Redox enzymes of red beetroot vacuoles ( Beta vulgaris L.)
Автор: Pradedova E.V., Nimaeva O.D., Salyaev R.K.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.10, 2014 года.
Бесплатный доступ
Years of research have shown that some of the redox elements (enzymes, coenzymes, and co-substrate) are isolated from each other kinetic and spatial manner (compartmentalization) in the eukaryotic cells. The redox elements forming the "highly" and "widely" specialized redox system are found in all cell structures: mitochondria, plastids, peroxisomes, apoplast, nucleus etc. In recent years the active involvement of the central vacuole in the maintenance of the plant cell redox homeostasis is discussed, actually the information about the vacuolar redox system is very small. The high-priority redox processes and "redox-specialization" of the vacuolar compartment are not known. We have begun a study of red beet-root vacuole redox systems ( Beta vulgaris L.) and have identified redox enzymes such as: phenol peroxidase (EC 1.11.1.7), superoxide dismutase (EC 1.15.1.1) and glutathione reductase (EC 1.8.1.7). This paper presents some of the characteristics of these enzymes and considers the probable ways of their functioning in vacuolar redox chains.
Beta vulgaris l, vacuole, glutathione transferase, peroxidase, redox chain, superoxide dismutase
Короткий адрес: https://sciup.org/14323915
IDR: 14323915
Текст научной статьи Redox enzymes of red beetroot vacuoles ( Beta vulgaris L.)
В основе окислительно-восстановительных процессов лежат окислительно-восстановительные реакции (Oxidation-Reduction reaction или Redox reaction). Для редокс-реакций характерно изменение степени окисления соединений вследствие переноса электронов между ними. При этом один из участников реакции выполняет функцию донора (восстановитель), а другой – акцептора электронов (окислитель). Окислитель и восстановитель образуют сопряженную пару – редокс-пару (Zholnin, 2012). Реакции этого типа наиболее распространены в биологических системах. Следует отметить, что редокс-реакции биологических систем заметно отличаются от редокс-реакций, протекающих в неорганических и органических системах. Для редокс-реакции биологических систем характерны высокая скорость и обратимость, что обусловлено непосредственным участием специализированных ферментов (оксидоредуктаз) (Ksenzhek, Petrova, 1986; Kritskii, Telegina, 2004). Участие ферментов, т.е. каталитических белков, свидетельствует в пользу генетического контроля за редокс-процессами в биологических системах (Rogozhin et al., 2004). А изменение экспрессии генов этих белков, которое довольно часто отмечают при действии определенных факторов, указывает на эпигенетический контроль (Martinovich, Cherenkevich, 2008).
Функционирование редокс-ферментов тесно сопряжено со специализированными коферментами, которые представлены редокс-парами (NAD/NADH; GSH/GSSG; Fe3+/Fe2+ и т.п.). Редокс-коферменты выполняют функцию акцепторов и переносчиков восстановительных эквивалентов, им свойственно обратимое окисление-восстановление. Существует четыре способа передачи электронов с помощью коферментов:1) прямой перенос электронов ( e– ) между элементами; 2) перенос в составе атома водорода ( H , e– + H+); 3) в форме гидрид-иона ( H~ ,
2 e– + H+); 4) перенос e– при прямом взаимодействии донора с кислородом. Таким образом, редокс-процессы могут протекать без участия и с участием кислорода, акцептирующего электроны (Murray et al . 1993; Zholnin, 2012).
Редокс-коферменты, как правило, – полифункциональны. Они взаимодействуют с разными ферментами (Kritskii, Telegina, 2004). Хотя некоторые из них могут специализироваться на переносе определенного окислительного эквивалента. Например, редокс-кофакторы Fe2+, Cu2+, Mn2+, Mo4+ и Co2+, а также кофермент гем, обеспечивают перенос e–, тогда как пиридиннуклеотиды (NAD+, NADP+) переносят главным образом Н~. Флавины (FAD; FMN) могут переносить два эквивалента: Н~ и e–. Хиноновые коферменты, глутатион, аскорбиновая и липоевая кислоты осуществляют перенос e– и H+ (H) (Saab– Rincon, Valderrama, 2009). Некоторой полифункциональностью могут обладать и редокс-ферменты, взаимодействующие с определенными коферментами. Однако для большинства из них характерны узкая специализация и субстратная специфичность (Kritskii, Telegina, 2004).
В клетках всех организмов, включая растения, редокс-ферменты катализируют многие ключевые реакции энергетического обмена и биологического окисления. Некоторые из них окисляют соединения, отнимая один электрон в свободнорадикальном катализе. Другие отнимают два электрона, при этом интермедиаты, как правило, стабилизируются активными сайтами ферментов, что позволяет избежать неконтролируемую генерацию реактивных продуктов (Saab-Rincon, Valderrama, 2009).
Редокс-ферменты подразделяют на несколько групп. Основанием для такого разделения служит совместное функционирование с определенными коферментами и косубстратами: 1) металлопротеины (содержащие медь, марганец, никель, молибден, железо-кластеры, а также гем) 2) флавин-содержащие белки; 3) пиридиннуклеотид-зависимые белки; 4) хинон-зависимые белки (Saab-Rincon, Valderrama, 2009). Наряду с этими группами выделяют группы ферментов, функционально связанные с определенными косубстратами. Например, выделяют группы глутатионзависимых ферментов, тесно взаимодействующих с глутатионом, или аскорбатзависимых ферментов, функционально связанных с аскорбиновой кислотой.
Что касается пространственного функционального сопряжения редокс-ферментов, кофакторов и косубстратов, то наиболее распространенным способом их взаимодействия можно назвать объединение в специализированные редокс-цепи, в которых осуществляется поэтапный или многоступенчатый перенос электронов от первичного субстрата к конечному акцептору (Jones, 2008). Поток электронов (редокс-путь) между элементами таких редокс-цепей зависит от констант скоростей реакций, от суммарного количества и распределения структурных элементов цепи, а также от их стандартных редокс-потенциалов (Melissa et al., 2008). Полагают, что окислительный стресс может происходить вследствие нарушения функционирования этих редокс-цепей при изменении путей переноса электронов (Cherenkevich et al., 2013). В то же время эффективность работы редокс-цепей, согласно мнению некоторых авторов, обеспечивается изоляцией в клеточных структурах (компартментацией) редокс-элементов, а также солокализованными с ними механизмами, координирующими их функции (Jones, 2008; Cherenkevich et al., 2013).
