Редкие опухоли. Метастатический рак носоглотки
Автор: Болотина Л.В.
Журнал: Злокачественные опухоли @malignanttumors
Статья в выпуске: 3S1 т.14, 2024 года.
Бесплатный доступ
Рак носоглотки (РН) является уникальной патологией, для которой характерна специфическая эндемичность, половые и возрастные особенности распределения, а также высокая чувствительность к консервативным методам лечения. Одной из особенностей РН является высокая ассоциация с вирусом Эпштейна-Барр (ВЭБ), что определяет особенность функционирования сигнальных путей и высокую иммуногенность опухоли. Статья посвящена анализу исследований химио-иммунотерапии в 1 линии лечения рецидивного / метастатического РН.
Рак носоглотки, вирус Эпштейна-Барр, химио-иммунотерапия
Короткий адрес: https://sciup.org/140307163
IDR: 140307163 | DOI: 10.18027/2224-5057-2024-14-3s1-14-18
Текст научной статьи Редкие опухоли. Метастатический рак носоглотки
Рак носоглотки (РН) может быть отнесен к орфанным заболеваниям в силу крайней редкости данной патологии. В структуре онкологической заболеваемости в мире РН занимает 23 позицию с удельным весом 0,6%. В 2022 году в мире было зарегистрировано 120416 новых случаев РН и 73476 смертей (21 место, 0,8%). Для РН характерна специфическая эндемичность. Так, заболеваемость среди европейской популяции составляет лишь < 1 на 100000, тогда как в Китае, Азии и Африке возрастает до 2,4; 1,8 и 1,3 на 100000 соответственно (1). Соотношение мужчин и женщин составляет 4,5:1. По данным мировой статистики в 2018 году заболеваемость среди мужского населения Юго-Восточной Азии составляла 6,4 на 100000 населения (2). В РФ заболеваемость на протяжении многих десятилетий находится на устойчивых показателях 0,3%–0,4% в общей структуре ЗНО. Пик заболеваемости приходится на 30 лет, однако в эндемичных регионах наблюдаются 2 возрастных пика 10–25 лет (Азия) и 50 лет (Африка). На основании исследований при семейном РН и популяционных исследованиях геномных ассоциаций было показано, что возникновение РН ассоциировано с наличием специфических гаплотипов HLA и множественных герминальных вариантов в хромосоме, кодирующей молекулы основного комплекса гистосовместимости 1 класса, а также нарушениях в генах, вовлеченных в сигнальный путь NOTCH (4–7).
Морфологическая структура РН представлена тремя гистопатологическими подтипами: I подтип — ороговевающий, высоко дифференцированный рак, на который приходится менее 5% РН, наиболее часто встречается в западноевропейской популяции и часто ассоциирован с ВПЧ. Два других подтипа связаны с инфицированием вирусом Эпштейна-Барр (ВЭБ): II подтип представлен неороговевающим плоскоклеточным раком, а III подтип — неороговевающим недифференцированным (носоглоточным) раком (8–12). Было показано, что для ВЭБ-ассоции-рованных раков характерно возникновение отдаленных метастазов, тогда как при ВПЧ-ассоциированном РН чаще возникают локо-регионарные рецидивы, но при этом фиксируются худшие показатели общей выживаемости (ОВ) (12). Проведенный популяционный скрининг в эндемичном регионе (Гонконг, n = 20142, мужчины в возрасте от 40 до 62 лет) позволил установить высокую негативную предсказательную значимость выявления ВЭБ. Так, при отсутствии в плазме крови ДНК ВЭБ вероятность того, что у пациента не возникнет РН приближалась к 100%. При этом обнаружение ДНК ВЭБ ассоциировалось с развитием РН в 11 % (13). Следует отметить, что сегодня не определен единый порог позитивности ВЭБ, который характеризует ВЭБ-ассоциированный РН. В разных исследованиях он колеблется от 500 до 4000 копий/мл. Для европейской популяции общепринятым порогом считается 500 копий/мл, а для эндемичных регионов — от 2000 копий/мл. В отдельных исследованиях отмечена прогностическая роль в отношении безрецидивной выживаемости (БРВ) и ОВ объема первичной опухоли (> 30 см или > 50 см3) и регионарных лимфоузлов (4–6 см), а также уровня ЛДГ (14–18).
