Rapid detection of Fusarium mycotoxins in wheat flour using thermoanalytical assays
Автор: Al-daoude A., Jawhar M.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.21, 2025 года.
Бесплатный доступ
Fungal mycotoxins pose a significant threat to the health of both human and livestock. Understanding the pressing need for a reliable and effective detection method. The current study aims to evaluate the presence of Fusarium head blight (FHB) mycotoxin contamination in wheat flour using thermogravimetry (TG) and differential scanning calorimetry (DSC) techniques. The TG curves of the contaminated flour samples exhibited three distinct mass losses steps, which corresponded to the thermal events when compared with the control samples . While, DSC analysis revealed the starch gelatinization process, with a decreasing in the gelatinization enthalpy (ΔH gel) for the three wheat genotypes in the contaminated flour samples ranging from 145 to 203 j/g compared to the non-contaminated ones which ranged from 195 to 254 j/g. These results warrant careful observation and analysis. The TG and DSC curves demonstrated high repeatability. Therefore, the thermal analysis approach presented here does not rely on any prior assumption about the thermal decomposition changes in wheat infected with F. culmorum. The current findings suggest that a combined TG and DSC approach could enable rapid, accurate, and sensitive detection of mycotoxins contamination in wheat flour.
Wheat, fusarium culmorum, mycotoxin, thermal analysis, dsc, tg
Короткий адрес: https://sciup.org/143184702
IDR: 143184702
Текст научной статьи Rapid detection of Fusarium mycotoxins in wheat flour using thermoanalytical assays
Wheat is a crucial source of carbohydrate and protein for both humans and animals. This crop routinely faces various fungal diseases each year across the globe. Unfortunately, these fungal interactions have raised significant concerns to the extremely determined effects of mycotoxins on human nutritional health and livestock feed (Gozzi et al., 2024). The Food and Agricultural Organization (FAO) estimates that each year approximately 25% of the world’s crops are contaminated with mycotoxins, which causes yearly losses of around one billion metric tons of food products Eskola et al., 2020). Therefore, mycotoxins monitoring becomes essential for maintaining crops quality and grain products in indoor storage facilities.
Mycotoxins pose a significant threat to food and feed safety, bringing them into focus and underscoring the need for reliable and efficient detection methods. The analysis of these toxic compounds is primarily conducted using high-performance liquid chromatography, following sample clean-up through solid phase extraction or immunoaffinity columns (Leite et al ., 2023). These assays demand high accuracy and precision, but they are also costly and time-consuming due to the complex sample preparation involved (Kromidas, 2016). Additionally, the intricate nature of the wheat grain matrix, combined with the diverse physical and chemical properties of various mycotoxins, necessitates effective detection methods for identifying co-occurring compounds (Gozzi et al. , 2024).
Fusarium head blight (F B), caused by Fusarium culmorum and other species, can significantly decrease both the yield and quality of wheat crops while producing mycotoxins that pose risks to food safety (Spanic et al. , 2018; Placinta et al. , 1999). During F B infection, the fungus spreads throughout the spikelet and eventually affects the entire head, leading to the decomposition of storage proteins and starch. As a result, the grains become shriveled and shrunk, ultimately reducing the yield and quality of the wheat crop (Nilsen et al. ., 2021; Yip et al. ., 2022).
Fusarium mycotoxins are generally regarded as thermally stable and can still be found in foods that have undergone thermal processing (Kabak, 2009; Qu et al., 2024). Currently, there is limited knowledge about the thermal degradation products of mycotoxins, as these are not typically detected in routine mycotoxin analysis. Standard detection methods are confined to a predetermined set of target analytes. Consequently, analytical chemists must develop suitable approaches for identifying and quantifying the thermal degradation products of mycotoxins across various food matrices (Leite et al., 2023).
The levels of water, protein, and starch in wheat kernels are closely linked to the degree of damage caused by Fusarium head blight (F B) (Wegulo, 2012). Wheat starch serves as the primary storage carbohydrate, comprising about 60–75% of grain and 70–80% of flour (Shevkani et al. , 2017). Literature often discusses starch gelatinization, which occurs when starch granules lose their ordered structure due to heat, making them accessible for enzymatic conversion (Bakri et al. , 2020; Kim et al. , 2021). This gelatinization process, influenced by the starch-to-water ratio, can be analyzed using differential scanning calorimetry (DSC). Additionally, thermogravimetry (TG) can provide insights into the behavior of starch granules during heating, which leads to depolymerization (Arabi et al. , 2012; Rangelov et al. , 2017).
