cells; (B) sodium selenite alone treatement (H&E 100X): showing normal glomerulus (1), and normal tubular cells; (C) mercury treatment (H&E100X): showing degeneration of tubular cells (1), loss of nuclus (2), degeneration of glomerulus (3); (D) combined treatment of mercuric chloride with sodium selenite (H&E100X): showing normal glomerulus (1), normal tubular cells (2).
Table 1: Changes in body and absolute and relative kidney weights of control and rats treated with selenium (Se), mercuric chloride, and combined treatment of mercuric chloride with selenium after 3 weeks of treatment.
|
Parameters |
Treatment groups |
|||
|
Control |
Se |
HgCl 2 |
HgCl 2 +Se |
|
|
Initial body weight (g) |
240.13±37.1 |
241.3±36.1 |
240.87±25.3 |
240.81±21.5 |
|
Final body weight (g) |
244.03±32.2 |
247.16±30.2 |
229.48±14.8 |
231.61±18.4 |
|
Absolute kidney weight (g) |
1.81±0.18 |
1.71±0.23 |
2.2±0.19*** |
1.86±0.26** |
|
Relative kidney weight (g/100g b.w) |
0.75±0.15 |
0.69±0.12 |
0.95±0.11*** |
0.8±0.1** |
Values are given as mean ± SEM for group of 8 animals each. *P≤0.05 compared to controls. **P≤0.01 compared to controls. ***P≤0.001 compared to controls.
Table 2: Changes in biochemical parameters of control and rats treated with selenium (Se), mercuric chloride, and combined treatment of mercuric chloride with selenium after 3 weeks of treatment.
|
Parameters |
Treatment groups |
|||
|
Control |
Se |
HgCl 2 |
HgCl 2 +Se |
|
|
Urea (g/l) |
0.47±0.062 |
0.47±0.062 |
0.89±0.039*** |
0.61±0.046*** |
|
Creatinine (mg/l) |
2.68±1.02 |
2.38±0.72 |
7.13±2.5 *** |
4.87±1.54* |
|
Uric acid (mg/l) |
21.67±4.10 |
23.93±4.63 |
45.7±2.22*** |
33.10±3.03*** |
|
LDH (U/l) |
409.25±208 |
399.64±175 |
606.53±204* |
540±160 |
|
Total Billirubin (mg/l) |
1.84±0.15 |
1.73±0.35 |
3.59±0.35*** |
2.56±0.19*** |
|
Direct Billirubin (mg/l) |
0.35±0.11 |
0.28±0.09 |
0.73±0.03*** |
0.53±0.10*** |
Values are given as mean ± SEM for group of 8 animals each. *P≤0.05 compared to controls. **P≤0.01 compared to controls. ***P≤0.001 compared to controls.
Table 3: Changes in Hematological parameters of control and rats treated with selenium, mercuric chloride, and combined treatment of mercuric chloride with selenium after 3 weeks of treatment.
|
Parameters |
Treatment groups |
|||
|
Control |
Se |
HgCl 2 |
HgCl 2 +Se |
|
|
WBC (103/µl) |
8.35±1.94 |
7.39±2.69 |
13.66±3.88** |
11.22±2.69 |
|
RBC (106/µl) |
10.08±0.61 |
9.8±0.92 |
9.18±0.59** |
8.61±0.34* |
|
HGB (g/dl) |
17.26±0.38 |
16.38±1.67 |
15.26±0.6*** |
15.36±0.64 |
|
HCT (%) |
56.05±1.37 |
53.6±5.32 |
47.66±3.06** |
48.28±3.06 |
|
MCH (Pg) |
17.16±1.05 |
17.38±0.84 |
18.38±0.45* |
17.83±0.81 |
|
MCV(Fl) |
55.59±2.04 |
54.79±2.81 |
51.18±3.06 |
51.46±3.81 |
|
MCHC (g/dl) |
30.71±0.97 |
30.74±1.15 |
33.39±9.76 |
31.82±0.95 |
|
PLT (103/µl) |
839.62±155 |
819.75±792 |
648.62±266* |
766±131 |
|
LYM (%) |
45.46±3.70 |
44.21±2.78 |
67.6±12.3 *** |
61.68±5.83* |
Values are given as mean ± SEM for group of 8 animals each. *P≤0.05 compared to controls. **P≤0.01 compared to controls. ***P≤0.001 compared to controls.