Важная роль проведенных экспериментальных исследований состоит в том, что авторам удалось обнаружить характерный для РН высокий уровень инфильтрации опухолевой ткани лимфоцитами. Это связывают с тем, что ВЭБ-ассоциированные опухолевые клетки при РН экспрессируют белки, выступающие в качестве мишеней для CD4 + и CD8 + T-лимфоцитов. Высокий уровень TILs оказался фактором благоприятного прогноза в отношении БРВ (HR 0,41, p < 0,001) и ОВ (HR 0,42, p < 0,01) Кроме того, экспрессия PD-L1 обнаруживается более чем на 90% опухолевых клеток (25–26). Экспрессия лиганда соотносится с неблагоприятным прогнозом. Так, в 2017 г. Li YF с соавторами был опубликован анализ исходов 120 пациентов РН, убедительно продемонстрировавший снижение показателей БРВ (р = 0,002) и ОВ (р = 0,023) при наличии экспрессии PD-L1.
Такие особенности РН явились основанием для изучения химио-иммунотерапевтических режимов в качестве 1 линии распространенного/метастатического процесса. К настоящему времени достаточно зрелые и убедительные данные РКИ 3 фазы доступны для 3 китайских анти-PD-1 агентов — тислелизумаба, торипалимаба и кам-релизумаба (регистрация которого по данному показанию в РФ запланирована на 2024 год). Во всех трех протоколах режим ХТ был представлен комбинацией гемцитабина и цисплатина (GemCis).
В исследовании RATIONALE-309 проводилось сравнение 4–6 курсов ХТ GemCis с комбинацией GemCis (4–6 курсов) + тислелизумаб, который после завершения ХТ продолжался в качестве поддерживающего лечения (28). Авторами продемонстрировано достоверное преимущество от применения ХИТ в отношении БРВ, медиана которой составила в экспериментальной и контрольной группе 9,6 мес. vs 7,4 мес., соответственно. (HR 0,50). Выигрыш от добавления тислелизумаба отмечался независимо от экспрессии PD-L1 и опухолевой нагрузки. Также было отмечено преимущество в отношении ВБП2 (не достигнута в экспериментальной группе vs 16,6 мес., HR 0,39) и тенденция к увеличению ОВ.
Исследование JUPITER-02 посвящено изучению эффективности ХИТ, включающей торипалимаб. В этом протоколе пациенты также получали до 6 курсов GemCis +/- то-рипалимаб. Далее экспериментальная группа продолжала поддерживающую терапию торипалимабом (29). Первичной конечной целью являлась ВБП. Опухоли 75%–76% больных являлись PD-L1-позитивными (PD-L1 ≥ 1% на опухолевых или иммунных клетках). Исследование оказалось успешным — медианы ВБП в экспериментальной и контрольной группах составили 21,4 мес. vs 8,2 мес., HR 0,52, p < 0,0001. Абсолютная разница превысила 1 год. Подгрупповой анализ продемонстрировал, что все группы получали выигрыш от добавления торипалимаба, в т. ч. независимо от уровня PD-L1 на опухолевых или иммунных клетках. Медиана ОВ в исследовательской группе не достигнута vs 33,7 мес. в контрольной группе, HR 0,63, p = 0,0083, 2-летняя ОВ 78,0 % vs 65,1 %, а 3-летняя ОВ 64,5% vs 49,2%, соответственно. Частота нежелательных явлений (НЯ) ≥ 3 степени (89,7% против 90,2%) и НЯ с летальным исходом (3,4% против 2,8%) была одинаковой в двух группах.
