Radiomics for diagnostic process and comprehensive treatment in glioblastoma: clinical case
Автор: Nikulshina Ya.O., Kolpakov A.V., Redkin A.N., Zakharov M.A.
Журнал: Международный журнал гуманитарных и естественных наук @intjournal
Статья в выпуске: 7-3 (70), 2022 года.
Бесплатный доступ
The paper highlights diagnostic and therapeutic options for glioblastoma. Glioblastoma is known to be a neuroepithelial malignant with an aggressive clinical course and extremely adverse prognosis. It is pointed out that contrast-enhanced magnetic resonance imaging (MRI) is the “gold standard” in glioblastoma diagnostics. Special attention is paid to radiomics that presents a multi-stage process involving image acquisition and pre-processing, segmentation, feature extraction and selection, and advanced statistics using machine learning algorithms. The aim of the study is to investigate objective numerical control options of pathological process dynamics and monitoring of the comprehensive glioblastoma treatment effectiveness in a particular patient according to the informative parameters of MR-images. Primary confirmation of objectifying diagnostic and treatment process in patient with glioblastoma according to the indicated statistical parameters of T2-weighted images was obtained. Further research should be aimed at the use of radiomics for planning, monitoring treatment of glioblastoma, predicting clinical outcomes.
Glioblastoma, radiomics, magnetic resonance imaging, radiotherapy, lesion
Короткий адрес: https://sciup.org/170195175
IDR: 170195175 | DOI: 10.24412/2500-1000-2022-7-3-35-40
Текст научной статьи Radiomics for diagnostic process and comprehensive treatment in glioblastoma: clinical case
Introduction . Glioblastoma is a neuroepithelial malignant, predominantly astrocytic brain tumor with an aggressive clinical course and extremely adverse prognosis [1, 2, 3]. According to the Central Brain Tumor Registry of the United States (CBTRUS), the incidence of glioblastoma is 14.7% among all central nervous system tumors and 47.7% among all malignant brain tumors [4].
Glioblastoma overall survival median is 14.6 months after standard treatment, including combination of surgery, radiotherapy and chemotherapy [1, 3, 4]. Various molecular genetic characteristics of glioblastoma in individual patients, as well as intratumoral heterogeneity, explain the relatively low effectiveness of standard treatment methods [1, 3, 4], which creates a problem in providing quality oncological care and indicates the need to develop a personalized approach to the diagnosis and treatment of glioblastoma.
Contrast-enhanced magnetic resonance imaging (MRI) is the “gold standard” in glioblastoma diagnostics. Due to the hyperpermeability of the immature tumor vessels, extra-vascular accumulation of contrast agent occurs with a shortening of T1-time and hyperintensive signal in T1-weighted images [2, 3]. On MR-images, glioblastoma characteristically appears as a fuzzy-contoured heterogenous structure lesion containing regions of unequal contrast agent accumulation surrounding the necrotic tumor center, which is hypointensive (dark) on T1-weighted MR-images [2, 3, 4]. Necrosis, the hallmark of glioblastoma, is associated both with the presence of thrombosed vessels and with high rate tumor cell proliferation, which leads to a mismatch between accelerated oxygen consumption and blood supply deficiency.
Perifocal vasogenic edema, signs of hemosiderin inclusion (hemorrhages in the tumor structure), mass-effect (deformation or displacement of adjacent structures) are also common features of glioblastoma MR-imaging, which reflect the structural tumor characteristics itself, as well as its microenvironment [3].
Since MR-images reflect structural tumor lesions that are inextricably linked with impaired metabolism of normal tissues, their quantitative assessment and informative parameters of MR-images can improve the in-tratumoral heterogeneity determination and studying the pathophysiological and molecular genetic mechanisms of a particular tumor [5, 6, 7].
Radiomics is a multi-stage process that includes image acquisition and pre-processing, segmentation, feature extraction and selection, and advanced statistics using machine learning algorithms [5, 6, 7, 8]. There are a lot of methods for describing and analyzing the object features in radiomic images: geometric characteristics analysis, local brightness differences analysis, statistical characteristics of textures analysis, etc. As a result, up to hundreds of features are extracted from one MR-image. Finally, the identified informative features, combined with clinical outcomes, are used as input data for classificational or prognostic models construction [5, 8, 9, 10].
The aim of the study is to investigate objective numerical control possibility of pathological process dynamics and control of the comprehensive glioblastoma treatment effec- tiveness in a particular patient according to the informative parameters of MR-images.