Идея специализированной компартментации редокс-цепей и в целом редокс-процессов в клетках основана на многочисленных фактах (Jones, 2008; Martinovich, Cherenkevich, 2008). На сегодня редокс-цепи выявлены практически во всех клеточных компартментах. Например, широко известны электрон-транспортные цепи сопряженного окисления (ЭТЦ) митохондрий и хлоропластов. Электрон-транспортные цепи (несопряженного окисления) с монооксигеназной активностью исследованы у микросом клеток животных и на мембранах эндоплазматического ретикулума растительных клеток (Berezov, Korovkin, 2008). Аскорбат-глутатионовый цикл, представляющий редокс-цепь последовательного переноса восстановительных эквивалентов от NADPH к глутатиону и аскорбиновой кислоте, выявлен у хлоропластов (Ksenzhek, Petrova, 1986). Электрон-транспортные цепи с участием тиоловых редокс-пар функционируют в цитозоле клеток животных и растений (Cherenkevich et al., 2013). Все перечисленные и многие другие редокс- процессы протекают с обязательным участием редокс-элементов, которые представлены кофакторами, коферментами, косубстратами, ферментами и редокс-группами (остатки цистеина и т.п. в составе белков и пептидов).
Придерживаясь идеи компартментации редокс-процессов, главными структурными единицами которых являются редокс-цепи, мы приступили к изучению редокс-элементов, способных совместно функционировать в редокс-цепях внутри такого клеточного компартмента, как центральная вакуоль. Редокс-системы и редокс-процессы этой клеточной структуры практически не исследуются, в связи с этим ничего не известно о «редокс-специализации» вакуоли. В вакуолярном содержимом клеток корнеплодов столовой свеклы ( Beta vulgaris L.) мы выявили активность таких редокс-ферментов, как: фенольная пероксидаза (EC 1.11.1.7), супероксиддисмутаза (EC 1.15.1.1) и глутатионредуктаза (EC 1.8.1.7). В настоящей работе представлены характеристики этих ферментов и рассмотрены вероятные способы их совместного функционирования во внутривакуолярных редокс-цепях.
MATERIALS AND METHODS
Объектом исследования служили корнеплоды столовой свеклы ( Beta vulgaris L. ), на стадии их физиологического покоя (осенний–зимний период).
Вакуоли выделяли из паренхимы корнеплодов с помощью макрообъемного метода, предложенного Leigh и Branton, и в дальнейшем модифицированного Саляевым и соавт. (Leigh,
Branton, 1976; Salyaev et al ., 1981).
Водные экстракты получали из тканей корнеплодов, для этого ткань гомогенизировали в среде: 100 мМ K-Na-фосфатный буфер (рН 7.0); 1 мМ дитиотрейтол; 1 мМ EDTA; 1% поливинилпирролидон.
Содержимое вакуолей извлекали , разрушая органеллы в гипотонической среде: 100 мМ K-Na-фосфатный буфер, рН 7.2. Фильтраты экстрактов тканей, а также экстракты вакуолей центрифугировали 20 мин при 15500 g .
Активность GR определяли в вакуолярных и тканевых экстрактах спектрофотометрическим методом (Anderson et al ., 1990). В том случае, когда исследовали рН-зависимость фермента, использовали реакционные среды с разным pH: 5.0–6.0–7.0–8.0. Ингибиторный анализ проводили с 1-хлор-2.4-динитробензолом (CDNB), его конечная концентрация в среде составляла 2 мМ (Bilzer et al ., 1984). Относительную активность GR выражали в мкмолях косубстрата на мг белка в мин.
Количество белка определяли по методу Bradford (Bradford, 1976).
Активность супероксиддисмутазы (SOD) определяли модифицированным способом (Nishikimi et al ., 1972).
Метод изоэлектрофокусирования белков (IEF) применяли для разделения изоформ пероксидазы и SOD. IEF проводили в полиакриламидном геле (PAAG) (T 5%; C 3%), который содержал 10% глицерина и 3% амфолитов (рН 3.5-10) (Fluka) (Gaal et al., 1980). Маркером служил калибровочный КИТ для IEF (Pharmacia). Экстракты, полученные из ткани корнеплода и вакуолярных фракций, подвергали частичной очистке, для этого проводили двух часовой диализ при 4ºС. Анализируемые пробы содержали в среднем 100 мкг белка.
С помощью электрофоретического метода выявляли изоферментный состав GR. Электрофорез белков осуществляли стандартным способом в 7.5% PAAG при неденатурирующих условиях (Gaal et al ., 1980).
Активность полифенолоксидазы (тирозиназы) в геле выявляли в реакционной среде, содержащей 60 мМ K-Na-фосфатного буфера (рН 5.0 или 8.0), 50 мМ пирокатехина (о-дигидроксибензола) (PC), 0.93 мМ парафенилендиамина (PDA) (Voskresenskaya et al ., 2006). В качестве ингибитора пероксидазных реакций использовали 30 мМ 1,3-диэтил-2-тиомочевину.
Визуализацию изоформ пероксидазы в PAAG осуществляли в той же среде, что и изоформы тирозиназы, или в среде, содержащей 100 мМ цитратно-фосфатный буфер (рН 5.0 или рН 8.0). Для развития реакции в среды вносили 0.01% Н 2 О 2 , 0.1 мМ 3,3’-диаминобензидин (DAB) или 50 мМ PC и 0.93 мМ PDA.
Активность SOD в PAAG визуализировали согласно методу Beauchamp-Fridovich (Beauchamp, Fridovich, 1971).
Для выявления активности GR в PAAG использовали два метода. Первый предусматривал развитие окраски в агаровом слое, который наносили на PAAG. Агаровый слой содержал: 200 мМ трис-HCl (pH 8.0); 1 мМ EDTA; 2 мМ 5.5'-дитиобис-2-нитробензойная кислота (DTNB); 1мМ GSSG; 0.5 мМ NADPH; 15 мг/мл агар. В этом слое в зонах локализации GR появлялось желтое окрашивание, вызванное образованием в ходе реакции 5-тио-2-нитробензойной кислоты (Levites, 1986). Другой способ предусматривал инкубацию гелей в темноте в реакционном растворе: 250 мМ трис-HCl (рН 7.5); 3.4 мМ GSSG; 0.5 мМ NADPH; 10 мг 3-(4.5-диметилтриазол-2-4)2.5-дифенилтетразо-лиум бромид (MTT); 10 мг 2.6-дихлорофенолин-дофенол (DCPIP) (Anderson et al., 1990). Маркером ферментативных реакций служила GR из пекарских дрожжей (Sigma).
