Purification and Biochemical Properties of Carboxylesterase from Saga Seeds (Adenanthera pavonina)
Автор: Shafia Hoor F., Nagesh Babu R.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.17, 2021 года.
Бесплатный доступ
Carboxyl esterase (E.C.No.3.1.1.1) was partially purified from Adenanthera pavonina (Saga) using ammonium sulfate fractionation (0-60%) and DEAE (diethyl aminoethyl) ion exchange chromatography, the purified enzyme was characterized. One major saga-esterase was identified with Fold purification of 29. Molecular weight of the Ap-esterase was determined using Sephadex G-25 gel filtration and SDS-PAGE (Sodium dodecyl sulfate polyacryamide gel electrophoresis) which was found to be 26.0 k Da. Optimal activity of the saga-esterase occurred when the pH 7.0 at a temperature of 55°C. The activation energy for the hydrolysis of α-naphthyl acetate was determined to be 1.10 kcal/ mol. The Michaelis Menton constant (Km) and Vmax of the saga-esterase was 0.4µmoles and 105 IU respectively. In addition, the isoelectric point is at pH > 9 and immuno-blot using polyclonal antibodies showed that the saga-esterase was widely distributed in seeds but not in leaves. The saga-esterase inhibited by organophosphate and carbamate pesticides, which can be substituted for acetylcholinesterase.
Carboxyl esterase, Adenanthera pavonina, Organophosphate, carbamate pesticide
Короткий адрес: https://sciup.org/143173876
IDR: 143173876
Текст научной статьи Purification and Biochemical Properties of Carboxylesterase from Saga Seeds (Adenanthera pavonina)
Two kinds of lipolytic enzymes (esterases and lipases) are known as а/p -hydrolases (EC 3.1.1.X) (Ollis et al., 1992). The esterases that are active towards α-naphthyl acetate and are inhibited by organo-phosphours compounds (di-isopropyl phosphoro fluoridate, diethyl p-nitrophenyl thio-phosphate and diethyl p-nitrophenyl phosphate) are classified as Carboxylesterases (E.C.No.3.1.1.1), that hydrolyze ester linkage (Holmes and Masters, 1967). The important characteristic is that they have high degree of esterolytic heterogeneity. A carboxylesterase (CXE) or carboxylic-ester hydrolase is an enzyme that catalyzes carboxylic ester and water into alcohol and carboxylate. The esterase’s have a fixed role in plant growth and development, stomatal movement, insecticidal resistance against infection etc., Carboxyl esterases show less substrate specificity and have been studied with a wide variety of biochemical techniques such as electrophoresis and characterization based on their reaction with substrate (Veerabhadrappa and Aravinda Upadhya, 1979). Their special catalytic properties make them useful for industrial applications such as detergent industry, oleochemical industry, pulp and paper industry and resolution of chiral drugs (Kawamoto et al., 1987; Panda and Gowrishankar 2005). However, the natural substrates for themajority of carboxylesterases remain unknown, the activity being characterized using synthetic substrates, such as α or β-naphthyl esters and p-nitrophenyl esters. ed sandal wood / Saga (Adenanthera pavonina) is a species of tree belongs to the family Leguminosae, subfamily Mimosoideae, used for its timber. This tree is Native to India and southern China, but now found throughout the tropics (Corner, 1997). Nutritional studies have shown one quarter of the seed weight to be oil with a high percentage of protein, and a fatty acid composition favouring high digestibility for both humans and livestock (Balogun and Fetuga, 1985). They are also used for medicinal purpose; studies show that bark extract of this tree can reduce Blood pressure in ats (Aduragbenro et al., 2009). Use of esterases as marker enzymes in genetic and population variation studies has promoted many biochemists to purify and study them. In the present study, purification, characterization and properties of novel carboxylesterase isolated from the saga seeds of is described.
MATERIALS AND METHODS
Enzyme extraction:
The seeds of Adenanthera pavonina were collected from Bangalore, Karnataka, India. The soaked seeds were defatted by acetone and 10% crude enzyme was extracted with 0.05M phosphate buffer of pH 7.0 by constant stirring for 45 minutes at 4°C. The extract was then centrifuged at 8,000 rpm for 10 minutes at 4°C. The supernatant was collected as crude enzyme extract for enzyme assay and further purification.
Enzyme Assay:
Esterase activity was assayed according to the method of Gomori as modified by Van Asperen (Van Asperen, 1962). One unit of enzyme activity was defined as the amount of enzyme that released 1-1 mol of product per min at pH 7.0 and 27 °C. Protein concentration was determined according to the method of Lowry et al. (Gomori, 1953), using bovine serum albumin (BSA) as standard.
