Purification, characterization and partial cDNA cloning of hightemperature stress-induced protein from French bean ( Phaseolus vulgaris)
Автор: Nagesh Babu R, Balaji K.N., Devaraj V.R.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.9, 2013 года.
Бесплатный доступ
In order to identify the components of high temperature response in French bean, three heat shock proteins induced under high temperature were purified to homogeneity by Carboxy methyl cellulose and sephadex G-100 chromatography followed by preparative SDS-PAGE. Two of these, Hsp1 and Hsp3 were further characterized by immuno-detection with polyclonal antibodies. Hsp3 exhibited ATPase and chaperone activity with malate dehydrogenase and citrate synthase. Partial cDNA for Hsp3 synthesized using the primer derived from amino-terminal sequence was cloned and expressed in Escherichia coli. The recombinant protein possesses ATPase activity, and showed thermal protection at 50°C in Escherichia coli. The translated partial cDNA showed homology with stress induced proteins including ATPases from higher plants. These results supported the fact that French bean response to high temperature stress involves Hsps as one of the principal components.
Cdna, french bean, hts, hsps
Короткий адрес: https://sciup.org/14323725
IDR: 14323725
Текст научной статьи Purification, characterization and partial cDNA cloning of hightemperature stress-induced protein from French bean ( Phaseolus vulgaris)
Temperatures above optimum are sensed as heat stress by all organisms. High temperature stress (HTS) disturbs cellular homeostasis and can lead to severe retardation of growth and development, and even death (Sachin et al., 2007, Jagadish et al., 2010). Thus, HTS is a serious threat to crop production worldwide (Hall, 2001 and Guy,
-
2008 ). Most crops are affected by daily fluctuations in high day and/or night temperatures. Rising temperatures may lead to altered geographical distribution and growing season of agricultural crops by allowing the threshold temperature for the start of the season and crop maturity to reach earlier (Porter, 2005). While some stages of plant
growth may be more sensitive to high temperature than others, there is an overall reduction in plant performance at temperatures higher than the optimal at specific stages (Singh, Grover, 2008) . Plants mount resistance to heat stress by eliciting specific metabolic adjustments. The temperature for the induction of heat stress response varies amongst different plant species. But an increase of 5-10°C over and above the ambient temperature is generally sufficient to elicit the heat stress response. Hsps are one of the major components of abiotic stress response, and have been reported from large number of plants. Plant species which are extensively analyzed for Hsps include Arabidopsis , Maize , Tomato , Rice and Wheat (Singh et al., 2008). Excluding sHsp family, role of the many other Hsps/chaperones in plant abiotic stress tolerance is rather limited (Sachin et al., 2007, Huang et al., 2008). This is despite the fact that Hsps/chaperone are expressed in plant not only under HTS, but also in response to a wide range of other environmental insults, such as; water, salinity, cold and oxidative stress (Veirling, 1991). Nevertheless, Hsps/chaperones’ role in protecting plants against stress and reestablishment of cellular homeostasis have been supported by experimental data in plants and other organisms (Sachin et al.,2007). During stress, many enzymes and structural proteins undergo deleterious structural and functional changes. Therefore, maintenance of proteins in their functional conformations, prevention of aggregation to non-native forms, refolding of denatured proteins to regain their functional conformation and removal of nonfunctional and potentially harmful polypeptides are major tasks of stressed plants (Seki et al., 2002). An increasing number of reports show that HTS in plants is accompanied by changes in the expression
of number of genes, and functional proteins. The expression profiles of 7,000 genes from the model plant, A. thaliana were determined under drought stress, wherein 277 up-regulated and 79 down-regulated genes were identified (Seki et al., 2002). The products of these genes not only function in stress tolerance, but also in regulation of gene expression and signal transduction during stress response (Shinozaki et al., 2006). Because of their importance in crop protection, these traits have been extensively investigated in recent years. Although there are no economically viable technologies to facilitate crop production under abiotic stress, a great deal of progress has been achieved in the production of plants tolerant to environmental stresses (Jagadish et al., 2010).
French bean ( Phaseolus vulgaris ), an important food crop in tropical countries is constantly exposed to abiotic stress. The crop has shown tremendous ability to respond to HTS (Nageshbabu et al., 2008). Vidal and others (Vidal et al., 1993) have detected transcripts of sHsps from French bean under HTS. A mitochondrial Hsp70, with demonstrated molecular chaperone activity has been isolated and characterized (Green, 1993). In the light of remarkable stress response exhibited by French bean, and lack of reports pertinent to role of high molecular weight Hsps, the present investigation was initiated. This paper attempts to understand the molecular basis of HTS response in French bean.
MATERIALS AND METHODS
Plant material and Temperature stress
Seeds of French bean (Phaseolus vulgaris) (S-9 genotype) were surface sterilized with 0.1% of mercuric chloride (30 s) and washed immediately with large volume of sterile distilled water. Seeds were sown in plastic trays containing vermiculite and irrigated daily. Six days old seedlings were grown in a controlled chamber at 25°C, HR 70%, and 16 h light: 8 h dark photoperiod. High-temperature stress was applied by induction treatment at 38-39°C for 2 h followed by exposure to 45-48°C for 8 h. After stress treatment, the whole plant material was used for studies. Protein concentration was determined according to the method (Bradford, 1976) using bovine serum albumin (BSA) as standard.