Но более интересными для нас представляются результаты РКИ CAPTAIN-1st, в связи с ожидаемой доступностью камрелизумаба в РФ (30). В протокол разрешалось включать пациентов с прогрессированием через полгода камрелизумаб плюс GP
Плацебо плюс GP
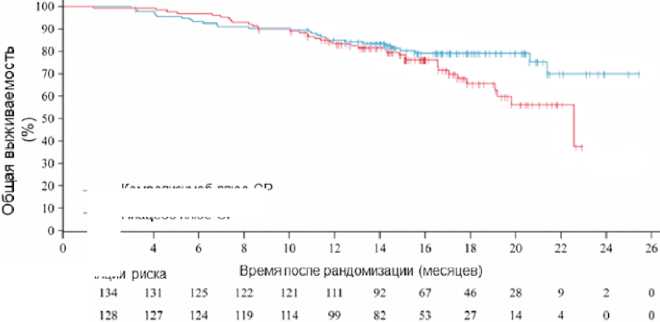
— Камрелизумаб плюс GP
----Плацебо плюс GP
Количество пациентов в популяции
GP — gemcitabine plus cisplatin.
Рисунок 1. Общая выживаемость по результатам анализа с дополненными данными и более после ранее проведенного лечения или при исходно метастатическом процессе. С учетом эндемичности региона в Китае в исследование было набрано 263 человека, распределенных на 2 группы, одна из которых получала стандартную ХТ GemCis, а вторая комбинацию ХТ с камрелизумабом. После завершения ХТ (4–6 циклов) экспериментальная группа продолжала ИТ камрелизума-бом до прогрессирования или неприемлемой токсичности. Первичной конечной точкой, как и во всех предшествующих РКИ, являлась ВБП. Более 70% участников основной группы имели ВЭБ + процесс, носоглоточный (неороговевающий недифференцированный) тип был установлен у 82%. У 52% отмечалось метастатическое поражение печени. Исследование достигло поставленной цели — мВБП оказалась достоверно выше при добавлении к ХТ камрелизумаба (9,7 мес. vs 6,9 мес., HR 0,52, р = 0,002). ЧОО в группе камрелизумаба превзошла показатель группы сравнения (87,3% vs 80,6%) с ожидаемо большей длительностью сохранения достигнутого ответа (8,5 мес. vs 5,6 мес.). При увеличении сроков наблюдения было зафиксировано, что к 1,5 годам без признаков прогрессирования оставались 25,3% и 13,4% больных основной и контрольной групп. Несмотря на то, что медиана ОВ в экспериментальной группе пока не достигнута, данный показатель удалось оценить в группе ХТ — 22,6 мес., HR 0,67. Таким образом, можно отметить очевидную тенденцию к увеличению ОВ при использовании ХИТ, которая начинает реализовываться после 16 месяцев (рис. 1).
Несомненно, важным аспектом является информация о том, что добавление камрелизумаба не увеличивало частоту нежелательных реакций 3 или более высокой степени тяжести. Наиболее часто среди токсических проявлений отмечались лейкопения (66% vs 70%), нейтропения (64% vs 66%), анемия (40% vs 44%), тромбоцитопения
(40% vs 40%). Серьезные НЯ регистрировались в 44% и 37% соответственно. Следует отметить характерную для камрелизумаба ТР — реактивную пролиферацию эндотелия кожных капилляров (РПЭКК), которая наблюдалась у 60% пациентов и в основном была представлена проявлениями 1–2 ст. тяжести. Также нельзя не упомянуть о том, что возникновение РПЭКК обычно является предиктором более высоких показателей ОВ (продемонстрировано при других ЗНО, в частности плоскоклеточном раке пищевода (31).
ВЫВОДЫ
-
1. РН является редкой патологией, имеющей четкую эндемичность, ассоциацию с ВЭБ, малое число активирующих мутаций, для которых разработаны таргет-ные препараты, что ограничивает число эффективных опций лекарственной терапии.
-
2. Для РН характерна высокая частота экспрессии PD-L1, что определяет потенциальную эффективность иммунотерапии.