Для оценки интактности органелл и их контаминации также использовали электрофоретический метод. Применяли стандартный метод электрофореза в неденатурирующих условиях. В качестве маркера цитозоля и клеточных структур (пластид и митохондрий) служила активность NAD- и NADP-зависимых малатдегидрогеназ (MDH). Активность MDH выявляли зимографическим методом в PAAG после электрофореза с помощью общепринятого метода (Levites, 1986).
Полученные в ходе экспериментов электрофореграммы фотографировали с помощью системы Digi Doc-it Imaging System («Bio-Rad», USA), а также сканировали на сканере (hp scanjet 5400c).
Все эксперименты были выполнены в 4-5 биологических повторностях. На графиках приведены средние арифметические значения и средние квадратичные отклонения величин активности фермента.
RESULTS AND DISCUSSION
Согласно сложившимся на сегодня представлениям, центральная вакуоль активно участвует в поддержании редокс-гомеостаза растительной клетки (Ferreres et al ., 2011). Однако вакуолярные редокс-системы, вовлекаемые в этот процесс, мало исследованы. Одним из наиболее известных редокс-ферментов вакуолярной локализации можно считать фенольную пероксидазу.
Гемсодержащий редокс-фермент пероксидаза
Несмотря на то, что пероксидаза выявлена в вакуолях у всех исследованных на сегодня растений, о ней не так много сведений, как можно было бы ожидать (Almagro et al ., 2009). Пероксидаза вакуолей, как и все фенольные пероксидазы (POX), является металлопротеином, гемсодержащим белком. Редокс-статус железа (Fe2+ или Fe3+) в белковопорфириновом комплексе POX изменятся в зависимости от каталитического цикла ферментативной реакции (Ferreres et al ., 2011).
POX окисляет соединения, восстанавливая H2O2, а также окисляет соединения кислородом. Для фермента характерен переход с пероксидазного каталитического цикла (окисление субстрата и восстановление H2O2), на оксидазный каталитический цикл (окисление субстрата O2). Особенность каталитического механизма POX в оксидазном цикле заключается в генерации свободных радикалов (О2", HO2', радикал органического субстрата) и Н2О2 (Chen, Schopfer, 1999; Rogozhin et al., 2004). В связи с этим полагают, что окислительные циклы POX могут регулироваться O2" и Н2О2, генерируемыми самим ферментом (Chen, Schopfer, 1999).
Способность окислять соединения кислородом, т.е. оксидазная активность, обнаружена у некоторых изоформ POX корней хрена, а также у POX клеточных стенок.
POX, выявленная нами в вакуолях корнеплодов столовой свеклы, также катализировала реакции оксидазного типа, которые были схожи с реакциями полифенолоксидаз (тирозиназ). Основные результаты этого исследования представлены в статье (Pradedova et al ., 2014).
Проведенные ранее спектрофотометрические исследования показали, что реакции оксидазного и пероксидазного окисления POX вакуолей клеток корнеплодов столовой свеклы зависели от условий pH. Для оксидазной активности, определяемой с пирокатехиом (PC), оптимальными были условия с pH 8.0–9.0. Эта активность POX в целом походила на активность полифенолоксидазы (тирозиназы). Однако для большинства полифенолоксидаз клеток растений, согласно данным литературы, оптимальными являются pH 5.0–5.5 (Li et al ., 2010).
Что же касается пероксидазных реакций POX вакуолей, то в наших исследованиях, определяемые с PC, PDA, DBA и H 2 O 2 они имели оптимумы при pH 5.0–6.0 (Pradedova et al ., 2014).
С помощью зимографического метода, позволяющего выявлять ферментативную активность в PAAG, установили, что все изоформы POX вакуолей клеток корнеплодов столовой свеклы, в отсутствие Н 2 О 2 , могли окислять PC, подобно тирозиназам (Рис. 1). Оксидазные реакции быстро развивались при pH 8.0 (Рис. 1а) и практически отсутствовали при pH 5.0 (Рис. 1б). Они также протекали в присутствие 1,3-диэтил-2-тиомочевины, акцептора H 2 O 2 , что подтверждало их оксидазную природу (Рис. 1г). Если в реакционные среды, содержавшие PC и PDA, вносили H 2 O 2 , то результат был прямо противоположным, пероксидазные реакции быстро протекали при pH 5.0 (Рис. 1в) и довольно медленно – при pH 8.0 (данные не приводятся). Для пероксидазных реакций POX вакуолей с DAB оптимальными также были pH 5.0–6.0 (Рис. 1д). Следует отметить, что ни с DAB, ни с PDA оксидазные реакции не развивались, ни при каких условиях pH. Что свидетельствует в пользу субстратной избирательности вакуолярных изоформ POX в оксидазном цикле, в котором они, по всей видимости, не взаимодействуют с бензидинами. Зависимость пероксидазных и оксидазных реакций от pH-условий, говорит о том, что наиболее вероятным механизмом, регулирующим переход POX вакуолей из пероксидазного цикла в оксидазный, является протонирование/депротонирование функциональных групп активного центра фермента.
Флавопротеин – глутатионредуктаза
Большое семейство флавопротеинов отличается многообразием. Общим для его представителей является сопряженное функционирование с изоаллоксазиновыми коферментами (FMN, FAD и др.), которые подвергаются окислению-восстановлению в ходе реакций. Восстанавливающими агентами для них служат в основном динуклеотиды (NADPH и NADH) (Saab-Rincon, Valderrama, 2009).
Флавопротеины широко распространены в биологических системах. Во многих организмах обнаружен высоко гомологичный флавопротеин – глутатионредуктаза (GR). Этот фермент из семейства флавинзависимых оксидоредуктаз восстанавливает дисульфид глутатиона (GSSG) до его сульфгидрильной формы (GSH). У растений глутатион (GSH) обнаружен во всех клетках и практически во всех клеточных компартментах (цитозоле, хлоропластах, митохондриях, вакуолях и т.д.) (Shao et al ., 2008). GSH является главными повсеместным небелковым тиолом, высокая химическая активность тиольной группы делает его эффективным восстановителем во многих окислительно-восстановительных процессах. Поскольку GR играет ключевую роль в поддержании высокого соотношения GSH/GSSG, от ее активности в определенной мере зависит редокс-гомеостаз клетки (Cherenkevich et al ., 2013).
GR растений, по сравнению с GR животных, изучена недостаточно. Не исследованы некоторые детали строения ферментативного комплекса, также неясны механизмы регуляции активности фермента, в полной мере не определена его компартментация (Noctor et al., 2011). В клетках растений GR обнаружена в цитозоле, митохондриях и пероксисомах, хотя основным местом локализации считают хлоропласты (Noctor et al., 2011). Следует отметить, что о GR вакуолей на сегодня практически нет информации (Rautenkranz et al., 1994)
Мы выявили активность GR в вакуолях клеток корнеплодов столовой свеклы. Она несколько превышала активность GR тканевого экстракта, которую использовали для сравнения (Рис. 2). Результаты нашего исследования не совпадали с ранее представленными результатам, согласно которым активность GR вакуолей проростков ячменя ( Hordeum vulgare L.) была в несколько раз ниже активности GR экстрактов ткани (Rautenkranz et al ., 1994).