Protein purification
All steps were performed at 4°C. Seedlings of French bean (Phaseolus vulgaris S-9), both control and stressed (150 g) were homogenized in 800 ml ice cold sodium phosphate buffer (50 mM, pH 7.5), containing 1 mM EDTA and 50 mM β-mercaptoethanol. Insoluble debris were removed by centrifugation (20,000×g for 20 min), and supernatant was subjected to (0-80%) ammonium sulfate fractionation. After centrifugation (20,000×g, 20 min) the precipitated protein was recovered in 50 mM sodium acetate buffer pH 5.5 (Buffer A) and dialyzed against the same buffer. The dialyzed samples (40 ml) were loaded on to a CM-Cellulose (1.5×30 cm), equilibrated with sodium acetate buffer (50 mM, pH 5.5). A linear gradient of 0-0.6 M NaCl in sodium acetate buffer pH 5.5 (16 ml/h) was used for elution. Active fractions from CM-Cellulose chromatography were pooled and subjected to gel-filtration on Sephadex G-100 (1Ф75 cm) column equilibrated with 50 mM sodium phosphate (pH 7.0). The column was developed with equilibration buffer. Pooled active fractions from Sephadex G-100 chromatography were subjected to preparative SDS-PAGE according to the method (Laemmli, 1970). Protein bands corresponding to differentially expressed proteins in stressed samples were excised and eluted using electro eluter. The proteins so obtained were checked for homogeneity by SDS-PAGE, and further characterized.
Production of antibodies
Two New Zealand rabbits weighing 5 kg were injected with 300 μg of purified Hsp1 (150 k Da) and Hsp3 (56 k Da) in Freund’s complete adjuvant subcutaneously. Subsequently, two booster injections of 150 μg each were given after every 15 days from previous injection. Eight days after each booster injection, rabbits were bled by ear vein puncturing. Blood was kept at room temperature for 3 h, and transferred to 4°C for 16 h. The serum was centrifuged for 15 min at 4500×g, and stored at -20°C. Anti-Hsp1 and -Hsp3 were purified by protein-A affinity column (Bangalore Genei) according to manufactures, instructions.
Purification of Hsps by affinity chromatography
Both Hsp1 and Hsp3 antibodies were immobilized on CNBr activated Sepharose CL-6B separately, and nonspecific sites were blocked by stirring with 2.0 M ethanolamine (pH 7.5) for 4 h at 4°C, the slurry was filtered and washed with excess of ice-cold 0.1 M sodium citrate buffer (pH 6.5). Beads were washed with PBS and coupled matrix was evaluated for its ligand capacity using different amount of antigens. 0-80% ammonium sulfate fractionate from heat stressed seedlings were subjected to affinity chromatography (2.0 ml). Bound Hsps were eluted with PBS containing 0.1% SDS. The preparations were checked for homogeneity and specificity by SDS-PAGE and Western blotting, respectively.
Western analysis
Affinity purified protein samples were separated on a 12% SDS-PAGE (13), and electro transferred onto PVDF membrane on a semi dry blotting unit.
The membrane was blocked with 5% defatted milk in TBS buffer (200 mM Tris-HCl, pH 8.0, 1.5 M NaCl), incubated for 1 h with a 1:500 diluted anti-Hsp3 antiserum (in 1x TBS) at 37 °C, and washed 3 times with TBST (TBS with 1% Tween-20) buffer. The peroxidase-conjugated anti-rabbit Ig G (Jackson Immunological, USA), were used at a dilution of 1:5,000 (v/v). The antigen-antibody complexes were detected using the enhanced chemiluminescence detection system (ECL Western Blot Detection Kit, Perkin Elmer).
N-terminal sequencing of purified Hsp3
Purified Hsp3 separated on SDS-PAGE was electro-blotted onto PVDF membrane (0.45 µm pore size, Millipore, Bedford, USA) in 10 mM CAPS buffer in 10% methanol (pH 8.3) using semi-dry transfer apparatus according to manufactures instructions. The Ponceau-S stained band on the PVDF membrane was excised and processed for N-terminal analysis in an automated amino acid sequencer (Shimadzu, Japan) equipped with C 18 column.
Prevention of thermal aggregation of malic dehydrogenase (MDH) and Citrate synthase (CS) by Hsp3
The aggregation of either MDH or CS upon heat denaturation was determined by measuring the apparent absorption due to light scattering at 360 nm in a Shimadzu UV-1600 PC UV-visible spectrophotometer equipped with electronically thermo-controlled cell holders (CPS-240A). Either malic dehydrogenase or CS was mixed at 25°C with varying amounts of Hsp3 in 50 mM HEPES-KOH, pH 7.5 (1 ml) in a stoppered quartz cell with a 10 mm light-path length. Where indicated, BSA, ribonuclease or v-crystallin was added instead of Hsp3 at the stated concentrations. The cells were incubated at 45°C and the light scattering was recorded continuously for 600 s.