Material and methods . Authors used clinical data of a patient underwent chemoradiotherapy course for glioblastoma in the Radiotherapy Department No.1. of Voronezh Region Budjetary Healthcare Institution “Voronezh Regional Clinical Oncological Dispensary” in 2021. Presented MRI-studies were performed in the Department of Diagnostic Radiology of the same hospital using magnetic resonance tomographs Philips Ingenia 1.5T and Philips Ingenia Ambition 1.5T. MR-images analysis was carried out at the Moscow State Technical University named after N.E. Bauman using the Matlab 2021 application package.
Clinical data . Patient S., born in 1986, diagnosed with glioblastoma G4 of the right frontal lobe. Histological diagnosis No. 22854 dated 09/20/2021: Glioblastoma G4.
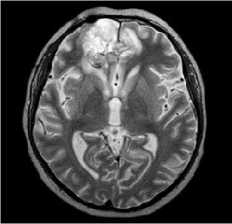
MRI dated 09/12/2021 (before surgical treatment):
Description: in the right frontal lobe, a large cystic-solid lesion is determined, with a pronounced mass-effect, extending to the temporal lobe. The total dimensions are 8.7x6.7x7.8 cm. The lesion compresses brain ventricular system; left lateral ventricle posterior horn is dilated. Median structures dislocation is 1.7 cm to the left. The right hemisphere sulci are narrowed. Conclusion : MR-picture of the right frontal lobe tumor (more likely, oligodendroglioma).
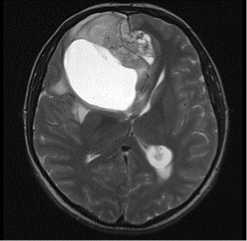
T2tra
T2tirm tra dark fluid
Figure 1. MRI before surgical treatment.
Patient S. was being treated at Neurosurgical department since 09/13/2021 to 09/29/2021 with the diagnosis: cystic-solid right frontal lobe tumor. Edema, dislocation of brain structures. Surgical treatment performed on 09/15/2021 included microsurgical removal of intracerebral right frontal lobe tumor with intraoperative ultrasound scanning.
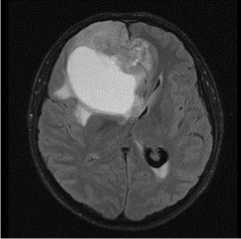
MRI dated 10/20/2021 (after surgical treatment):
Description: in the right frontal lobe spreading to the left frontal lobe, a post- operative cavity with hemosiderin deposits measuring 51x49x28mm is determined. The walls of the post-op cavity unequaly accumulate contrast agent. Perifocal edema appears measuring 53x68x40mm. Parasagittally along the posterior and inferior contours of the post- op cavity in the right frontal lobe, there is an altered MR-signal lesion in FLAIR-weighted images, measuring 17x36x33 mm, pushing the left frontal lobe. Conclusion: glioblastoma G4, condition after surgical treatment. MR-picture of residual tumor.
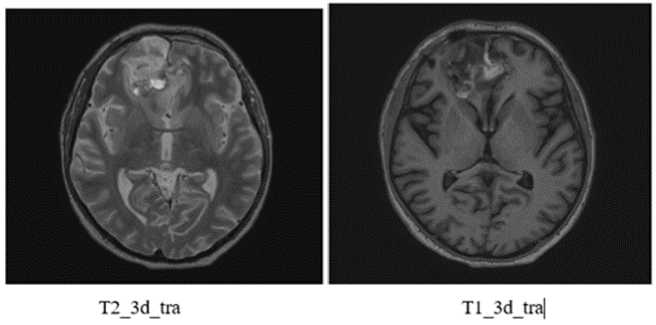
Figure 2. MRI after surgical treatment
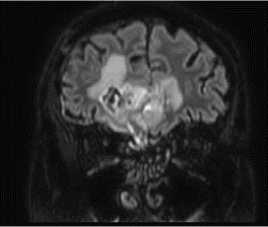
In the Department of Radiation Therapy No.1. patient S. underwent chemoradiotherapy from 11/01/2021 to 12/17/2021, specifically a 33-day-course of IMRT-radiation therapy on the right frontal lobe residual tumor tissue on Varian Halcyon linear particle accelerator with single focal dose 1.8 Gy and total focal dose 59.4 Gy. Chemotherapy: Temozolomide in capsules, 140 mg per day, a total of 4620 mg for the entire period of treatment; Dexa-methazone 12 mg intramuscular daily.