Специфичные внутриклеточные ингибиторы или активаторы для GR до сих пор не обнаружены. Однако установлено, что некоторые синтетические соединения могут подавлять ее активность. Например, нитрофураны и ароматические нитросоединения. Довольно сильный эффект на GR оказывают ароматические нитросоединения, к которым относится CDNB. При наличии CDNB в реакционных средах в конечной концентрации 2 мМ ферментативная активность GR вакуолей и тканевого экстракта заметно снижалась (Рис. 2). Следует отметить, что для GR животных был установлен неконкурентный тип ингибирования CDNB (Bilzer et al ., 1984).
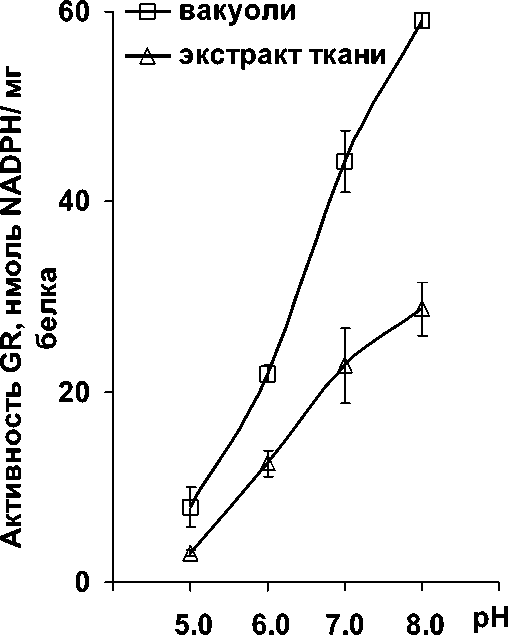
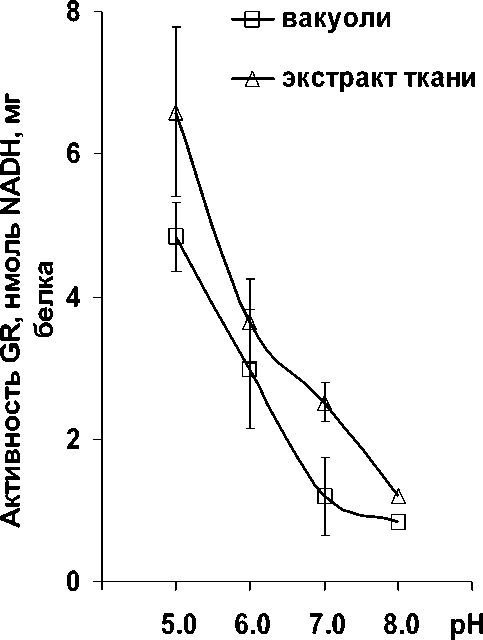
Уровень ферментативной активности GR в нашем исследовании зависел от природы динуклеотида. Известно, что в реакциях восстановления GSSG с участием GR косубстратом-восстановителем может служить и NADPH, и NADH. При этом ферментативная активность с NADPH в несколько раз выше, чем с NADH. Сродство фермента к пиридиннуклеотидам определяют условия pH. Для NADPH требуются нейтральные и слабощелочные условия, а для NADH – кислые (Shigeoka et al., 1987; Rautenkranz et al., 1994). Действительно активность GR вакуолей и экстрактов тканей корнеплодов столовой свеклы с NADPH была выше при pH 7.0– 8.0, а с NADH – при pH 5.0 (Рис. 3А и Б). Обращало на себя внимание то, что в целом уровень активности с NADPH был почти в 10 раз выше, чем с NADH.
Результаты нашего исследования совпадали с результатами других авторов, которые также показали, что оптимумы pH для GR с NADPH у самых разных растительных организмов варьируют в пределах от 7.0 до 8.0. Например, для GR сосны ( Pinus strobus L.) оптимальными были pH 7.3 и 7.8, а эвглены ( Euglena viridis ) – pH 8.2 (Shigeoka et al ., 1987; Anderson et al ., 1990). При этом изоформы GR многих организмов взаимодействуют с NADH как с альтернативным донором электронов. Например, для GR шпината ( Spinacia oleracea L.), дрожжей ( Saccharomyces cerevisiae ) и Escherichia coli при восстановлении GSSG наряду с NADPH субстратом может служить и NADH (Vanoni et al ., 1990).
Электрофоретическое исследование активности GR в PAAG мы проводили двумя разными методами. С их помощью в экстрактах ткани и содержимом вакуолей выявили две изоформы GR с относительными Rf 0.36 и Rf 0.430.047 (Рис. 4в). Маркером ферментативной активности служила активность GR пекарских дрожжей (Sigma), величины Rf для ее изоформы в условиях нашего эксперимента составляли 0.330.36 (Рис. 4б). Значения Rf GR пекарских дрожжей оказались близки к значениям Rf одной из изоформ GR вакуолей и экстрактов ткани. Следует отметить, что две изоформы GR были показаны и у других растений, например, в листьях Hordeum vulgare и Arabidopsis thaliana (Anderson et al., 1990; Marty et al., 2009).
В целом, проведенное нами исследование биохимических свойств GR вакуолей в сравнении с GR водных экстрактов ткани, указывало на специфичность ферментативных реакций. Высокий уровень активности GR вакуолей, сравнимый с уровнем активности фермента экстрактов ткани, говорил о том, что эта активность не могла быть следствием контаминации фракций изолированных вакуолей содержимым цитозоля. Однако чтобы убедиться в чистоте вакуолярных фракций и исключить вероятность взаимодействия органелл с вакуолями, а также везикуляцию тонопласта с одновременным захватом цитоплазматического содержимого, в ходе изолирования органелл, мы определили в исследуемых образцах активность других внутриклеточных ферментов. Наиболее подходящими маркерами для того исследования являются NAD- и NADP-малатдегидрогеназы (МDH). Известно, что NADP-МDH (EC 1.1.1.82) – специфична для пластид (Edwards et al., 1985), тогда как NAD-МDH (EC 1.1.1.37) обнаружена во многих клеточных компартментах (пероксисомах, глиоксисомах, митохондриях, пластидах, микросомах и цитозоле) (Yudina, 2012). Для выявления активности этих ферментов в экспериментальном материале использовали чувствительный и наглядный зимографический метод. С его помощью активность NAD-МDH и NADP-МDH была выявлена в тканевом экстракте корнеплодов свеклы (Рис. 5б). Однако в вакуолярных фракциях она не обнаружена (Рис. 5а), что исключало контаминацию изолированных вакуолей.