Reactivation (refolding) of citrate synthase (CS)
Thermal inactivation of citrate synhtase was carried out at 47°C for 30 min and chemical denaturation of CS was carried out in 8 M guanidine hydrochloride. Reactivation was initiated by 100 fold dilution in 100 mM Tris-HCl (pH 8.0) and 10 mM KCl (Sere et al.,1963) in presence of 50 μg of Hsp3 . 25 µl aliquots were assayed for citrate synthase activity (Lee et al.,1995).
Activity of ATPase
ATPase activity was measured in terms of free inorganic phosphate released from ATP according to (Chan et al.,1986). The reaction mixture containing 10 mM MOPS buffer (pH 6.5), 2 mM ATP, 1 mM MgCl 2 , and purified Hsp3 protein (0.8 μg) was incubated at room temperature for 30 min. The reaction was stopped by adding dye solution (water: 0.081% malachite green: 5.7% ammonium molybdate in 6 N HCl: 2.3% polyvinyl alcohol in 2:2:1:1, v/v). The absorbance at 620 nm was measured after the addition of sodium citrate.
Cloning and expression of French bean Hsp3
Cell viability
In order to measure cell viability under high temperatures, exponentially growing E. coli cells transformed with Hsp3 or control were cultured with 1 mM IPTG for 8 h at 50°C. Aliquots (100 µl) of the cultures were drawn at 1, 2, 3 and 4 h after their transfer to 50°C, and serial dilutions were plated on LB agar containing ampicillin. Their viability was determined by counting the colony forming units (CFU) after the plates were incubated overnight at 37°C. These counts were plotted as the percentage of CFUs formed after heat treatment relative to the number of CFUs formed in equivalent, untreated cultures.
RESULTS
Purification and characterization Hsp1 and Hsp3 induced by HTS
Comparison of protein band patterns of 0-80% ammonium sulphate fractionates from high temperature stressed and control seedlings indicated the presence of five new bands corresponding to apparent molecular masses of 105, 66, 56, 43 and 18 k Da (Figure1.A) in stressed samples. These bands persisted up to 72 h after heat shock treatment. Three of the five new bands were recovered from CM-Cellulose elution peaks (Figure1.B). The CM-Cellulose purified fractions contained proteins of 105 (Hsp 1), 66 (Hsp 2) and 56 (Hsp 3) k Da. The fractions from CM-Cellulose ion exchange chromatography were resolved into individual proteins by preparative SDS-PAGE. Hsp 2 was heterogeneous as judged by silver staining, therefore was not processed further. The other two proteins Hsp1 and Hsp3 were homogenous as judged by SDS-PAGE and silver staining (data not shown). The purified proteins were used as antigens, and the antibodies, raised against Hsp1 and Hsp3 were used for affinity chromatographic purification. Both, Hsp1 and Hsp3 exhibited good immunogenicity (Figure 1.C) as 2 µg of antigen concentration and were detected by 1:2000 dilution of antiserum (by ELISA). The purified antibodies coupled to CNBr-activated sepharose 6-LB also exhibited better binding to antigens. Each ml of affinity matrix bound ~0.8 μg of antigen (Hsp1 and Hsp3) (Figure 1.D). N-terminal sequence of Hsp3 was found to be GKGGNFGAL. This sequence on searching for homologues, matched with stretch of seven amino acids (GKGGNFG) from a fungal ATPase (XP-569263 ) and kinase (NP-001059761) from rice plants.
Suppression of thermal aggregation of MDH and CS by Hsp3
Biochemical properties of Hsp3 were investigated further to evaluate its probable role in stress response. The chaperone activity was measured in terms of inhibition of aggregation rate of the model substrates, malate dehydrogenase (MDH) or citrate synthase (CS) by light scattering experiments. When MDH or CS was heated to 45°C in the absence of Hsp3, the rate of aggregation increased during thermal stress. In contrast, when these model substrates were heated in the presence of Hsp3, aggregation rates were reduced in a dose dependent manner. Over a period of 600 s, only 57% of MDH and 65% CS aggregations were observed in presence of Hsp3 (Figure 2.A and B) . These results indicated that Hsp3 is a strong molecular chaperone that can reduce the heat induced aggregation of substrates and is effective even at a low concentration. The purified Hsp3 and rHsp3 exhibited a significant ATPase activity (Figure 2. C) .
Protection of CS activity by Hsp3
The ability of Hsp3 to protect CS activity and its potential to reactivate CS were examined in-vitro . CS was assayed over 60 min at 45°C without Hsp3.