Enzyme purification:
The crude extract was precipitated by using ammonium sulfate fractionation (0-60%). The mixture was kept at 4°C for 1 hour and the precipitated protein was collected by centrifugation at 10,000 rpm for 20 min. The pellet was dissolved in phosphate buffer of pH 7.0 and dialyzed. The dialyzed sample was loaded to an anion exchanger DEAE column. The column was equilibrated with Tris pH 8.0. The Bound proteins were eluted using 0.6M NaCl in same buffer at a flow rate of 1 ml min-1. 50 fractions of 1.5ml each were collected; the presence of protein was monitored by measuring absorbance at 280 nm. Fractions were examined for esterase activity using α-naphthyl acetate. The Molecular weight (Mr) of the purified enzyme was determined using Sephadex G-25 gel filtration column, equilibrated with Tris pH 8.0 and the enzyme was eluted using same buffer with a flow rate of ~ 12ml/h.
Native PAGE and activity staining:
Enzyme purity were monitored by Native-PAGE with Coomassive Brillant Blue staining. Non-denaturing polyacrylamide gel electrophoresis was performed at
4°C by using 5% stacking gel and a 12% separating gel. Esterase activity on polyacrylamide gels was detected by the method of Hunter and Markert (1957). The gels were stained for esterase activity after electrophoresis in a solution containing 50 mL of 0.05 M sodium phosphate buffer, pH 7.0, 20 mg of Fast blue and 10 mg of α-naphthyl acetate.
SDS-PAGE :
SDS-PAGE was performed using a modification of the method of Laemmli (1970). For the stacking and separating gels, the acrylamide content was 6.5% and 12%, respectively. A volume of 20 μL was loaded onto each lane. The protein bands were observed by staining Coomassie brilliant Blue 250. For determination of pI IEF 3-9 PhastSystem gels were used. The purified target proteins were verified via Western blot analysis with polyclonal antibody.
Preparation of anti-Ap-Esterase antiserum:
Purified saga-esterase was used to prepare anti-saga-esterase immune sera. abbits were injected four times with 25 g of antigen mixed with an equal volume of adjuvant (monophosphoryl lipid A and trehalose dicorynomycolate; Sigma) at days 1, 15, 30, and 60. Sera were collected at various time points after the last injection, and the specificity of the antibodies was tested by Western blotting.
Electrophoresis and immunoblotting :
Equal amounts of protein (10 or 20 g) were then separated by 12% SDS-PAGE and transferred onto a Protran nitrocellulose transfer membrane. The membranes were then saturated with 1% bovine serum albumin (BSA) in PBS, 0.1% Tween 20 and probed by incubation overnight with either rat antiserum raised against saga-esterase at a 1/2,000 dilution or rabbit anti-saga-esterase immune sera After extensive washing, the membranes were then incubated with anti-rat or antirabbit immunoglobulin G (IgG) conjugated to alkaline phosphatase, respectively.
Inhibitor Specificity:
The gels were preincubated with 1×6 10-6 M solution of organo-phosphates and carbamates inhibitors for 30 min, washed with 0.05 M sodium phosphate buffer, pH 7.0 and stained for esterase activity
Effect of substrate concentration on esterase activity:
A substrate saturation curve was obtained by plotting the substrate concentration versus esterase activity in the presence of α-naphthyl acetate as substrate. The Michaelis-Menten constant (Km) and the maximum velocity of the reaction (Vmax) were calculated from Lineweaver-Burk plot (Lineweaver, Burk, 1934).
Effects of pH and temperature on enzyme activity:
Esterase activity was measured at different pH from 2 to 9, the activity was expressed in terms of µmoles/minute and plotted to find out optimum pH. Esterolytic activity, as a function of temperature was determined in a temperature range of 15-97°C, from which Arrhenius plot was constructed to find out Activation energy.
Determination of kinetic parameters: The Michaelis constant (Km) and the maximum reaction velocity (Vmax) of the saga-esterase was determined at different substrate concentrations. They were evaluated by plotting the data on a Lineweaver-Burke doublereciprocal graph (1/Vo) versus (1/[S]) (Lineweaver, Burk, 1934).