DISCUSSION lipid peroxidation level and the stimulation of GSH-
In the present study, oxidative stress induced by Px, GST and catalase activities. Accordingly,
HgCl2 was evidenced in kidney of rats by increase in oxidative stress induced by HgCl2 has been previously reported (Lund et al., 1993; Sener et al.,
2007). As a consequence of lipid peroxidation biological memranes are affected causing cellular damage, release of LDH to extracellular media. And increased activity of this enzyme in the blood (Kaplan and Pesce, 1996). Renal damage observed in rats exposed to HgCl2 was also evidenced by increase in the plasmatic levels of urea, creatinine and uric acid, which are renal markers of damage. It has been reported that mercury can cause hematological alteration, such as hemolysis of red blood cells (Zolla et al., 1997). In this study, toxicity induced by HgCl2 was marked by hematological alterations in rats, demonstrated by the reduction in erythrocyte, hemoglobin and hematocrit counts, indicating anemia. To support the hypothesis of hemolytic anemia, billirubin content was evaluated. Rats treated with HgCl2 presented an increase in billirubin content, one of the most sensitive parameters that reflect the prognosis of acute hepatic disease (Batra and Acharya, 2003). In the present study, the hyperbillirubinemia observed is mostly of the indirect (unconjugated) category. This could be attributable to hemolysis. Which demonstrate no variance on size of erythrocytes, and MCV, MCH and MCHC values, excluding evidence of iron deficient anemia (Brugnara, 2000). A reduction in other hematological parameters patelet and increase in leukocyte, lymphocyte counts were demonstrated in HgCl2 exposed rats. It is know that mercury can induceabnormal responses in the immune system, including leukocyte count, a marker of cellular defense (Fribergand Enestrom, 1991; WHO, 1991). In the present study, serum urea, creatinine, uric acid levels were significantly increased after 3weeks mercuric chloride (0.25mg/kg), showing insufficiency of renal function. Studies in animals have established that tubular injury plays a central role in the reduction of glomerular filtration rate in acute tubular necrosis. Two major tubular abnormalities could be involved in the decrease in glomerular function in mercuric chloride treated rats: obstruction and backleak of glomerular filtrate (Girardi and Elias, 1995). The alterations in glomerular function in mercuric chloride treated rats may also be secondary to ROS(reactive oxygen species), which induce mesangial cells contraction, altering the filtration surface area and modifying the ultrafiltration coefficient factors that decrease the glomerular filtration rat (Stohs and Bagchi, 1995; Zalups, 2000). No significant change in renal and hematological parameters after treatment of sodium selenite in mercuric chloride treated rats showed the evidence for correction of toxicity developed by mercuric chloride treatment. The activity of GSH-Px, GST and catalase that can clear to protect the cells from being injured represents the competence of clearing free radicals from the organism. MDA content manifests the level of lipid peroxidation, and then indirectly represents the level of damage of the cell of renal mitochondria. Evaluating from GSH, MDA levels and GSH-Px, GST, catalase activities in kidney of rats. Hg alone significantly decreased GSH level, GSH-Px, GST, catalase activities and increased MDA content along with histological damage in kidney.