The activity of CS decreased to 70% compared to control. In the presence of Hsp3, a 3-fold protection of CS activity was observed during HTS stress (Figure 2. D)
Expression and characterization of recombinant French bean Hsp3
Degenerate oligonucleotide derived from the N-terminal amino acid sequence (GKGGNFGAL) of Hsp3 from French bean efficiently performed primer function in RT-PCR experiments and yielded a 1050 bp cDNA fragment. The PCR product was sequenced and deposited in the Gene Bank database (DQ862779). Sequence analysis of the cDNA showed that there were two open reading frames of 600 and 450 bps. The PCR product (600 bp) was successfully cloned at EcoR1 site of pGEM®-T Easy Vector. Sequencing of the clone suggested a molecular mass of 29 k Da. The molecular mass that differed from the apparent mass of Hsp3 (56 k Da), indicated the partial nature of cDNA. Analysis of the deduced amino acid sequence of the partial cDNA revealed that it contained sequences identical to the N-terminal sequence of the purified Hsp3. The cDNA corresponding to the Hsp3 when sub-cloned into pET32A and expressed in E, coli, yielded a His-tagged fusion protein. The fusion protein purified by Ni-NTA affinity column exhibited a molecular mass of 35 k Da, which included a mass of 13 k Da due to thrombin (Figure 3.A) . Immunodetection of recombinant Hsp3 with anti Hsp3 antibody showed a single immunoreactive band corresponding to an apparent molecular mass of 35 k Da. Blots derived from induced control vector did not show any reactivity with anti Hsp3 antibodies (Figure 3.B) .
In-vivo chaperone activity of French bean Hsp3
To characterize the chaperone function of Hsp3 in vivo , we conducted thermotolerance assays.
Serially diluted bacterial cells harboring either exhibited better growth than those harboring the empty plasmid or Hsp3 plasmid were exposed to empty plasmid (Figure 3.C and D). This result
25°C to 50°C on agar plates. When cells were exposed to an extreme temperature of 50°C, cells harboring recombinant plasmid containing Hsp3
indicated that Hsp3 confers thermotolerance to bacterial cells over a period of 1 h.
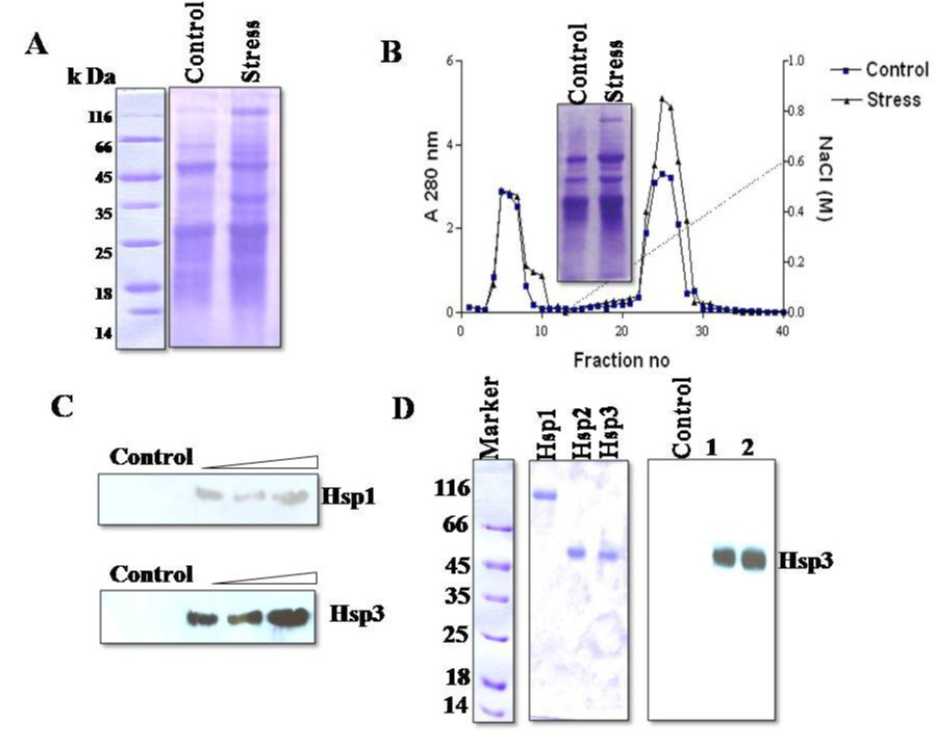
Figure 1. A . SDS-PAGE pattern of soluble proteins from control and temperature stressed French bean seedlings; 20 μg proteins each from control and stressed samples were resolved on SDS-PAGE (12%) and stained with Coomassie Brilliant blue R-250. Arrows indicate major proteins over-expressed under heat shock.
B . CM Cellulose cation exchange chromatography of ammonium sulfate fractionates from control and heat stressed French bean seedlings; 2.0 mg each of fractionates from control and stressed seedlings were loaded onto 50 ml column and eluted with a linear gradient of 0-0.6M NaCl. Fractions of 4 ml each were analyzed for Hsps and protein content, respectively, by SDS-PAGE and absorption at 280 nm.
C . Immuno detection of Hsp-1 and Hsp-3. 10, 15, and 20 μg each of affinity purified Hsps and 10 μg of ammonium sulphate fractionate from control were resolved on SDS–PAGE transferred on to PVDF membrane and detected using specific polyclonal antibodies.
D. SDS-PAGE patterns of affinity purified Hsp1 and Hsp3 proteins (10 μg each). Panel-1; Proteins were stained with Coomassie Brilliant blue R-250. Panel-2; Full length gel was blotted and proteins were detected with ani-Hsp3 antibody.