RESULTS AND DISCUSSION
Carboxylesterases were purified and characterized from different sources including plants, animals, and micro-organisms. The saga esterase, although not exhaustively analyzed, falls into a broad class of esterases that hydrolyse maximally shorter chain acyl compounds. In the present study, the purified esterase was obtained by ammonium sulfate saturation and ion exchange chromatography. Preliminary studies of the carboxylesterase obtained from saga seeds indicated the presence of one major esterase by activity staining. The three-step enzyme purification is summarized in Table 1. Considerable loss of saga-esterase activity was observed by pH and acetone precipitation. On the other hand, ammonium sulphate fractionation showed significantly better yield and increased fold purification. The ammonium sulphate fraction was subjected to DEAE ion-exchange chromatography using CM-cellulose. The elution profile of results single peak, the enzyme was purified 29-fold and the specific activity was found to be 0.29 IU/mg protein using DEAE column Fig
1 A. The pure enzyme showed a single band on native PAGE with activity staining (Figure 1 B). The Molecular weight of the saga esterase was estimated to be around 26.0 k Da by SDS-PAGE and Sephadex G-25 (Figure1 C and Fig 2 A). Most of the carboxylesterases isolated and purified from animal and insect sources were reported to possess high molecular weight and were oligomeric in nature consisting of two or more subunits. The isolelectric pH values of purified saga-esterase was 8.5 and they showed binding affinity to an anion exchanger at pH 8.3. The basic isoelectric pH values and binding affinity indicates the presence of large proportions of acidic amino acids. Our results were an agreement with previously reported carboxylesterases isolated from plants and animals have low isoelectric pH values and contain large proportions of acidic amino acids. The purification of carboxylesterases in most of the cases involved the use of anion exchangers (Upadhya et al., 1985; Govindappa et al., 1987; Sreerama et al., 1991). An optimum pH 7.0 was obtained for saga esterase Fig. 2 B. and was stable between pH 4.0-9.0. The optimum pH obtained for most of the purified carboxylesterases range from pH 7 to 9. Among plant carboxylesterases, an optimum pH of 7.0 was obtained for sorghum and barley (Sae et al., 1971; Burger et al., 1970) and 7.5 for finger millet and S. grantii (Upadhya et al., 1985; Govindappa et al., 1987; Sreerama et al., 1991). The saga-esterase, was found to be optimally active at 45 and 37°C Fig. 2 C. Though the enzyme was completely active at 60°C, the energy of activation of the enzyme was 1.10 kcal/mol for hydrolysis of α-naphthyl acetate. The optimum temperature obtained for other plant carboxylesterases such as barley, Finger millet, S. grantii and Cucurbita pepo was also in the above range (Burger et al., 1970; Upadhya et al., 1985; Govindappa et al., 1987; Sreerama et al., 1991). Km and Vmax values of the esterase from saga-esterase were determined from the linear regression analysis of 1/V versus1/[S]. Using α-naphthyl acetate as substrate at varying concentrations, Km and Vmax values were calculated to be 0.4 µmoles and 105 IU respectively Figure 1 D. The analysis of Km showed more affinity towards than naphthyl acetate naphthyl propionate. The purified fractions of bean, pea, finger millet and S. grantii carboxylesterases also showed similar types of substrate specificities exhibiting preferential action towards acetate esters (Burger et al., 1970; Upadhya et al., 1985; Govindappa et al., 1987; Sreerama et al., 1991). To determine the sensitivity and specificity of saga-esterase on pesticides, organophosphorus and carbamate (at concentrations of 2×10-3 ~2×10-9mol-1), the inhibition curve shown in the Figure. 2 D. Single sigmoid curves were obtained with both the organophosphate inhibitors tested. The results showed that both organophosphorus and carbamate pesticides can inhibit purified saga esterase on dose dependent manner. At the same pesticide concentration, the inhibition rate of purified saga-esterase was found to be significantly higher than that of the crude. Carboxylesterases (EC 3.1.1.1), (Carboxyl ester hydrolase), are inhibited by OPs and prefer aliphatic esters, generally of longer fatty acid chain than acetate. The esterases were inhibited only by OPs and hence were classified as carboxylesterases. Similar observations were noticed in case of insect carboxylesterases (Govindappa et al., 1987; Sreerama et al., 1991). and plant carboxylesterases (Burger et al., 1970).