-
3. Камрелизумаб, наряду с другими препаратами группы анти-PD-1 агентов, согласно данным РКИ, является важным компонентом лекарственной терапии 1 линии распространенного РН. Режим ХИТ увеличивает эффективность по сравнению с ХТ GP (ЧОО, ВБП).
-
4. Добавление камрелизумаба не увеличивает частоту нежелательных реакций степени 3 или более высокой степени тяжести.
-
5. С учетом планирующейся регистрации камрелизумаба в РФ схему GP + камрелизумаб следует рассматривать в качестве приоритетной лечебной опции 1 линии терапии (после регистрации камрелизумаба в РФ).
Список литературы Редкие опухоли. Метастатический рак носоглотки
- Bray F., Laversanne M., Sung H., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024;74(3):229-263. https://doi.org/10.3322/caac.21834
- Chua M.L.K., Wee J.T.S., Hui E.P., Chan A.T.C. Nasopharyngeal carcinoma. Lancet 2016;387(10022):1012-1024. https://doi.org/10.1016/S0140-6736(15)00055-0
- Злокачественные новообразования в России в 2014 году (заболеваемость и смертность). Под ред. А.Д. Каприна, В.В. Старинскиого, Г.В. Петровой. М.: МНИОИ им. П.А. Герцена-филиал ФГБУ «ФМИЦ им. П.А. Герцена» Минздрава России, 2016.250 с.
- Bej J.X., Jia W.H., Zeng Y.X. Familial and large-scale case-control studies identify genes associated with nasopharyngeal carcinoma. Semin Cancer Biol 2012;22(2):96-106. https://doi.org/10.1016/j.semcancer.2012.01.012
- Lu S.J., Day N.E., Degos L., et al. Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature 1990;346(6283):470-471. https://doi.org/10.1038/346470a0
- Dai W., Zheng H., Cheung A.K.L., et al. Whole-exome sequencing identifies MST1R as a genetic susceptibility gene in nasopharyngeal carcinoma. Proc Natl Acad Sci USA 2016;113(12):3317-3322. https://doi.org/10.1073/pnas.1523436113
- Yu G., Hsu W.L., Coghill A.E., et al. Whole-exome sequencing of nasopharyngeal carcinoma families reveals novel variants potentially involved in nasopharyngeal carcinoma Sci Rep 2019;9(1):9916. https://doi.org/10.1038/s41598-019-46137-4
- Lo E.J., Bell D., Woo J.S., et al. Human papillomavirus and WHO type I naso- pharyngeal carcinoma. Laryngoscope 2010;120(10):1990-1997. https://doi.org/10.1002/lary.21089
- Maxwell J.H., Kumar B., Feng F.Y., et al. HPV-positive/p16-positive/EBV- negative nasopharyngeal carcinoma in white North Americans. Head Neck 2010;32(5):562-567. https://doi.org/10.1002/hed.21216
- Chan Y.H., Lo C.M., Lau H.Y., Lam T.H. Vertically transmitted nasopharyngeal infection of the human papillomavirus: Does it play an aetiological role in nasopharyngeal cancer? Oral Oncol 2014;50(5):326-329. https://doi.org/10.1016/j.oraloncology.2013.12.025
- Dogan S., Hedberg M.L., Ferris R.L., et al. Human papillomavirus and Epstein- Barr virus in nasopharyngeal carcinoma in a low- incidence population. Head Neck 2014;36(4):511-516. https://doi.org/10.1002/hed.23318
- Stenmark M.H., McHugh J.B., Schipper M., et al. Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. Int J Radiat Oncol Biol Phys 2014;88(3):580-588. https://doi.org/10.1016/j.ijrobp.2013.11.246
- Chan K.C.A., Woo J.K.S., King A., et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 2017;377(6):513-522. https://doi.org/10.1056/NEJMoa1701717
- Feng M., Wang W., Fan Z., et al. Tumor volume is an independent prognostic indicator of local control in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Radiat Oncol 2013;8:208. https://doi.org/10.1186/1748-717X-8-208