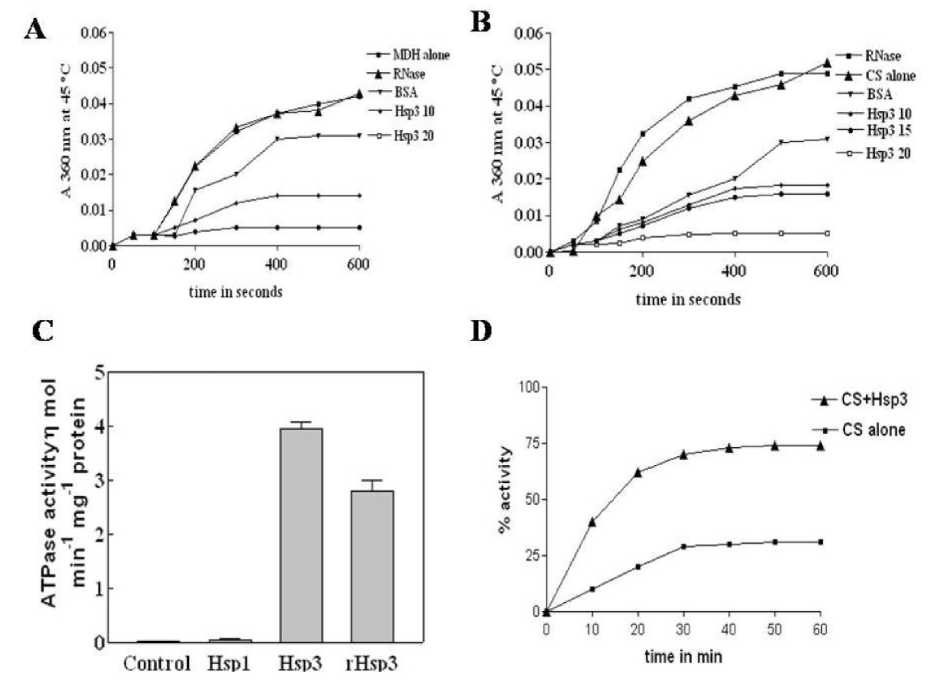
Figure 2. A . Prevention of aggregation of malic dehydrogenase (MDH) at 45°C by Hsp3. MDH was mixed in the absence or presence of various concentrations of Hsp3 at 25°C and shifted to 45°C for 10 min.
Relative light scattering was measured for 600 s at 360 nm. For comparison ribo-nuclease (RNase) or BSA was used instead of Hsp3.
B . Prevention of aggregation of citrate synthase (CS) at 45°C by Hsp3. CS was mixed in the absence or presence of various concentrations of Hsp3 at 25°C and shifted to 45°C for 10 min. Relative light scattering indicative of substrate aggregation was measured as the apparent absorbance at 360 nm. For comparison ribo-nuclease or BSA was used instead of Hsp3.
C . Protection of CS activity by Hsp3. Heat denatured CS was incubated either in the presence or absence of Hsp3, extent of reactivation was expressed as % CS activity for 60 min.
D . ATPase activity in ammonium sulfate fractionates of control seedlings and affinity purified Hsp1 and Hsp3 and recombinant Hsp3.
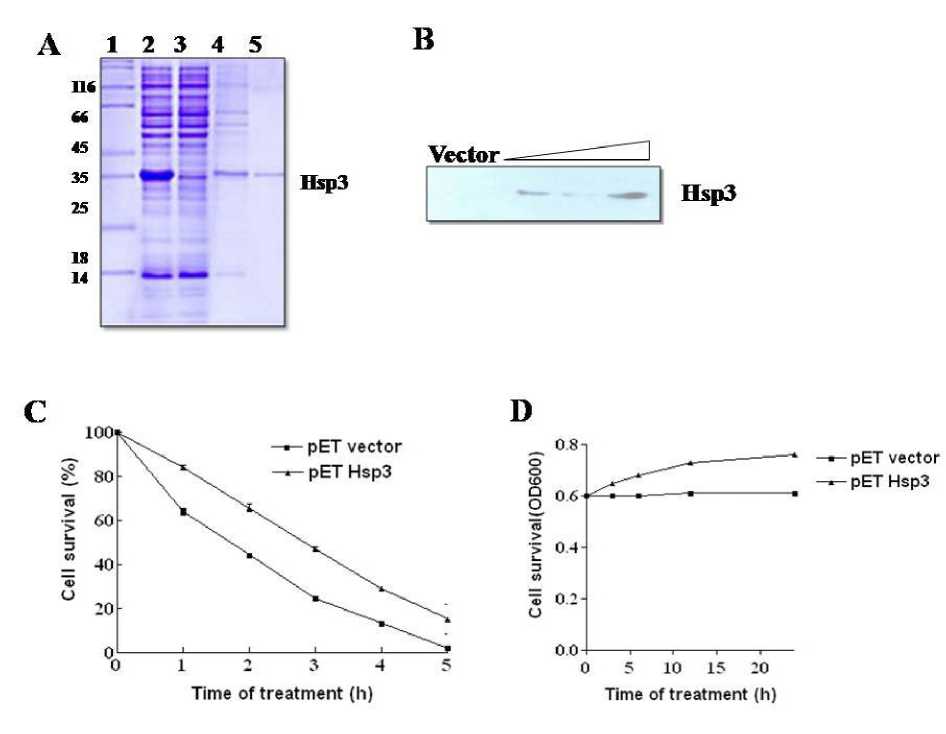
Figure 3. A . Affinity purification of recombinant Hsp3 by Ni2+-sepharose chromatography; Different fractions were separated on 12% SDS-PAGE, and stained with Coomassie Brilliant Blue. Lane-1 molecular weight markers, Lane-2; induced cell lysate, Lane-3; flow through, Lane 4 and 5; elution 1 and 2.
