Regenerative ability and micropropagation of Petunia hybrida in vitro
Автор: Borovaya Svetlana A., Boginskaya Natalia G.
Журнал: Овощи России @vegetables
Рубрика: Селекция, семеноводство и биотехнология растений
Статья в выпуске: 6 (68), 2022 года.
Бесплатный доступ
Scientific relevance. The garden petunia, Petunia hybrida, is a popular and wide spread ornamental crop from the family Solanaceae. It is a promising model plant for molecular and genetic research. In vitro micropropagation plays an important role in the distribution of the garden petunia because the survivability and quality of seed material decreases significantly in every subsequent generation. Besides, micropropagation reduces the cost of production substantially. Considering that very few researchers addressed this question in the Russian Federation, this direction of research is still worthy of attention. Materials and methods. The experiments were conducted by the Laboratory of Breeding and Genetic Research on Field Crops at FSBSI “Federal Scientific Center of Agricultural Biotechnology of the Far East named after A.K. Chaiki”. Seeds of Petunia hybrida (double-flowered) were used as primary explants. Liquid bleacher ACE diluted with distilled water in the proportion 1:9 was used as a sterilizing agent (the working solution contained 0.50% NaOCl). The total time of exposure was 15 minutes. The primary explants were subcultured onto a hormone-free Murashige and Skoog basal medium containing 20 g/L sucrose and 6 g/L agar. Isolated in vitro objects were cultured in test tubes with cotton-gauze plugs at an illuminance of 4000 lx, a temperature of 22-25 °C, and a 16h photoperiod in a culture room. The duration of one passage was 60 days. Micropropagation was carried out using 710 mm cuttings with one or two nodes. The pot culture of the regenerants was established under controlled conditions in a light room (photoperiod was 16 hours, temperature was 23°С). Results. The optimal method for introducing Petunia hybrida into cell culture is the use of seeds treated with the solution of bleacher ACE that was diluted with distilled water in the proportion 1:9. The optimal time of exposure is 15 minutes. Petunia hybrida demonstrated a high regeneration rate on the hormone-free MS medium - it had a fast growth and development rate, and good rhizogenesis; the reproductive rate was 8.77. For the micropropagation of the garden petunia, it is advisable to use cuttings of test tube plants, which should be placed onto a hormone-free MS medium. The test tube plants of Petunia hybrida acclimatized successfully on a soil substrate. This shows the high plasticity of the culture.
Petunia hybrida, micropropagation, sterilizing agent, regenerants, in vitro, pot culture
Короткий адрес: https://sciup.org/140296493
IDR: 140296493 | УДК: 635.9:(581.143.5+573.6) | DOI: 10.18619/2072-9146-2022-6-24-28
Текст научной статьи Regenerative ability and micropropagation of Petunia hybrida in vitro
urrently biotechnological methods are widely used both for propagating valuable genotypes (cell, tissue and organ cultures, micropropagation, etc.) and for creating a new genetically unique starting material (cell selection, the use of somaclonal variations, the development of induced mutants, etc.). The potential of plant regeneration has been exploited for a long time to multiply clones, cuttings and graftings, thus being a foundation for many research studies [1]. The method of micropropagation is employed for commercial purposes all around the world, though the regenerative ability of plants varies significantly among genotypes [2-4].
A topical research direction is the development of methods and approaches for introducing crops into cell culture and for micropropagating plants. Besides their esthetic value, such plants can be used as indicators of viral infection for monitoring viruses of many important agricultural crops. The garden petunia is one of these plant species. P. hybrida , a species from the family Solanaceae ,includes about 30 subspecies [5]. It has colorful flowers, which bloom for a long time. The esthetic value of the garden petunia makes it an economically important species cultivated all around the world [6,7]. Petunia seeds comprise 30% of the global seed production of floriculture crops [8]. Additionally, the garden petunia is a promising model plant for molecular biology, plant genetics, etc. [9]. P. hybrida has been used as genetic material for somatic hybridization, molecular, biochemical, and cytogenetic research studies, as well as functional analysis [10]. It is a plant-differentiator of viral infections for other crops. Besides, Petunia hybrida might be used as a research object for studies on thebiosynthesis of flavonoids, plant improvement, and selfincompatibility [11-13].