Co-administration of Hg and Se significantly increased GSH level, and activities of GSH-Px, GST, catalase and decreased MDA content. The effect of Hg and Se interaction depended on the molar ratio of these elements admintrated to animals. The maximal effect of Se on Hg induced nephrotoxicity was observed when Se was given the same mol as Hg. Hg can give rise to free radicals that induce lipid, protein, and DNA oxidation. Hg has a great affinity for SH groups of proteins and enzymes that are crucial in cell metabolism. Woods et al. (1990a, b) have reported that in vitro Hg+2 both hinders the antioxidant potential of GSH and yields reactive species via thiol complexation. Lipid oxidation yields hydroperoxides and aldehydes, such as MDA (Perottoni et al., 2004a). Endogenous antioxidant enzymes such as GSH-Px, GST, and catalase are involved in the protection against oxidative stress and lipid peroxidation in kidney (Pokorny, 1987). Induction of these antioxidant enzymes indicates an adaptive onset of the redox defence system, whereas inhibition is thought to contribute to oxidative stress in mouse brain following mercury intoxication (Yee and Choi, 1994; Hussain et al., 1997). Se can enhance antioxidant ability by enhancing activities of antioxidant enzymes and by increasing contents of the antioxidants. Rotruck et al. (1973) and Xia et al. (2003) reported that Se is crucial in several enzymes with physiological antioxidant properties, including GSH-Px and thioredoxin. Besides, the ability of Se to reduce Hg toxicity has been extensively investigated (Cuvin-Aralar and Furness, 1991; Sasakura and Suzuki, 1998; Perottoni et al., 2004a). It have been demonstrated that HgCl2 lower the activity of the selenoenzyme GSH-Px in the renal mitochondria after prolonged treatment. Their direct inhibitory action, preseumably via covalent reaction with the selenol group of the selenocysteine residue (Chandiene and Tappel, 1984) is one mechanism whereby thy impair the activity of GSH-Px and possible other selenoenzymes, following prolonged exposure. Selenite treatment prevented HgCl2 induced decline in GSH-Px activity in the renal mitochondria of rats (Santos et al., 1997). The protective effect of Se against Hg induced nephrotoxicity may be related to the formation of a Se-Hg complex. This conclusion is based on previuos studies demonstrating that pre-treatment with sodium selenite increased whole retention of Hg, conceivably due to the formation of inert Se-Hg complexes (Perottoni et al., 2004b). And the complexes reduced the availability of Hg (Sasakura and Suzuki, 1998; Gailer et al., 2000). Yoneda and Suzuki (1997) also demonstrated that Se forms an equimolar complex with Hg in the plasma which subsequently binds to selenoprotein P. simultaneous administration equimolar doses of sodium selenite prevented not only methyl mercury induced increased of oxidized glutathione, inhibition of GSH-Px in kidney (Hoffman and Heinz, 1998), but also histological and functional damage in kidney as well (Magos et al., 1987; Perottoni et al., 2004a,b). Although the exact mechanism of mercuric chloride induced nephrotoxicity is not well understood, several studies suggested the involvement of free radicals. Oxidative stress develops when the disturbances between reactive oxygen forms are produced in excess and the factors preventing their harmful effect occur. It has been show in various studies that mercuric chloride administrations are associated with increased formation of free radicals, and with heavy oxidative stress. This will lead to oxidative damage cell components e.g proteins, lipids and nucleic acids (Boya et al., 1999). HgCl2 inhibits activities of antioxidant enzymes (GSH-Px, GST, and catalase) and there is depletion of cellular thiols (Song et al., 1998) in rat kidney and testes suggesting that HgCl2 toxicity results from generation of reactive oxygen species. Selenite metabolite are similar to thiols, and therefore compounds that react with thiols are expected to react also with selenols (Sheen and Ajith, 2003) may be the one mechanism to restore the activity of antioxidant. Mercuric chloride induced nephrotoxicity is associated with increased level of MDA. MDA and 4-HNE (4-hydroxy-2-nonenal) are the end products produced by the decomposition of W3 and W6 polyunsaturated fatty acids (Seppanen et al., 2004; Valko et al., 2005) due to HgCl2 administration, platinum sulphydryl group complexes formed are taken up by renal cells and stabilized by intracellular GSH for several hours, in case of intracellular GSH depletion the complexes undergo the rapid transformation to receive metabolites, this depletion seems to be the prime factor that permits lipid peroxidation and impair antioxidant enzymes (Stohs and Bagchi, 1995). Nephroprotectant by the exogenous selenite might be directly related to its antioxidant activity.
Selenite, an important exogenous source for endogenous selenium compounds, is known to be extensively biotranformed (Duque, 1991). It undergoes glutathione dependent reduction to form, consecutively, GS-Se-SG, GS-she, and hydrogen selenide (HSeH). The last is methylated sequentially, resulting in production of methylselenol (CH 3 SeH),then the volatile and expirale dimethyl selenide(CH 3 SeCH 3 ), and finalytrimethylselenonium ion (CH 3 ) 3 Se, which is excreted in urine. Selenide is also the precursor for selenocysteine and thus is needed for synthesis of selenoproteins. Additionally, selenide is also a toxic metabolite of selenite, whereas methylselenol is thonght to be involved in both toxicity and anticarcinogenic activity (Tsen and Tappel, 1958; Spallholz, 1994) of selenite.