B . Immuno-detection of recombinant Hsp3; 10 μg of recombinant protein along with vector control was separated on SDS-PAGE (12%), and blotted onto PVDF membrane and detected by ECL.
C . Viability of E. coli expressing Hsp3 at 50 °C; Cells carrying Hsp3 or pET32A were incubated at 50 °C for 5 h. Per cent survival of the heat-treated cells in terms of colony forming unit relative to control (pET32A) was plotted against time of treatment.
D . In-vivo thermo-tolerance/chaperone activity of rHsp3. E. coli transformed with control vector and recombinant plasmids were incubated at 37°C for 2 h after addition of IPTG. After induction, cells were transferred directly to 50°C and incubated for 24 h, and cell viabilities were measured at 600 nm.
DISCUSSION
Plant adaptation to environmental stress is regulated through multiple physiological mechanisms at the cellular, tissue, and whole-plant levels (Ito et al., 2006). Expression of stress proteins is an important adaptation to cope with environmental stresses (Gerold et al., 2009). Most of the stress proteins are soluble in water and therefore contribute to stress tolerance, presumably via hydration of cellular structures. Although heat shock proteins are exclusively implicated in heat stress response, certain other proteins are also involved in the response (Wahid et al., 2007). French bean showed overlapping, and independent responses to high temperature and salt stress in terms of biochemical components (Nagesh babu et al., 2008) . Identification of three different Hsps of molecular mass of 105, 66 and, 56
k Da indicated their up regulation under HTS, and the existences of multiple forms of these proteins, which may sub serve different functions. The identified proteins are similar to other Hsps, accumulated under different abiotic stresses in variety of plants [Wahid et al., 2007, Singh 2008). The induction of Hsps specifically during HTS was further confirmed by the immunoblot analysis, wherein the blots from controls did not show any reactivity with either anti-Hsp1 or anti-Hsp3 antibodies. Although anti-Hsp1 antibodies showed cross reactivity with Hsp3 protein, the anti-Hsp3 antibodies did not detect Hsp1. HTS and consequent accumulation of misfolded proteins in plant cells induces synthesis of chaperones (Liberek et al., 2008). The induced chaperones bind unassembled or misfolded proteins, and prevent their aggregation. The measured in-vitro chaperone activity of Hsp3 in presence of model substrates, MDH and CS, wherein Hsp3 strongly suppressed the heat-induced aggregation of MDH and CS suggested its role in homeostatsis during applied stress. CS is very sensitive to thermal inactivation; activity is almost completely lost following incubation at 47°C for 30 min. Refolding of either thermally or chemically unfolded or inactivated CS was significantly enhanced by the addition of Hsp3. Although 50% CS activity was maintained during high temperature stress, the presence of Hsp3 was a prerequisite for partial CS reactivation. This result is in agreement with data obtained in plant Hsp 18 (Liberek et al., 2008). However, the activity of Hsp3 as a chaperone was not as efficient as the α-crystallin. The observed molecular chaperone activity and apparent molecular mass of 56 k Da of Hsp3 suggested that is a distinct from known molecular chaperones. Hsps are also known to function as ATPases through a highly conserved N- terminal ATPase domain, which has been found to play a crucial role in thermotolerance (Zhang et al., 2009). Significant chaperone and ATPase activity and molecular weight of Hsp3 suggested that it is comparable to either Hsp70 or Hsp90 family members from plants, which have been found to play a crucial role in thermo-tolerance (Young et al., 2001, Wahid, 2007, Zhang et al., 2009). The similarity of French bean Hsp3 in functional properties to other known Hsps is also corroborated from its N-terminal sequence homology.
Genetic engineering for increased thermotolerance by enhancing heat shock protein synthesis in plants has been achieved in a number of plants species (Katiyar et al., 2003, Cho et al., 2007 and Ashraf, 2010). To explore the possibility of exploiting the gene coding for Hsp3 in transgenics, partial cDNA was cloned and expressed in E. coli. The rHsp3 was found in cell free extract and was recognized by antibodies raised against purified French bean Hsp3 induced under HTS. Hsp3 is not only repaired misfolded proteins but also induced thermotolerance in cells. Over-expressing the rice Hsp90 in E. coli enhanced thermotolerance by preventing the denaturation of bacterial proteins under heat stress. Similarly, when French bean Hsp3 was over-expressed in E. coli, the thermotolerance was enhanced. The demonstrated ATPase activity and better survival rates of E .coli carrying Hsp3 cDNA demonstrated that the recombinant protein acts as a molecular chaperone in vivo. However, the viabilities of E. coli cells carrying the empty vector and those carrying Hsp3 decreased abruptly after 1 h at 50°C due to instability of many house-keeping gene products and membrane structure. This implies that partial Hsp3 gene weakly assists in the refolding of denatured proteins under high temperature. Similar observation has been made in tobacco overexpressing NtHsp70-2 (Gerold et al., 2009, Zhang et al., 2009).