To the best of our knowledge, there are no previous studies evaluating and optimizing thermogravimetry (TG) and differential scanning calorimetry (DSC) assays for the detection of mycotoxins in wheat flour infected with Fusarium head blight (F B). The aim of the present work was to investigate the occurrence of Fusarium mycotoxins in wheat flour under potentially epidemic F B conditions across three wheat different genotypes, utilizing TG and DSC techniques.
MATERIALS AND METHODS
Isolation of fungus
The virulent isolate of F. culmorum (FSY 12) described by Alazem (2007) was used in this study. The fungus was grown in 9-cm Petri dishes containing potato dextrose agar (PDA, DIFCO, Detroit, MI. USA) for 10 days, at 22 ±1°C in the dark to allow mycelial growth.
Plant materials
The experiments were conducted under growth room conditions using three different wheat genotypes (Cham 3, 4 and 6) differ in their different reaction. Seeds were surface-sterilized with 5% sodium hypochlorite solution for 5 min and then washed three times in sterile distilled water. They were planted in plastic pots (20-cm) filled with sterilized peatmoss in three replicates. Each experimental unit consisted of five pots of 15 seedlings per genotype. Pots were placed under growth chamber conditions at temperatures of 22-24°C day/ 18-20°C night.
Infection procedure and sample preparation
Inoculum was prepared as described by Bekele (1987), briefly; with sharp pointed tweezers a small piece of cotton soaked with Fusarium suspension was placed between the glumes of a spikelet in contact with the anthers and the stigma of a floret. The cotton size was about one fifth of a glume and the amount of the soaked inoculum about 15µl. The experiment was repeated twice. For the experiments, healthy grains without visible damage or discoloration (F B) was selected as control samples. Seeds were tested for the absence or presence of F. culmorum by incubation on PDA media as above. ealthy and infected seeds samples were ground to the consistency of flour using a coffee grinder. The ground wheat flour samples were homogenized separately and stored at –20 °C until further analyses.
Thermal Analyses
TG and DSC analyses were performed using a Differential Scanning Calorimetry (SETARAM, France) at a -130 0C to 250 0C rate (10 0C/min), Gas Flow: N2 -100 ml/min and crucible aluminum 30 µl. Sealed aluminum crucibles were used in order to study the gelatinization process with a 5:1 (water: starch w/w) ratio prepared directly by weighting 2,0 mg of each starch and 10 mL of water was added; the aluminum crucible was sealed and after one hour a new DSC curve was realized.
RESULTS AND DISCUSSION
The TG and DSC curves for both infected and noninfected wheat flour are presented in Figures 1 and 2.
The TG curves indicate that mass losses in the infected flour occur in three distinct steps, each associated with specific thermal events. The first mass loss, observed between 60–270 °C, is attributed to dehydration and takes place in a single step. The second mass loss, occurring between 290–430 °C, corresponds to a change in the thermal peak and is ascribed to the oxidation of organic matter. The final mass loss, which occurs between 420–580 °C, is a gradual process associated with a small, broad heat change between 270 and 430 °C, culminating in a sharp peak at 410 °C, indicative of the complete oxidation of organic matter. These three distinct steps were notably more pronounced in the infected wheat flour compared to the control samples (Fig. 1).
The DSC curves for indium and the empty aluminum crucible were used as references. The characteristics of the transitions are illustrated in Figure 2 and detailed in Table 1. The results indicate that sharp changes in the DSC curves of the infected wheat flour were observed at temperatures ranging from 85 to 94 °C (Fig. 2). Notably, both the onset and peak temperatures were higher in the infected flour compared to the control samples (Table 1).
Additionally, the Δ _gel enthalpy was reduced in the infected flour across the three wheat genotypes, ranging from 145 to 203, in contrast to non-infected flour, which ranged from 195 to 254. This decrease in Δ _gel may be attributed to the degradation of starch chains caused by modifications during infection; consequently, less energy is required for starch gelatinization (Klein et al. , 2013). Nus and Shashikumar (1993) reported that fungal pathogens degrade plant cell wall polymers during the infection process to obtain nutrients and facilitate cell penetration and spread through plant tissue. This could explain the observed changes in thermal curves, likely due to the degradation of hemicelluloses in the cell wall by F. culmorum shortly after infection.