Table-1 : Purification of Saga carboxylesterases from Adenanthera pavonina
|
Sample |
Total volume (ml) |
Total activity (mkmol/min) |
Total soluble protein (mg) |
Specific activity (IU/mg) |
Fold purification |
% Yield |
|
Crude |
57 |
21.3 |
3207 |
0.00664 |
1 |
100 |
|
0-60% (NH 4 ) 2 SO 4 saturation (precipitate) |
7 |
20.4 |
1953 |
0.01 |
1.51 |
95.7 |
|
DEAE ion exchange (flow through 0.6M NaCl |
4 |
0.45 |
1.529 |
0.29 |
29 |
2.11 |
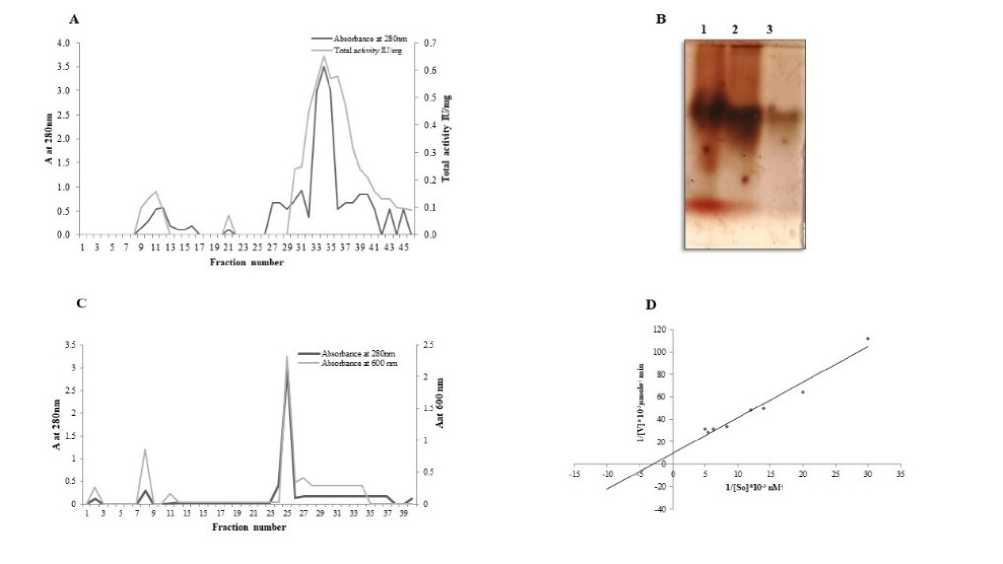
Figure 1: A. Elution pattern of carboxylesterase from DEAE ion exchange chromatography using 0.6 M NaCl. B. Native
PAGE activity staining: lane 1, crude enzyme extract; line 2, esterase purified by ammonium sulfate; lane 3: esterase purified by DEAE Ion exchange chromatography. C. Elution pattern of carboxylesterase from Sephadex G-25 gel filtration chromatography using Tris pH 8.3 D. km and Vmax determination of saga-esterase with 1-naphthyl acetate as substrate
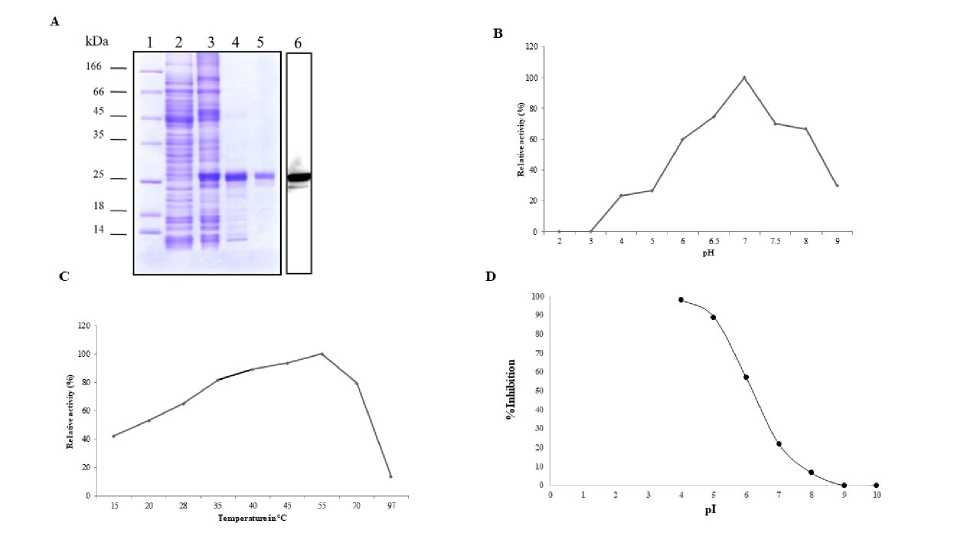
Figure 2: A. SDS–PAGE pattern of Lane-1 standard proteins Lane-2 Crude Enzyme extract Lane-3 Ammonium sulphate fractionated Lane -4 DEAE Bound fraction Lane -4 Sephadex G-25 Gel permeation Chromatography eluted fraction and Lane-6 Immunoblot of saga esterase conjugated with anti-saga-esterase antibody B. Activity of saga-esterase at different pH. C. Activity of saga-esterase at different temperatures. D. Inhibition curves for the hydrolysis of a-naphthyl esters saga-esterase in the presence of organophosphate (Dichlorvos)
In addition, the marked inhibition by organophosphates and carbamates represents our investigations revealed the interesting characteristics of saga esterase viz, low molecular weight, high stability and high affinity towards short chain naphthyl which exhibits pivotal role in the hydrolysis of short chain fatty acid esters during plant growth and development and used as an indicator of excessive pesticide residues in samples.