In conclusion, identification of three different Hsp proteins suggested the involvement of multiple molecular forms of these proteins during HTS in French bean. The biochemical and molecular properties of purified and recombinant Hsp3, which exhibited similarity to Hsps from other plants, demonstrated its role in thermo-tolerance.
REFRENCES
Ashraf M. (2010) Inducing drought tolerance in plants. Recent advances Biotechnology Advances . 28 : 169-183.
Bradford M.M. (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem . 72 : 248-254.
Chan K.M., Delfert D., Junger K.D. (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Analytical Biochemistry . 157(2) : 375380.
Cho K., Song J.B. (2007) ATP-independent thermo-protective activity of Nicotiana tabacum heat shock protein 70 in E. coli . J. Biochem and Mol Biol . 40 : 107-112.
Gerold Beckers J.M., Jaskiewicz M., Liu Y., William R., Sheng U., He Y., Zhang S., and Conratha U. (2009) Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. The Plant Cell . 21(3) : 944-953
Green P.J. (1993) Control of mRNA stability in higher plants. Plant Physiol . 102 : 1065-1070.
Guy C., Kaplan F., Kopka J., Selbig J. and Hincha D.K.
(2008) Metabolomics of temperature stress ,
Physiologia Plantarum. 132(2) : 220-235.
Hall A.E. (2001) Crop Responses to Environment. CRC Press, LLC, Boca. Raton Florida
Huang B. and Xu C. (2008) Identification and characterization of proteins associated with plant tolerance to heat stress. Journal of Integrative Plant Biology . 50(10) : 1230-1237.
Ito Y., Karsura K., Maruyama K., Taji T., Kobayashi M., Seki M.M, Shinozaki K, Yamaguchi-Shinozaki K. (2006) Functional analysis of rice DREB/CBF-type transcription factors involved in cold-responsive Gene expression in transgenic Rice. Plant and Cell Physiol . 47 : 141-153.
Jagadish S.V.K., Muthurajan R., Oane R., Wheeler T. R., Heuer S., Bennett J. and Craufurd P.Q, (2010) Physiological and proteomic approaches to address heat tolerance during anthesis in rice ( Oryza sativa L.). J ournal of Experimental Botany . 61 : 143-156.
Katiyar-Agarwal S., Agarwal M., Grover A., (2003) Heat-tolerant basmati rice engineered by overexpression of hsp101. Plant Mol Biol . 51 : 677686.
Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature . 227 : 680-686.
Lee G., Pokala N., Vierling E. (1995) Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J. Biol Chem . 270 : 10432-10438.
Liberek K., Lewandowska A., Zietkiewicz S. (2008) Chaperones in control of protein disaggregation. EMBO J . 27(2) : 328-335.
Nagesh Babu R., Devaraj V.R. (2008) High temperature and salt stress response in French bean (Phaseolus vulgaris). Australian Journal of Crop Science . 2(2) : 40-48.
Porter J.R. (2005) Rising temperatures is likely to reduce crop yields. Nature . 436 : 174-179.
Sachin K., Larkindale J., Lee U., Koskull P., Vierling D.E. and Scharf K.D. (2007) Complexity of the heat stress response in plants. Current Opinion in Plant Biology. 10(3) : 310-316.
Seki M., Narusaka M., Ishida J., Nanjo T., Fujita M., Oono Y., Kamiya A., Nakajima M., Enju A., Sakurai T. (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J . 31: 279292.
Sere P.A., Brazil H., Gonen L. (1963) The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta chem. Scand . 17 : 129-134.
Shinozaki K., and Yamaguchi-Shinozaki K. (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stress. Annual Review of Plant Biology 57 : 781-803.
Singh A., Grover A. (2008) Genetic engineering for heat tolerance in plants. Physiol. Mol. Biol. Plants . 14 : 155-165.
Vidal V., Ranty B., Dillenschneider M., Charpenteau M., Ranjeva R. (1993) Molecular characterization of a 70 k Da heat shock proteins of bean mitochondria. Plant J . 3 : 143150
Vierling E. (1991) The heat shock response in plants. Annu Rev Plant Physiol Plant Mol Biol . 42 : 579620.
Wahid A., Gelani S., Ashraf M., Foolad M.R. (2007) Heat tolerance in plants: An overview. Environmental and Experimental Botany . 61 : 199-223.
Wang, W., Vinocur, B., Shoseyov, O., Altman, A. (2004) The role of plant heat-shock proteins/molecular chaperones in the abiotic stress response. Trends in Plant Science 9(5) : 244-252.
Young T.E., Ling J., Geisler-Lee C.J., Tanguay R.L., Caldwell C., Gallie D.R. (2001) Developmental and thermal regulation of maize heat shock protein, Hsp101. Plant Physiol . 127 : 777-791.