Основная функция GR, как уже упоминалось, заключается в поддержании пула восстановленного глутатиона. GSH обнаружен практически во всех клеточных структурах, в том числе и в вакуолях (Noctor et al., 2011). Ранее при исследовании транспорта глутатиона в вакуоли отмечали, что скорость переноса дисульфидной формы (GSSG) заметно выше скорости переноса сульфгидрильной формы глутатиона (GSH). Транспорт GSSG усиливался при окислительном стрессе (Queval et al., 2011). В связи с этим было высказано предположение о важной роли вакуоли в поддержании редокс-статуса глутатиона в растительной клетке (Tommasini et al., 1993). В качестве основного метаболического пути транспортируемого в вакуоли GSSG рассматривали его гидролиз γ-глутамилтранспептидазами и дипептидазами (Zechmann et al., 2006; Noctor et al., 2011). Однако результаты наших экспериментов указывают на другой возможный путь метаболизма GSSG в вакуолях, а именно на его активное восстановление до GSH вакуолярными GR.
Металлофермент супероксиддисмутаза
Во фракциях вакуолей, изолированных из корнеплодов столовой свеклы, мы определили активность супероксиддисмутазы (SOD). Основные результаты этого исследования были опубликованы (Pradedova et al ., 2009).
Сопоставив уровни активности SOD вакуолей и экстракта ткани корнеплодов, установили, что активность вакуолярного фермента превосходила активность SOD тканевого экстракта, что продемонстрировано на рисунке 6. Высокий уровень ферментативной активности мог указывать на значительное количество фермента в вакуолях.
При помощи электрофоретических методов с применением ингибиторного анализа, в вакуолярном соке была выявлена активность Cu,Zn-SOD. Хотя в клетках корнеплода столовой свеклы обнаружены все формы SOD: Mn-SOD; Fe-SOD и Cu,Zn-SOD (Pradedova et al., 2009). Вакуолярная Cu,Zn-SOD, как показали результаты исследования с помощью метода IEF, была представлена тремя изоформами с низкими значениями pI (от 4.5 до 3.0), заметно отличавшимися от pI вакуолярных анионных пероксидаз, значения которых варьировали в пределах от 7.0 до 4.5 (Рис. 7). Выявляемая на зимограммах активность SOD не являлась неспецифической реакцией пероксидаз, способных замедлять накопление формазана, проявляя SOD-подобную активность (Giannopolitis, Ries, 1977; Pradedova et al., 2014). Следует отметить, что многочисленные изоформы Cu,Zn-SOD у других растительных объектов тоже были анионными (Wingsle et al., 1991).
Ранее Cu,Zn-SOD в вакуолях была показана в A. thaliana с помощью метода протеомного анализа (Carter et al., 2004). Используя методы спектрофотометрии и электрофореза мы подтвердили присутствие этого фермента в вакуолях, на примере вакуолей Beta vulgaris. Присутствие в вакуолярном содержимом редокс-ферментов предполагает формирование редокс-цепей
Все биологические системы содержат редокс-элементы, которые участвуют в физиологической регуляции и клеточной сигнализации, а также в преобразовании и обмене макромолекул. Многие из них – полифункциональны (Coleman, Rehm, 2000). Например, FAD, NAD(P)H, GSH и др. взаимодействуют со многими ферментами. Некоторой полифункциональностью обладают и оксидоредуктазы, взаимодействующие с различными коферментами и зачастую с различными субстратами. «Узкую специализацию» редокс-элементы (кофакторы, косубстраты и ферменты) приобретают в результате объединения в редокс-цепь, предназначенную для выполнения определенной функции. Таким образом, координация активности редокс-элементов осуществляется через редокс-цепи (Jones, 2008; Melissa et al ., 2008).
Такими редокс-цепями переноса электронов могут быть ферментативные белковые комплексы (например, NADPH-оксидаза), ассоциированные с мембранами ЭТЦ, ферментативные цепи и циклы (например, тиольные цепи и аскорбат-глутатионовый цикл), и т.п. В одних случаях редокс-элементы объединяются в цепь транслокации электронов посредством растворимых и мобильных переносчиков-косубстратов (например, аскорбата и глутатиона) (Jones, 2008). В других, основным фактором, определяющим формирование функционально специализированных редокс-цепей, является компартментация (изоляция в клеточных структурах). Некоторые узко специализированные цепи переноса электронов образуются только в определенных клеточных структурах, например, ЭТЦ хлоропластов, митохондрий, микросом. Другие, напротив, могут функционировать в любой части клетки. Например, электронный поток, происходящий между АФК и антиоксидантами, обнаружен практически во всех клеточных компартментах.
Наличие тех или иных редокс-элементов в определенном компартменте позволяет предположить вероятную организацию цепей переноса электронов и вероятные «общие узлы» такого переноса (как правило, ими являются мобильные доноры электронов), что может служить основанием для формирования представления о функциональной активности и специализации компартмента (Jones, 2008; Melissa et al., 2008). Так, присутствие NADPH-оксидазного комплекса на поверхности плазмалеммы, а SOD и POX в клеточной стенке клеток растений, предполагает активную генерацию H2O2 в апопласте. При этом очевиден редокс-путь (поток электронов): сначала от NADPH к O2, что приводит к формированию O2•–; затем при дисмутации 2O2•– с участием SOD – к H2O2; и далее при участии пероксидазы – к H2O (Gasaryan et al., 2000; Baranenko, 2006). Нарушение последовательности переноса электронов между элементами такой редокс-цепи вызывает спонтанное неконтролируемое взаимодействие АФК со структурными молекулами как самого компартмента, так и с внутриклеточными компонентами (в случае миграции H2O2 в цитоплазму), что в итоге может привести к развитию окислительного стресса (Cherenkevich et al., 2013).
В вакуолярном компартменте с участием выявленных редокс-ферментов также можно ожидать формирование редокс-цепей. Например, таких:
-
1) POX (Fe3+/2+) → 2O 2 •¯ + SOD (Cu2+/0) → H 2 O 2 + POX (Fe3+/2+) → 2H 2 O.