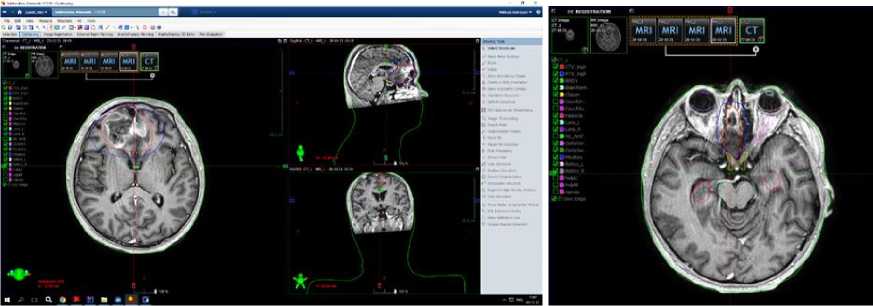
Figure 3. Radiotherapy planning: clinical target volume (CTV) and planning target volume (PTV) contouring in axial T1-contrast-enhanced-weighted images
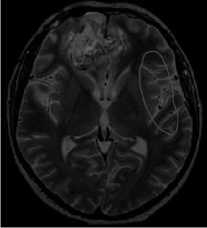
MRI dated 01/21/2022 (after comprehensive treatment):
Description: in previously determined localization in the right frontal lobe with spread to the left frontal lobe, a post-op cavity with hemosiderin deposits measuring 48x45mm (previously 52x52mm) is determined. There is a less pronounced heterogeneous accumulation of contrast agent. A perifocal changes region remains 59x54 mm (previously 62x51 mm). Parasagittally along the posterior and inferior post-op cavity contours in the right frontal lobe, a region of altered MR-signal on FLAIR T2-weighted images with dimensions of 24x11mm (previously 26x17mm) remains, pushing the left frontal lobe. Conclusion: condition after surgical treatment, chemoradiotherapy. The MR-picture of residual tumor in the right frontal lobe, in comparison with the study dated October 20/ 2021, shows a slight decrease in all previously determined changes size.
T23dtra
VFLAIRcorSENSE
Figure 4: MRI after chemoradiotherapy.
Data analysis . As an initial stage of the study, T2-weighted MR-images recorded before surgery, after surgery, and after chemoradiotherapy course were analyzed. As informative lesion signs on images, the statistical characteristics of brightness local distribution of the lesion images were analyzed, which are described by statistical texture parameters [11]:
-
- the intensity average value in the region (hereinafter referred as the average value);
-
- the intensity standard deviation in the region (hereinafter referred as the standard deviation);
-
- texture smoothness;
-
- the third moment characterizing asymmetry of the intensity histogram;
-
- entropy [11].
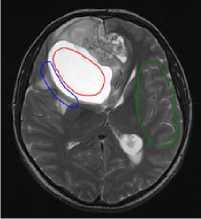
Figure 5 shows examples of analyzed images. Affected regions are highlighted in red, transitional regions in blue, and normal tissue regions in green.
a
b
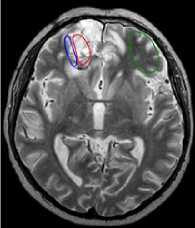
c
Figure 5. Examples of analyzed MR-images: a) before operation; b) after operation; c) after comprehensive treatment.
The results of texture parameters calculations are presented in tables 1-3.