Taking into account the results of this study, it is concluded that HgCl2 administration produces severe nephrotoxicity in rats, increase in the serum: urea, creatinine, uric acid and bilirubin levels, and activity of antioxidant enzymes and GSH level were decreased in kidney along with increase the lipid peroxidation level. Co-administration with sodium selenite, show significant modification in the activity of antioxidant enzymes. We suggest that the use of sodium selenite may offer a beneficial strategy against HgCl2 toxicity.
Список литературы Protective role of sodium selenite on mercuric chloride induced oxidative and renal stress in rats
- Aebi, H. (1984) Catalase in vitro. In: Colowick, S. P., Kaplan, N.O. (Eds.), In: Methods in Enzymology, vol. 105. Academic press, New York, pp. 121-126.
- Batra, Y., Acharya, S.K. (2003) Acute liver failure: prognostic markers. India. J. Gastroenterol. 22, 66-68.
- Block, K., Birringer, M., Jiang, W.Q., Nakahodo, T., Thompson, H.J., Toscano, P.J., Uzar, H., Zhang, X., Zhu, Z.J. (2001) Allium chemistry: synthesis, natural occurrence, biological activity, and chemistry of Se-alk(en)ylselenocysteines and their gamma-glutamyl derivatives and oxidation products. J. Agric. Food chem. 49, 458-470.
- Bradford, M.A. (1976) Rapid and sensitive method for the quantities of microgram quantities of protein utilizing the principale of protein-dye binding.Anal.biochem. 72, 248-54.
- Brugnara, C. (2000) Reticulocyte cellular indices: a new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit. Rev. Clin. Lab. Sci. 37, 93-130.
- Buege, J.A., Aust, S.D. (1984) Microsomal lipid peroxidation. Methods.enzymol. 105, 302-10.
- Cabanero, A.I., Madrid, Y., C’amra, C. (2004) Selenium and mercury bioaccessibility in fish samples: on in vitro digestion method. Anal.Chim.Acta 526, 51-61.
- Chandiene, J and Tappel, A.L. (1984) Interaction of gold(I)with the active site selenium-glutathione peroxidase. J. Inorg. Biochem. 20, 313-325.
- Chen, C.Y., Qu, L.Y., Zhao, J.J., Lin, S.P., Deng, G.L., Li,B.,Zhang, P.Q., Chai, Z.F. (2006) Accumulation of mercury, selenium and their binding proteins in porcine kidney and liver from mercury-exposed areas with the investigation of their redox responses. Sci. Tot. Environ. 366, 627-637.
- Cuvin-Aralar, M.L.A., Funess, R.W. (1991) Mercury and selenium interaction: a review. Ecotoxicol. Environ. Saf. 21, 348-364.
- Diplock, A.T., Watkims, W.J., Heurson, M. (1986) Selenium and heavy metals. Ann. Clin. Res. 18, 55-60.
- Duque, I., Garcia-Escribano, C., Rodriguez-Puyol, M., Diez-Marques, M.L. (1992) Effects of reactive oxygen species on cultured rat mesangial cells and isolated rat glomeruli.Am. J. Physiol. 263, F466-F473.
- Flohe, L., Gunzler, W.A. (1984) analysis of glutathione peroxidase. Methods enzymol. 105, 114-21.
- Fowler, B.A., Woods, J.S. (1977) Ultrastructural and Biochemical changes in renal mitochondria during chronic oral methylmercury exposure: the relationship to renal function. Exp.Mol.pathol. 27, 403-412.
- Friberg, L., Enestron, S. (1991) Toxicology of inorganic mercury. In: Dayan, A.D., Hertel, R.F., Heseltine, E., Kazantzis, G., Smith, E.M., Van der venne, M.T. (Eds.), immunotoxicity of metals and immunotoxicitology. Plenum Press, New York, PP. 163-173.