CONCLUSION
In this study, the carboxylesterases from saga was identified and characterized. Ap esterase is classified as a carboxylesterase by substrate and inhibitor specificity. The Ap esterase has the optimal catalytic activity at pH 7.0 at a temperature of 55°C and the activation energy found to 1.10 kcal/ mol. The Molecular eight and isoelectric pH is 63.0 k Da and 9 respectively. The Ap esterase activity can be inhibited by organophosphates and carbamate pesticides. This suggests Ap esterase as a new enzyme source belongs to the type B esterase group, which can be substituted for AChE to detect organophosphorus and carbamate by enzyme inhibition.
ACKNOWLEDGMENTS
Список литературы Purification and Biochemical Properties of Carboxylesterase from Saga Seeds (Adenanthera pavonina)
- Aduragbenro D. A. Adedapo, Yeside O. Osude, Adeolu A. Adedapo J. Olanrewaju Moody, Ayotunde S. Adeagbo Olumayokun, A. Olajide and Janet M. Makinde. (2009). Blood Pressure Lowering Effect of Adenanthera pavonina Seed Extract on Normotensive Rats: Rec. Nat. Prod. 3:2 82-89
- Balogun, A.M. and B.L. Fetuga. (1985). Fatty acid composition of seed oils of some members of the Leguminosae Family. Food Chemistry, 17(3): 175-82.
- Burger, W.C., Prentice, Neville., Moeller, Mary. (1970). Peptide hydrolase C in germinating barley. Plant Physiol. 46, 860-861.
- Corner E. J. H., (1997) Wayside Trees of Malaya: Vol I, Malayan Nature Society, 4th ed., Gomori, G. (1953). Human esterases. J. Lab. Clin. Med. 42 (3), 445–453
- Govindappa, T., Govardhan, L., Jyothy, P.S., Veerabhadrappa, P.S., (1987). Purification and characterisation of a carboxylesterase from the latex of Synadenium grantii Hook, ‘f’. J. Biosci. 12 (1), 71-86
- Holmes, R.S. and Masters, C.J. (1967). The developmental multiplicity and isoenzyme status of cavian esterases. Biochim. Biophys. Acta. 132: 379-399.
- Hunter, R. L., & Markert, C. L. (1957). Histochemical demonstration of enzymes separated by zone electrophoresis in starch gels. Science, 125(3261), 1294-1295.
- Kawamoto T, Sonomoto K, Tanaka A. (1987) Esterification in organic solvents: Selection of hydrolases and effects of reaction conditions. Biocatalysis, 1: 137-145.
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680-685.
- Lineweaver H, Burk D. (1934) The determination of enzyme dissociation constants. J Am Chem Soc; 56: 658-666
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., (1951). Protein measurement with the Folin phenol reagent’’. J. Biol. Chem. 193(1), 265–275.
- Ollis DL, Shea E, Cygler MB, Dijkstra B, Frolow F. (1992) The α/β hydrolase fold. Protein Eng, 5: 197-211.
- Panda T, Gowrishankar BS. (2005) Production and applications of esterases. Appl Microbiol Biot, 67: 160-169.
- Sae SW, Kadoum AM, Cunningham BA (1971) Purification and some properties of Sorghum grain esterase and peroxidase. Phytochemistry 10: 1–8.
- Sreerama, L., Veerabhadrappa, Patnagere.S., (1991). Purification and properties of carboxylesterases from the mid-gut of the termite Odentotermes horni. W. Insect Biochem. 21 (8), 833-844
- Upadhya, G., Govardhan, L., Veerabhadrappa, P. S., (1985). Purification and properties of a carboxylesterase from germinated finger millet (Eleusine coracana Gaertn.). J. Biosci., 7(3 and 4), 289-301.
- Van Asperen, K. (1962) A study of housefly esterases by means of a sensitive colorimetric Method, J. Insect Physiol. 8: 401 - 416.
- Veerabhadrappa, P. S., & Upadhya, G. A. (1979). Changes in soluble esterases during germination of ragi (Eleusine coracana Gaertn.). Indian Journal of Experimental Biology, 17(7), 640-643.