Zhang Z.L., Zhu J.H., Zhan Q.I., Cai Y.B. (2009) Molecular characterization of an ethephon-induced Hsp70 involved in high and low-temperature responses in Hevea brasiliensis. Plant Physiology and Biochemistry . 47 : 954-959.
Список литературы Purification, characterization and partial cDNA cloning of hightemperature stress-induced protein from French bean ( Phaseolus vulgaris)
- Ashraf M. (2010) Inducing drought tolerance in plants. Recent advances Biotechnology Advances. 28: 169-183.
- Bradford M.M. (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72: 248-254.
- Chan K.M., Delfert D., Junger K.D. (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Analytical Biochemistry. 157(2): 375-380.
- Cho K., Song J.B. (2007) ATP-independent thermo-protective activity of Nicotiana tabacum heat shock protein 70 in E. coli. J. Biochem and Mol Biol. 40: 107-112.
- Gerold Beckers J.M., Jaskiewicz M., Liu Y., William R., Sheng U., He Y., Zhang S., and Conratha U. (2009) Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. The Plant Cell. 21(3): 944-953
- Green P.J. (1993) Control of mRNA stability in higher plants. Plant Physiol. 102: 1065-1070.
- Guy C., Kaplan F., Kopka J., Selbig J. and Hincha D.K. (2008) Metabolomics of temperature stress, Physiologia Plantarum. 132(2): 220-235.
- Hall A.E. (2001) Crop Responses to Environment. CRC Press, LLC, Boca. Raton Florida
- Huang B. and Xu C. (2008) Identification and characterization of proteins associated with plant tolerance to heat stress. Journal of Integrative Plant Biology. 50(10): 1230-1237.
- Ito Y., Karsura K., Maruyama K., Taji T., Kobayashi M., Seki M.M, Shinozaki K, Yamaguchi-Shinozaki K. (2006) Functional analysis of rice DREB/CBF-type transcription factors involved in cold-responsive Gene expression in transgenic Rice. Plant and Cell Physiol. 47: 141-153.
- Jagadish S.V.K., Muthurajan R., Oane R., Wheeler T. R., Heuer S., Bennett J. and Craufurd P.Q, (2010) Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). Journal of Experimental Botany. 61: 143-156.
- Katiyar-Agarwal S., Agarwal M., Grover A., (2003) Heat-tolerant basmati rice engineered by over-expression of hsp101. Plant Mol Biol. 51: 677-686.
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227: 680-686.
- Lee G., Pokala N., Vierling E. (1995) Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. J. Biol Chem. 270: 10432-10438.
- Liberek K., Lewandowska A., Zietkiewicz S. (2008) Chaperones in control of protein disaggregation. EMBO J. 27(2): 328-335.
- Nagesh Babu R., Devaraj V.R. (2008) High temperature and salt stress response in French bean (Phaseolus vulgaris). Australian Journal of Crop Science. 2(2): 40-48.
- Porter J.R. (2005) Rising temperatures is likely to reduce crop yields. Nature. 436: 174-179.
- Sachin K., Larkindale J., Lee U., Koskull P., Vierling D.E. and Scharf K.D. (2007) Complexity of the heat stress response in plants. Current Opinion in Plant Biology. 10(3): 310-316.
- Seki M., Narusaka M., Ishida J., Nanjo T., Fujita M., Oono Y., Kamiya A., Nakajima M., Enju A., Sakurai T. (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 31: 279-292.
- Sere P.A., Brazil H., Gonen L. (1963) The citrate condensing enzyme of pigeon breast muscle and moth flight muscle. Acta chem. Scand. 17: 129-134.
- Shinozaki K., and Yamaguchi-Shinozaki K. (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stress. Annual Review of Plant Biology 57: 781-803.
- Singh A., Grover A. (2008) Genetic engineering for heat tolerance in plants. Physiol. Mol. Biol. Plants. 14: 155-165.
- Vidal V., Ranty B., Dillenschneider M., Charpenteau M., Ranjeva R. (1993) Molecular characterization of a 70 k Da heat shock proteins of bean mitochondria. Plant J. 3: 143-150
- Vierling E. (1991) The heat shock response in plants. Annu Rev Plant Physiol Plant Mol Biol. 42: 579-620.
- Wahid A., Gelani S., Ashraf M., Foolad M.R. (2007) Heat tolerance in plants: An overview. Environmental and Experimental Botany. 61: 199-223.
- Wang, W., Vinocur, B., Shoseyov, O., Altman, A. (2004) The role of plant heat-shock proteins/molecular chaperones in the abiotic stress response. Trends in Plant Science 9(5): 244-252.
- Young T.E., Ling J., Geisler-Lee C.J., Tanguay R.L., Caldwell C., Gallie D.R. (2001) Developmental and thermal regulation of maize heat shock protein, Hsp101. Plant Physiol. 127: 777-791.
- Zhang Z.L., Zhu J.H., Zhan Q.I., Cai Y.B. (2009) Molecular characterization of an ethephon-induced Hsp70 involved in high and low-temperature responses in Hevea brasiliensis. Plant Physiology and Biochemistry. 47: 954-959.