При переходе POX на оксидазный реакционный цикл вероятным продуктом первичного и вторичного окисления может быть O2• . Две молекулы супероксидного анион-радикала с помощью SOD образуют H2O2. Взаимодействуя с каталитическим центром POX, пероксид водорода, как уже упоминалось выше, переводит ферментативный цикл на пероксидазное окисление, в ходе которого он восстанавливается до H2O (Chen, Schopfer,1999). Главными редокс-элементами в такой цепи переноса являются гем пероксидазы и ионы меди SOD, а восстановительными эквивалентами – e– и H (e- + H+).
-
2) POX (Fe3+/2+) → 2O 2 •¯ + SOD (Cu2+/0) → H 2 O 2 + GSH → GSSG + GR (FAD-NADН) → GSH.
Образованный при совместном функционировании POX и SOD пероксид водорода может диффундировать на значительное расстояние от POX. В этом случае восстановителем для H 2 O 2 может быть GSH, который, как было показано ранее, содержится в вакуолях (Shao et al ., 2008; Noctor et al ., 2011). Пул восстановленного глутатиона могут поддерживать вакуолярные GR. Главными редокс-элементами в такой редокс-цепи будут: гем пероксидазы, ионы меди SOD, GSH, FAD глутатионредуктазы и NADН. Восстановительные эквиваленты этой цепи: e–, H и H~.
-
3) POX (Fe3+/2+) + GSH → GSSG + GR (FAD-NADН) → GSH
Известно, что донором электронов в пероксидазных реакциях POX может быть GSH (Rogozhin et al ., 2004). Если такая реакция протекает в вакуолярном пространстве, то один из путей метаболизма образующегося дисульфида GSSG, по всей видимости, будет его восстановление вакуолярными GR. Главными редокс-элементами в этом случае будут гем пероксидазы, GSH, FAD
глутатионредуктазы и NADН, а восстановительными эквивалентами – e– , H~ и H+ среды.
-
4) GSSG + GR (FAD-NADН) → GSH + H 2 O 2 → GSSG → GR (FAD-NADН).
В вакуоли из цитозоля, как было установлено, активно транспортируется GSSG, предположительно для утилизации (Zechmann et al ., 2006; Noctor et al ., 2011). Однако в вакуолях присутствует GR, поэтому возможен иной путь его метаболизма. Дисульфид глутатиона может восстанавливаться в вакуолярном пространстве и включаться в локальные редокс-процессы. В связи с этим будет формироваться специализированная редокс-цепь, основные элементы которой – GSH, FAD глутатионредуктазы и NADН, а восстановительные эквиваленты – H~ и H+ среды.
Убеждение в том, что редокс-элементы организуют редокс-пути, которые зависят от центральных или ключевых редокс-пар (например, NAD(P)Н, GSH, тиоредоксин и др.) и значительно отличаются функционально и кинетически, усиливается изобилием информации о редокс-зависимом контроле над физиологическими и сигнальными процессами (Jones, 2008). В связи с этим в последние годы все чаще говорят о необходимости моделирования редокс-путей (Jones, 2008; Melissa et al ., 2008; Cherenkevich et al ., 2013).
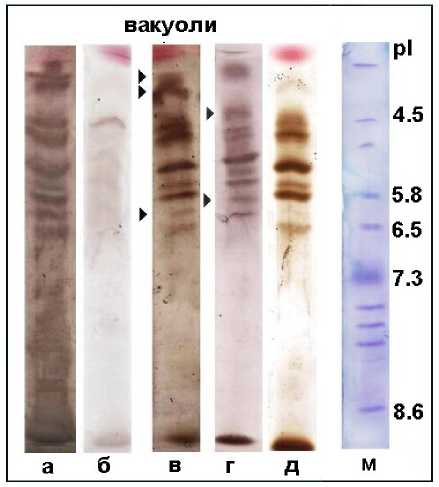
Рисунок 1. Сравнение изоферментного состава пероксидаз вакуолей, катализирующих реакции пероксидазного и оксидазного типа. PAAG после IEF: а – тирозиназная активность с PC и PDA, pH 8.0; б – тирозиназная активность с PC и PDA, pH 5.0; в – пероксидазная активность с PC и PDA, рН5.0; г – тирозиназная активность с PC и PDA в присутствии 1,3-диэтил-2-тиомочевины, акцептора H 2 O 2 , pH 8.0; д – пероксидазная активность с DAB, pH 5.0; м – белковые маркеры для IEF (Pharmacia).
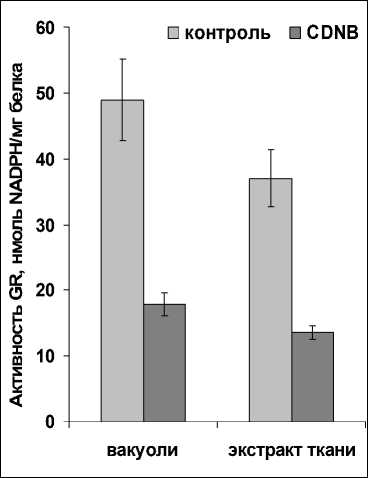
Рисунок 2. Активность глутатионредуктазы в вакуолях и тканевом экстракте, и ее ингибирование CDNB (2 мМ).
А Б
Рисунок 3. Изменение активности глутатионредуктазы в зависимости от pH-условий: А – с NADPH
(100 мкМ). Б – с NADH (100 мкМ).
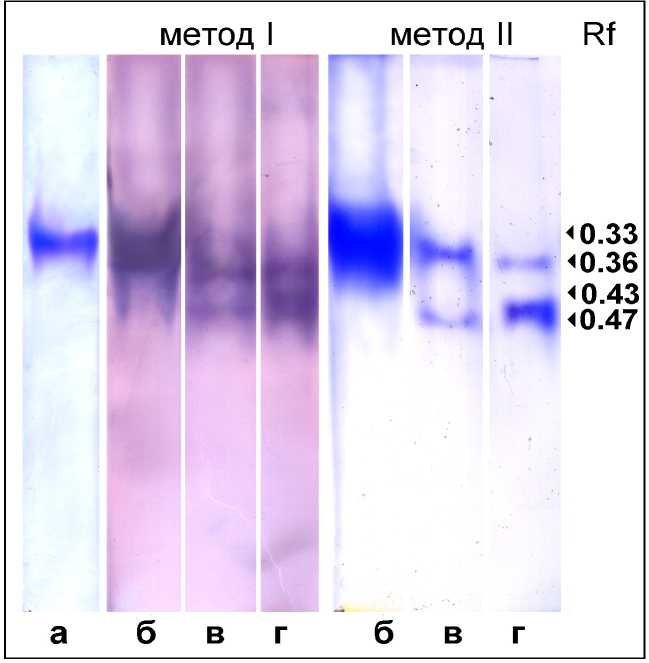
Рисунок 4. Определение активности глутатионредуктазы (GR) в PAAG двумя методами: метод I – с применением MTT и DCPIP; метод II – с DTNB (ферментативная реакция с DTNB проявляется в виде желтого окрашивания, на рисунке для улучшения качества изображения цвет изменен на синий). а – GR пекарских дрожжей, окрашенная Кумасси R-250, служила маркером электроподвижности; б – GR пекарских дрожжей в качестве маркера ферментативной активности; в – активность изоформ GR вакуолей; г – активность изоформ GR экстрактов ткани.