Table 1. Experimental values obtained of gelatinization enthalpy (Δ gel), onset temperature (T0), and peak temperature (Tp) for studies flour of three wheat genotypes infected with F. culmorum
Genotype ΔHgel
|
Cham 3 |
||
|
I |
159.03 |
|
|
II |
203.44 |
|
|
Cham 4 |
||
|
I |
192.44 |
|
|
II |
254.33 |
|
|
Cham 6 |
||
|
I |
145.84 |
|
|
II |
195.56 |
I: Infected and II: Non-infected
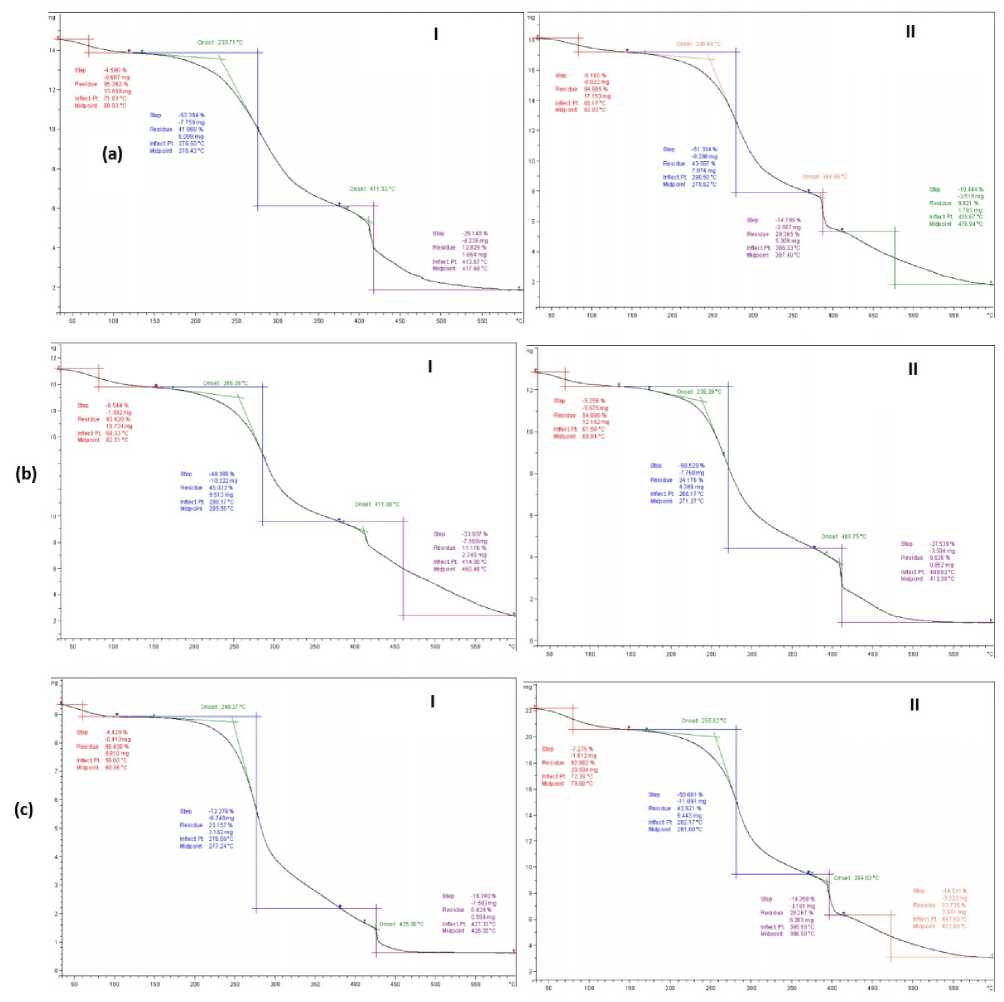
Figure 1: TGA curves of; (I) infected and (II) non-infected seed flour of three wheat genotypes (a: Cham 3, b; Cham 4 and c: Cham 6) with F. culmorum
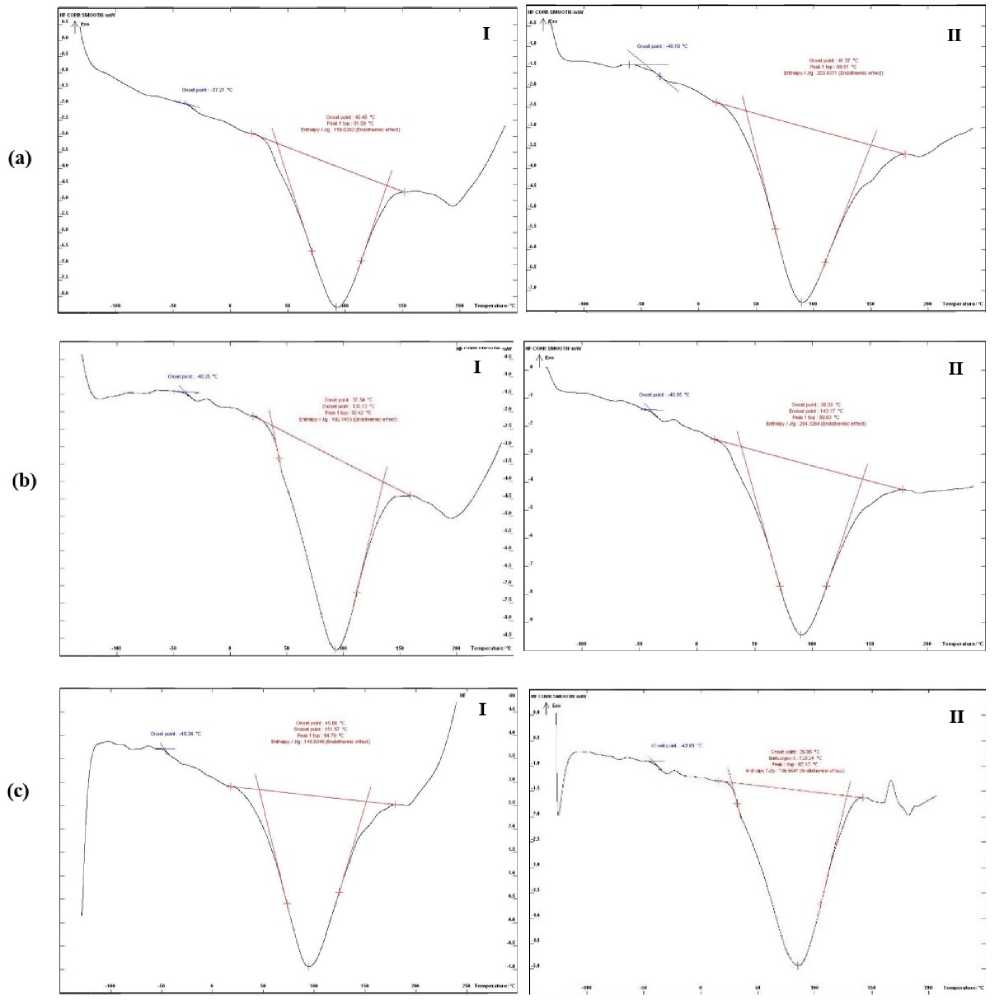
Figure 2: DSC curves of; (I) infected and (II) non-infected seed flour of three wheat genotypes (a: Cham 3, b; Cham 4 and c: Cham 6) with F. culmorum
On the other hand, literature suggests that the thermal peak of DSC-starch transitions (gelatinization and fusion of the most stable crystallites) typically occurs at around 60 °C. owever, as water content decreases, this peak shifts to higher temperatures (Münzing, 1991; Temesgen et al. , 2024). Given that the water content in the wheat flour sample is approximately 12%, the starch transition endotherm is expected to occur at a temperature higher than 70 °C with a low Δ _gel.
Therefore, the higher DSC peak parameters observed in the infected flour (85–94 °C) may be attributed to starch transition parameters influenced by Fusarium mycotoxins.
CONCLUSION