Table 1. Statistical textural parameters of homogeneous lesion regions
|
Parameter |
Before operation |
After operation |
After complex treatment |
|
Mean |
1218.3±37.4 |
641.2±52.2 |
631.3±47.4 |
|
Standard deviation |
33.8±21.3 |
106.3±21.2 |
84.2±23.0 |
|
Smoothness (x10-6) |
0.37±0.49 |
2.73±1.01 |
1.78±1.02 |
|
Third Moment (x10-4) |
-1.10±2.44 |
-0.23±1.15 |
-0.27±0.74 |
|
Uniformity |
0.017±0.004 |
0.005±0.001 |
0.006±0.001 |
|
Entropy |
6.24±0.42 |
7.91±0.31 |
7.65±0.24 |
Table 2. Statistical texture parameters of transition regions
|
Parameter |
Before operation |
After operation |
After comprehensive treatment |
|
Mean |
826.2±65.5 |
527.4±51.0 |
484.3±23.4 |
|
Standard deviation |
301.9±61.8 |
119.9±15.8 |
112.3±17.3 |
|
Smoothness (x10-6) |
22.1±9.0 |
3.4±0.9 |
3.0±0.9 |
|
Third Moment (x10-4) |
4.73±2.01 |
0.62±1.83 |
-0.07±1.19 |
|
Uniformity |
0.0027±0.0008 |
0.0039±0.0007 |
0.0040±0.0005 |
|
Entropy |
8.97±0.35 |
8.25±0.24 |
8.18±0.14 |
Table 3. Statistical textural parameters of normal tissue regions
|
Parameter |
Before operation |
After operation |
After comprehensive treatment |
|
Mean |
303.6±24.9 |
327.9±30.7 |
298.1±29.9 |
|
Standard deviation |
49.6±12.8 |
71.0±21.9 |
62.9±21.9 |
|
Smoothness (x10-6) |
0.81±0.23 |
1.28±0.64 |
1.03±0.58 |
|
Third Moment (x10-4) |
0.46±0.49 |
1.19±0.84 |
0.73±0.75 |
|
Uniformity |
0.0081±0.0026 |
0.0066±0.0026 |
0.0081±0.0032 |
|
Entropy |
7.28±0.39 |
7.63±0.49 |
7.37±0.52 |
Results. As a result of texture parameters values analysis, the statistical significance of differences in the standard deviation, smoothness, and the third moment of affected, normal tissue and transition regions intensity on T2-weighted MRI images recorded before surgical treatment, after surgical treatment, and after a full course of chemoradiotherapy was established. Thus, as a result of clinical case analysis, the primary confirmation of objectifying diagnostic and treatment process in patient with glioblastoma according to the indicated statistical parameters of T2-weighted images was obtained.
Conclusion . The goal of further research in this direction is the use of radiomics for planning, monitoring treatment of glioblastoma, for predicting clinical outcomes, as well as predictive analysis of comprehensive treatment response, which would help open up prospects for the precision (personalized) oncological care development in patients with glioblastoma.
Список литературы Radiomics for diagnostic process and comprehensive treatment in glioblastoma: clinical case
- Yakovlenko Yu.G. Glioblastomas: the current state of the problem // Medical Bulletin of the South of Russia. 2019. №4.
- Zolotova S.V., Khokhlova E.V., Belyashova A.S. Study of the metabolic features of primary glioblastomas by SPECT-CT with Tc-MIBI with an assessment of their impact on the disease prognosis // Problems in neurosurgery. - 2019. - T. 83.2. - P. 17-26. doi: 10.17116/neiro20198302117.
- Omuro A., DeAngelis L.M. Glioblastoma and other malignant gliomas: a clinical review // JAMA. - 2013. - V. 310 (17). - P. 1842-50. doi: 10.1001/jama.2013.280319.4.
- Ostrom Q.T., Gittleman H., Truitt G., et al. CBTRUS Statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015 // Neuro Oncol. - 2018. - V. 20 (suppl_4). - P.iv1-iv86. doi: 10.1093/neuonc/noy131.
- Incoronato M., Aiello M., Infante T., et al. Radiogenomic Analysis of Oncological Data: A Technical Survey // International journal of molecular science. 2017. 24 (3): 14-21. doi:10.3390/ijms18040805.
- Zanfardino M., Franzese M., Pane K., et. al. Bringing radiomics into a multi-omics framework for a comprehensive genotype-phenotype characterization of oncological diseases // Journal of Translational Medicine. 2019. 34 (3): 26-38. doi: 10.1186/s12967-019-2073-2.
- Czarnek N.M., Clark K., Peters K.B., et al. Radiogenomics of glioblastoma: A pilot multi-institutional study to investigate a relationship between tumor shape features and tumor molecular subtype // Tourassi GD, Armato SG (eds). 2016. doi: 10.1117/12.2217084.
- Mazurowski MA, Clark K., Czarnek NM, et al. Radiogenomics of lower-grade glioma: al-gorithmically-assessed tumor shape is associated with tumor genomic subtypes and patient outcomes in a multiinstitutional study with The Cancer Genome Atlas data // Journal of neuro-oncology. 2017. 133 (1): 27-35. doi: 10.1007/s11060-017-2420-1.
- Miller JJ, Shih HA, Andronesi OC, et al. Isocitrate dehydrogenase-mutant glioma: Evolving clinical and therapeutic implications // Cancer. 2017. 123 (23): 4535-4546. doi: 10.1002/cncr.31039.
- Wang Y., Zhang T., Li S., et al. Anatomical localization of isocitrate dehydrogenase 1 mutation: a voxel-based radiographic study of 146 low-grade gliomas // European journal of neurology. 2015. 22 (2): 348-354. doi: 10.1111/ene.12578.
- Gonzalez R., Woods R. Digital image processing. M.: Technosfera, 2005. 1072 p.