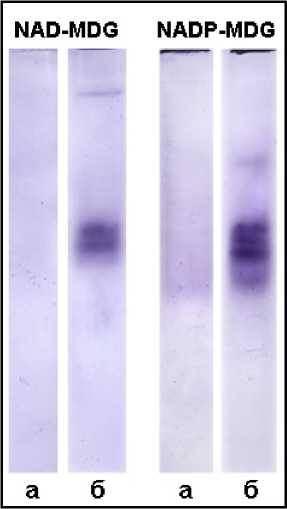
Рисунок 5. Определение интактности вакуолей и чистоты вакуолярных фракций.
Ферментативными маркерами служили NAD-МDH и NADP-МDH, активность которых определяли в PAAG после электрофореза. а – содержимое изолированных вакуолей б – экстракт ткани клеток корнеплодов столовой свеклы.
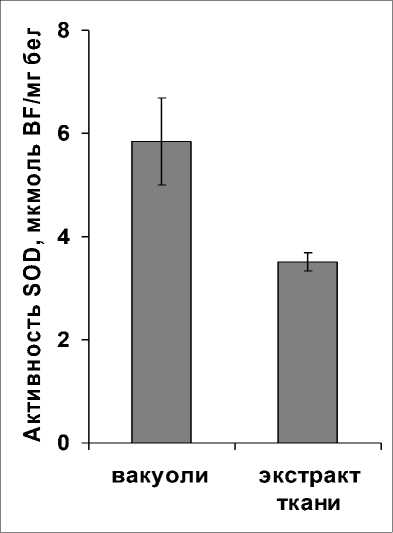
Рисунок 6. Уровень активности супероксиддисмутазы (SOD) во фракциях изолированных вакуолей и тканевом экстракте клеток корнеплодов столовой свеклы. Активность рассчитана на количество образующегося в реакции биформазана (BF).
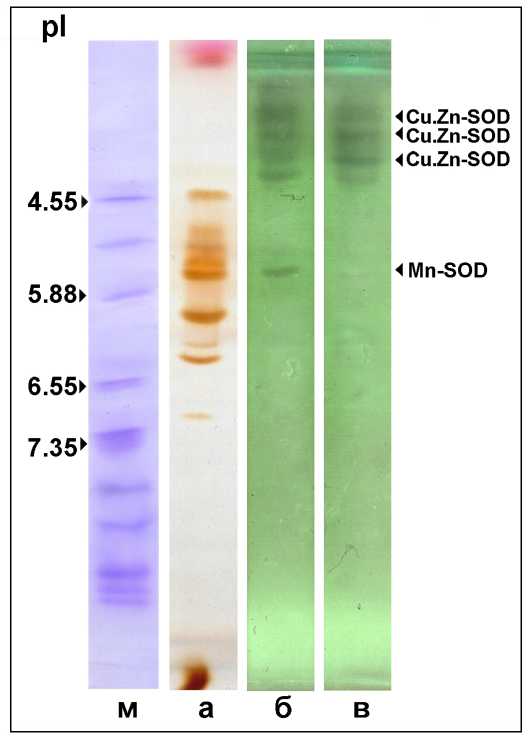
Рисунок 7. Определение супероксиддисмутазной активности во фракциях изолированных вакуолей и тканевом экстракте в PAAG после IEF с помощью зимогафического метода. м – белковые маркеры (Pharmacia); а – активность пероксидазы в тканевом экстракте; б – активность SOD в тканевом экстракте; в – активность SOD в вакуолях. (Изображение зимограмм SOD негативное).
На основании результатов проведенного исследования можно сделать следующие выводы. Во-первых, в центральной вакуоли, как в любом клеточном компартменте, протекают редокс-реакции с участием редокс-ферментов. Во-вторых, исследованные редокс-ферменты вакуолей могут формировать специализированные редокс-цепи, в рамках которых проходят редокс-пути переноса электронов. Моделирование вакуолярных редокс-цепей позволит выявить приоритетные редокс-процессы, происходящие в вакуоли, что расширит представление о ее роли в редокс-метаболизме и, возможно, редокс-сигналинге растительной клетки. ACKNOWLEDGMENT
Исследование выполнено при поддержке
Российского фонда фундаментальных исследований (грант № 14-44-04059 р_сибирь_а).
Список литературы Redox enzymes of red beetroot vacuoles ( Beta vulgaris L.)