- Gailer, J., George, G.N., Pickering, I.J., Madden, S., Prince, R.C., Yu, E.Y., Denton, M.S., Aposhian, H.V. (2000) Structural basis of the autagonism between inorganic mercury and selenium in mammals. Chen. Res. Toxicol. 13, 1135-1142.
- Girardi, G., Elias, M.M. (1995) Mercuric chloride effects on rat renal redox enzymes activities: SOD protection. Free radic. Biol. Med. 18, 61-66.
- Goyer, R.A., Rhune, B.C. (1975) Toxic changes in mitochondrial membranes and mitochondrial function. In: Trump, B.F., Arstilla, A.U. (Eds.), Pathobiol. Cell Mem. Academic Press, New York.
- Habig, W.H., Pabst, Jakoby, W.B. (1974) Glutathione-S-transferase the first step in mercapturic acid formation. J. Biol. Chem. 249, 7130-9.
- Hoffman, D.J., Heinz, G.H. (1998) Effects of mercury and selenium on glutathione metabolism and oxidative stress in mallard ducks. Environ. Toxicol. Chem. 17, 161-166.
- Hussain, S., Rodgers, D., Duhart, H.A., Ali, S.F. (1997) Mercuric chloride-induced reactive oxygen species and its effect on antioxidant enzymes in different regions of rat brain. J. Environ. Sci. Health 32B, 395-409.
- Juresa, D., Blanusa, M., Kostil, K. (2005) Simultaneous administration of sodium selenite and mercuric chloride decrease efficacy of DMSA and DMPS in mercury elimination in rats. Toxicol.Lett. 155, 97-102.
- Kaplan, L.A., Pesce, A.J. (1996) Clinical chemistry-theory. Analysis and correlation. Mosby-Year Book. PP. 609-610.
- Karin, A.and Elias, A.S.J. (2003) Rapid induction of cell death by seleniul-compromised thioredoxinreductase I but not by the fully active enzyme containing selenocysteine. J. Biol. Chem. 278, 15966-15972.
- Lund, B.O., Miller, D.M, Woods, J.S. (19991) Mercury-induced H2O2 production and lipid peroxidation in vitro in rat kidney mitochondria. Biochem.Pharmacol.42, S181-S187.
- Lund, B.O., Miller, M.D., Woods, J.S. (1993) Studies on Hg (II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rakidney mitochondria. Biochem.Pharmacol. 45, 2017-2024.
- Magos, L., Clarkson, T.W., Spanow, S., Hudson, A.R. (1987) Comparison of the protection given by selenite, selenomethionine and biological selenium against the renotoxicity of mercury. Arch. Toxicol. 60, 422-426.
- Paller, M.S. (1985) Free radical scavengers in mercuric chloride-induced acute renal failure in the rat. J. lab.Clin.Med. 105, 459-463.
- Parizek, J., Ostadalova, I. (1967) The protective effect of small amounts of selenite in sublimate intoxication. Experientia 23, 142-143.
- Perottoni, J., Lobato, L.P., Silveira, A., Rocha, J.B.T., Emanuelli, T. (2004a) effects of mercury and selenite on δ-aminolevulinatedehydratase activity and on selected oxidative stress parameters in rats. Environ. Res. 95, 166-173.
- Perottoni, J., Rodrigues, O.E.D., Paixao, M.W., Zeni, G., Labato, L.P., Braga, A.L., Rocha, J.B.T., Emanuelli, T. (2004b) Renal and hepatic ALA-D activity and selected oxidative stress parameters of rats exposed to inorganic mercury and organoselenium compounds. Food Chem. Toxicol. 42, 17-28.
- Pokorny, J. (1987) Major factors affecting auto-oxidation. In: Chan, H.W.(Ed.), Autooxidation of unsaturated lipids. Academic Press. London, PP. 141-207.
- Sasakura, C., Suzuki, K.T. (1998) Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J. Inorg.Biochem. 71, 159-162.
- Schafer, F.Q. and Buetner, G.R. (2001) Redox state of the cell as viewed through the glutathione disulfide/glutathione couple. Free radical. Biol. Med. 30, 1191-1212.
- Sener, G., Sehirli, O., Tozan, A., Velioglu-ovuç, A., Gedik, N., Omnrtag, G.Z. (2007) Gingobiloba extract protects against mercury (II)-induced oxidative tissue damage in rats. food chem. Toxicol. 45, 543-550.