This study revealed significant thermal decomposition changes in flour samples contaminated with F. culmorum compared to the controls. The TG curves of the contaminated flour exhibited mass losses in three distinct steps, allowing for the observation of dehydration and changes in thermal patterns relative to the controls. Additionally, the DSC curves illustrated starch gelatinization and quantified the Δ _gel enthalpy in both infected and non-infected wheat seeds, which warrants careful consideration.
While these techniques do not precisely identify the specific Fusarium toxins present in wheat flour, they hold important implications for preventing mycotoxinproducing fungi and mycotoxins from entering the food supply chain, thus reducing risks to human health. Furthermore, the combination of TG and DSC may serve as a reliable diagnostic tool for the rapid screening of large sample sizes, providing insights into potential mycotoxin contamination from various fungal species across a wide range of agricultural and food products.
ACKNOWLEDGMENT
The authors thank all members of the IAEA-CRP (Nuclear Techniques to Support Risk Assessment of Biotoxins and Pathogen Detection in Food and Related Matrices “D52044”) for their advice during the course of the work, and assistance within this manuscript. We wish to acknowledge also the assistance of Dr. SASANYA, James Jacob (IAEA - Food Safety and Control Section) as a Project Officer of the CRP. Thanks also extended to the Director General of AECS and the ead of Biotechnology Department for their help throughout the period of this research.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.