- Almagro L., Gomez Ros L.V., Belchi-Navarro S., Bru R., Ros Barcello A. and Pedreno M.A. (2009) Class III peroxidases in plant defense reactions. J. Exp. Bot., 60, 377-390
- Anderson J.V., Hess J.L. and Chevone B. (1990) Purification, characterization and immunological properties for two isoforms of glutathione reductase from eastern white pine needles. Plant Physiol., 94, 1402-1409
- Baranenko V.V. (2006) Superoxide dismutase in plant cells. Tsitologiya (Rus.), 48, 465-474
- Beauchamp C. and Fridovich I. (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem., 44, 276-287
- Berezov T.T. and Korovkin B.F. (1998) Biological chemistry: Uchebnik. 3rd (ed.), Revised. and suppl. M.: Medicine, 704 p
- Bilzer M., Krauth-Siegel R.L., Schirmer R.H., Akerboom T.P.M., Sies H. and Schulz G.E. (1984) Interaction of a glutathione S-conjugate with glutathione reductase kinetic and X-ray crystallographic studies. Eur. J. Biochem., 138, 373-378
- Bradford M. (1976) A rapid and sensitive method for the quantitation of protein utilising the principal of protein-dye binding. Anal. Biochem., 72, 248-254
- Carter C., Pan S., Zouhar J., Avila E.L., Girke T., Raikhel N.V. (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant cell., 16, 3285-3303
- Chen S. and Schopfer P. (1999) Hydroxyl-radical production in physiological reactions a novel function of peroxidase. Eur. J. Biochem., 260, 726-735
- Cherenkevich S.N., Martinovich G.G., Martinovich I.V., Gorudko I.V. and Shamova E.V. (2013) Redox regulation of cellular activity: concepts and mechanisms. Bulletin of the National Academy of Sciences of Belarus. Series of Biological Scien., 1, 92-108
- Coleman J. and Rehm K.H. (2000) Visual biochemistry: Transl. German. M.: Mir, 469 p
- Edwards G.E., Nakamoto H., Bunell J.N. end Hatch M.D. (1985) Pyruvate, Pi dikinase and NADP-malate dehydrogenase in C4 photosynthesis: properties and mechanism of light/dark regulation. Annu. Rev. Plant Physiol., 36, 255-286
- Ferreres F., Figueiredo R., Bettencourt S., Carqueijeiro I., Oliveira J., Gil-Izquierdo A., Pereira D.M., Valentao P., Andrade P.B., Duarte P., Barcelo A.R. and Sottomayor M. (2011) Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: an H2O2 affair? J. Exp. Botany., 62, 2841-2854
- Gaal O., Medgyesi G.A. and Vereczkey L. (1980) Electrophoresis in the separation of biological macromolecules. New York: John Wiley & Sons Ltd, 422 p
- Giannopolitis C.N. and Ries S.K. (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol., 59, 309-314
- Jones D.P. (2008) Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol., 295, 849-868
- Kritskii M.S. and Telegina T.A. (2004) Coenzymes and evolution of the RNA world. Uspekhi Biologicheskoi Khimii, 44, 341-361
- Ksenzhek O.S. and Petrova S.A. (1986) The electrochemical properties of the redox-reversible biological systems. M.: Nauka, 152 p
- Leigh R.A., Branton D. (1976) Isolation of vacuoles from root storage tissue of Beta vulgaris L. Plant Physiol., 58, 656-662
- Levites E.V. (1986) Genetics of plant isozymes. Nauka: Sibir. Dep., 144 p
- Li J.L.Y., Sulaiman M., Beckett R.P. and Minibayeva F.V. (2010) Cell wall peroxidases in the liverwort Dumortiera hirsuta are responsible for extracellular superoxide production, and can display tyrosinase activity. Physiol. Plantarum., 138, 474-484
- Martinovich G.G. and Cherenkevich S.N. (2008) Redox processes in cells: Monograph. Mn.: BSU, 159 p
- Marty L., Siala W., Schwarzlander M., Fricker M.D., Wirtz M., Sweetlove L.J., Meyer Y., Meyer A.J., Reichheld J.P. and Hell R. (2009) The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A., 106, 9109-9114
- Melissa K., Go Y.M. and Jones D.P. (2008) Non-equilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med., 44, 921-937
- Murray R., Grenner D., Meyes P. and Rodwell V. (1993) Biochemiya cheloveka (Harper's biochemistry. 2 volumes. V. 1. Translat. Eng.M.: Mir, 384 p
- Nishikimi M., Rao N.A. and Yagi K. (1972) The occurrence of superoxide anion in the reaction of reduced phenasine methosulfate and molecular oxygen. Biochem. Biophys. Res. Comm., 46, 849-856
- Noctor G., Queval G., Mhamdi A. Chaouch S. and Foyer C.H. (2011) Glutathione. The Arabidopsis Book. Published By: The American Society of Plant Biologists URL: http://www.bioone.org/doi/full/10.1199/tab.0142, 9, 2-32
- Pradedova E.V., Isheeva O.D. and Salyaev R.K. (2009) Superoxide dismutase of palnt cell vacuoles. Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology, 3, 24-32
- Pradedova E.V., Nimaeva O.D., Trukhan I.S. and Salyaev R. K. (2014) Tyrosinase and superoxide dismutase activities of peroxidase in the vacuoles of beet roots. Russian J. of Plant Physiol., 61, 70-79
- Queval G., Jaillard D., Zechmann B. and Noctor G. (2011) Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell and Environ., 34, 21-32
- Rautenkranz A.F., Li L., Machler F., Martinoia E. and Oertli J.J. (1994) Transport of ascorbic and dehydroascorbic acids across protoplast and vacuole membranes isolated from barley (Hordeum vulgare l. cv Gerbel) leaves. Plant Physiol., 106, 187-193
- Rogozhin V.V., Verkhoturov V.V. and Rogozhina T.V. (2004) Peroksidaza: stroenie i mekhanizm deistviya (Peroxidase: structure and mechanism of action). Irkutsk: Irkutsk. Gos. Tech. Univ., 200 p
- Saab-Rincon G. and Valderrama B. (2009) Protein engineering of redox-active enzymes. Antioxidants & redox signaling, 11, 167-189
- Salyaev R.K., Kuzevanov V.Y., Khaptogaev S.B. and Kopytchuk V.N. (1981) Isolation and purification of vacuoles and vacuolar membranes from plant cells. Russ. J. Plant Physiol., 25, 1295-1305
- Shao H. B., Chu L. Y., Lu Z. H. and Kang C. M. (2008) Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci., 4, 8-14
- Shigeoka S., Onishi T., Nakano Y. and Kitaoka S. (1987) Characterization and physiological function of glutathione reductase in Euglena gracilisz. Biochem. J., 242, 511-515
- Tommasini R., Martinoia E., Grill E., Dietz K.J. and Amrhein N. (1993) Transport of oxidized glutathione into barley vacuoles: evidence for the involvement of the glutathione-S-conjugate ATPase. Zeitschrift Naturforsch., 48, 867-871
- Vanoni M.A., Wong K.K., Ballou D.P. and Blanchard J.S. (1990) Glutathione reductase: comparison of steady-state and rapid reaction primary kinetic isotope effects exhibited by the yeast, spinach, and Escherichia coli enzymes. Biochemistri, 29, 5790-5796
- Voskresenskaya, O.L., Alyabysheva, E.A. and Polovnikova, M.G. (2006) Bol'shoi praktikum po bioekologii, Ch. 1 (Large Manual on Bio-Ecology), Ioshkar-Ola: Mariisk. Gos. Univ., 107 p
- Wingsle G., Gardestrom P., Haligren J.E. and Karpinski S. (1991) Isolation, purification, and subcellular localization of isozymes of superoxide dismutase from scots pine (Pinus sylvestris L.) Needles1. Plant Physiol., 95, 21-28
- Yudina R.S. (2012) Malate dehydrogenase in plants: Its genetics, structure, localization and use as a marker. Advances in Bioscience and Biotechnology, 3, 370-377
- Zholnin A.V. (2012) General chemistry: a textbook. V.A., Popkov, A.V., Zholnina (ed). M.: GEOTAR Media, 400 p