- Seppanen, K., Soininen, P., Salonen, J.T., Lotjonen, S., Laatikainen, R. (2004) Does mercury promote lipid peroxidation? An in vitro study concerning mercury, copper, and iron in peroxidation of low-densitylipoprotein. Biol. Trace Elem. Res. 101, 117-132.
- Sheen, N. and Ajiyh, T.A. (2003) Prevention of nephrotoxicity induced by antioxidant drug cisplatin, using ganodermalucidum, a medicinal mushroom occurring in south india. Curr. Sci. 85, 478-482.
- Sorg, O., Schilter, B., Howegger, P., Monnet-Tschudi, F. (1998) Increased vulnerability of neurons and glial cells to low concentrations of methylmercury in a prooxidant situation. Actaneuropathol. 96, 621-627.
- Spallholz, J.E. (1994) On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 17, 45-64.
- Stacey, N.H., Kappus, H. (1982) Cellular Toxicity and lipid peroxidation in response to mercury.Toxicol.Appl.pharmacol. 63, 29-35.
- Stohs, S.J., Bagchi, D. (1995) Oxidative mechanisms in the toxicity of metal-ions. Free Radic. Biol; Med. 18, 321-336.
- Strubell, O., Kremer, J., Tilse, A., Keogh, J., Pentz, R. (1996) Comparative studies on the toxiciry of mercury, cadmium, and copper toward the isolated perfused rat liver. J. Toxicol. Enviro.Health. 47, 267-283.
- Tsen, C.C, Tappel, A.L. (1958) Catalytic oxidation of glutathione and other sulfhydryl compounds by selenite. J. Biol. Chem. 233, 1230-1232.
- Valko, M., Morris, H., Cronin, M.T. (2005) Metals, Toxicity and oxidative stress. Curr. Med. Chem. 12, 1161-1208.
- Weckbercker, G., Cory, J.G. (1988) ribonucleotidereductase activity and growth of glutathione-depended mouse leukaemia L 1210 cells in vitro. Cancer let.40, 257-264.
- WHO. (1991) Environmental health criteria 118: inorganic mercury-environmental aspects. World health organization, geneva, Switzerland, PP. 115-119.
- Woods, J.S. (1989) Mechanism of metal-induced alterations of cellular heme metabolism.Comments Toxicol. 3, 3-25.
- Woods, J.S., Calas, C.A., Aichre, L.D. (1990b) Stimulation of porphyrinogen oxidation by mercuric ion. Promotion of oxidation from the interaction of mercuric ion, glutathione, and mitochondria-generated hydrogen peroxide. Mol. Pharmacol. 38, 261-266.
- Woods, J.S., Calas, C.A., Aichre, L.D., Robinson, B.H., Mailer, C. (1990a) Stimulation of porphyrinogen oxidation by mercuric ion.Evidence of Free radical formation in the presence of thiols and hydrogen peroxide. Mol. Pharmacol. 38, 253-260.
- Xia, L., Nordman, T., Olsson, J.M., Damdimopoulos, A., Bjorkhembergman, L., Nalvarte, T., Eriksson, L.C., Armer, E.S.J, Spyron, G., Bjornstedt, M. (2003) The mammalian selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J. Biol. Chem. 278, 2141-2146.
- Yee, S., Choi, B. (1994) Methylmercury poisoning induces oxidative stress in the mouse-brain. Exp. Mol. Pathol. 60, 188-196.
- Yonaha, M.S.M, Sagai, M. (1983) Induction of lipid peroxidation in rats by mercuric chloride. Lif.Scie. 32, 1507-1514.
- Yoneda, S., Suzuki, K.T. (1997) Detoxification of mercury by selenium by binding of equimolar Hg-Se complex to a specific plasma protein. Toxicol. Appl. Pharmacol. 143, 274-280.
- Zalups, P.K. (2000) Molecular interactions with mercury in the kidney. Pharmacol. Rev. 52, 113-143.
- Zolla, L., Lupid, G., Bellelli, A., Amiconi, G. (1997) Effect of mercuric ions on human erythrocytes.Relationships between hypotonic swelling and cell aggregation. Biochem. Biophys. Acta 1328, 273-280.