- He Y.X., Wang Y., Cao P.F., et al. Prognostic value and predictive threshold of tumor volume for patients with locally advanced nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. Chin J Cancer 2016;35(1):96. https://doi.org/10.1186/s40880-016-0159-2
- Huang C.L., Chen Y., Guo R., et al. Prognostic value of MRI-determined cervical lymph node size in nasopharyngeal carcinoma. Cancer Med 2020;9(19):7100-7106. https://doi.org/10.1002/cam4.3392
- Lin J.C., Liang W.M., Jan J.S., et al. Another way to estimate outcome of advanced nasopharyngeal carcinoma - is concurrent chemoradiotherapy adequate? Int J Radiat Oncol Biol Phys 2004;60(1):156-164. https://doi.org/10.1016/j.ijrobp.2004.03.002
- Tang L.Q., Li C.F., Li J., et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst 2015;108(1):djv291. https://doi.org/10.1093/jnci/djv291
- Li Y.Y., Chung G.T.Y., Lui V.W.Y., et al. Exome and genome sequencing of nasopharynx cancer identifies NF-κB pathway activating mutations. Nat Commun 2017;8:14121. https://doi.org/10.1038/ncomms14121
- Lin D.C., Meng X., Hazawa M., et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet 2014;46(8):866-871. https://doi.org/10.1038/ng.3006
- Wong K.C.W., Hui E.P., Lo K.W., et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol 2021;18(11):679-695. https://doi.org/10.1038/s41571-021-00524-x
- Tsang C.M., Lui V.W.Y., Bruce J.P., et al. Translational genomics of nasopharyngeal cancer. Semin Cancer Biol 2020;61:84-100. https://doi.org/10.1016/j.semcancer.2019.09.006
- Bruce J.P., To K.F., Lui V.W.Y., et al. Whole-genome profiling of nasopharyngeal carcinoma reveals viral-host co-operation in inflammatory NF-κB activation and immune escape. Nat Commun 2021;12(1):4193. https://doi.org/10.1038/s41467-021-24348-6
- Lili L., Zhang Y., Fan Y., et al. Characterization of the nasopharyngeal carcinoma methylome identifies aberrant disruption of key signaling pathways and methylated tumor suppressor genes. Epigenomics 2015;7(2):155-173. https://doi.org/10.2217/epi.14.79
- Fang W., Zhang J., Honget S., et al. EBV-driven LMP1 and IFN-gamma up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014;5(23):12189-12202. https://doi.org/10.18632/oncotarget.2608
- Outh-Gauer S., Alt M., Le Tourneau C., et al. Immunotherapy in head and neck cancers: a new challenge for immunologists, pathologists and clinicians. Cancer Treat. Rev 2018;65:54-64. https://doi.org/10.1016/j.ctrv.2018.02.008
- Li Y.F., Ding J.W., Liao L.M., et al. Expression of programmed death ligand-1 predicts poor outcome in nasopharyngeal carcinoma. Mol Clin Oncol 2017;7(3):378-382. https://doi.org/10.3892/mco.2017.1318
- Yang Y., Pan J., Wand H., et al. Tislelizumab plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer: A multicenter phase 3 trial (RATIONALE-309). Cancer Cell 2023;41(6):1061-1072.e4. https://doi.org/10.1016/j.ccell.2023.04.014
- Mai H.Q., Chen Q.Y., Chen D., et al. Toripalimab Plus Chemotherapy for Recurrent or Metastatic Nasopharyngeal Carcinoma: The JUPITER-02 Randomized Clinical Trial. JAMA 2023;330(20):1961-1970. https://doi.org/10.1001/jama.2023.20181
- Yang Y., Qu S., Li J., et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2021;22(8):1162-1174. https://doi.org/10.1016/S1470-2045(21)00302-8
- Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator‘s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2020:21(6):832-842. https: https://doi.org///doi.org/10.1016/S1470-2045(20)30110-8