The garden petunia is mainly grown from seeds and micropropagation plays an important role in their production. Taking into account that the quality of plants decreases in every subsequent generation, the preservation of F 1 progeny is crucial for future multiplication and survivability [14]. Furthermore, propagating ornamental plants under field conditions is limited by such factors as disease spread, considerable expenditures, an adverse effect on environment owing to pesticide and fertilizer application, a need in a large land area, etc [15]. These factors emphasize the importance of cell culture and micropropagation for the cultivation of floriculture crops. The micropropagation of F 1 P. hybrida plants raises a possibility for the preservation of their qualitative characteristics, e.g. flower color and longevity, plant shape, and increases the success rate of plant multiplication. By contrast, the germination rate among F 1 hybrids is only 50-60% in the case of an ordinary planting of petunia seeds in soil [16].
Some foreign authors describe successful cases of petunia micropropagation in vitro through direct organogenesis of shoot tips [17], seeds [18], and nodal explants [15] and through indirect organogenesis from stem segments and leaf pieces [19], internodes [5], and shoot tips [20]. According to several researchers, the best medium for direct organogenesis of P. double explants from shoot tips and nodes is an MS basal medium supplemented with 1 mg/L of 6-benzylaminop-urine (BA) and 1 mg/L of 1-naphthaleneacetic acid (NАА) [15]. A maximum elongation of shoots was observed on an MS medium supplemented with 0.2 mg/L of gibberellic acid (GA). Seedlings grown under these conditions produced numerous leaves and nodes. Auxins, such as 1-naphthaleneacetic acid (NAA) and indole-3-butyric acid (IBA), added to an MS medium in a concentration of 0.2 mg/L and 1 mg/L, respectively, were the most beneficial for the root formation of P. hybrida.
Leaf bases, internodes and cotyledons of petunia seedlings are used to obtain callus [5]. The most successful protocol for the propagation of P. hybrida in vitro was implemented via the culturing of internodes on an MS medium supplemented with 0.5 mg/L of BA. The organogenesis of the shoots that were obtained using Petunia leaf blades was noted to be influenced by the explant size, the configuration and the duration of exposure to BA [21]. Z.A. Farooq et al. [14] reveals that the maximum induction of callus can be reached by using BA in a dose of 1.0 mg/L and 2,4-dichlorophenoxyacetic acid (2,4-D) in a dose of 1.5 mg/L. The highest regeneration of callus was achieved on an MS medium enriched with 2.0 mg/L of kinetin and 0.5 mg/L of IBA. The proliferation of P. hybrida was highest on media contenting 0.5 mg/L of BA and 0.5 mg/l of IBA. The best root formation was observed on an MS medium containing 1.0 mg/L of IBA.
According to D. Kulpa et al. [22], the garden petunia should be propagated in vitro on an MS medium supplemented with 0.5 mg/l of gibberellic acid. Plants propagated under these conditions grow to a considerable height and produce numerous leaves and lateral shoots. Indole-3-acetic acid (IAA) added to an MS medium in a dose of 0.5 and 1.0 mg/dm3 was most beneficial for the root formation of the petunia Flash Red × atkinsiana D. Don. These petunia plants had long numerous roots. The optimal medium for the induction of flowering in petunia plants is an MS medium supplemented with 0.5 mg/dm3 of kinetin. The use of kinetin resulted in the formation of the highest number of flowers with a normal morphological structure.
Considering that biotechnological approaches might reduce the cost of production significantly and there have been very few research studies on petunia tissue culture in the Russian Federation, this direction of research appear to be worthy of attention.
The goal of this research was to study morphological characteristics of Petunia hybrida regenerants and the micropropagation of petunia plants in vitro .
Materials and methods
Seeds of Petunia hybrida (double-flowered) were bought from the agrobusiness holding company “POISK” (the producer “Tsentr-Ogorodnik”, Llc, Russia). To be introduced into the culture, the seeds were sterilized with newly prepared bleacher ACE diluted with distilled water in the proportion 1:9 (the working solution contained 0.50% NaOCl). Container with the seeds was stirred up at regular intervals. The total time of exposure was 15 minutes. The plant material was rinsed with autoclaved distilled water three times for five minutes each time in a laminar flow cabinet. The primary explants were cultured onto a hormone-free Murashige and Skoog basal medium [23] containing 20 g/L sucrose and 6 g/L agar. The pH of the saline medium was brought to 5.7-6.0 using 1 n KOH. Isolated in vitro objects were cultured in test tubes with cottongauze plugs at an illuminance of 4000 lx, a temperature of 22–25 °C, and a 16h photoperiod in a culture room. The duration of one passage was 60 days. Micropropagation was carried out using single-node cuttings 7-10 mm in length. The box, laboratory glassware and instruments employed were prepared andsterilized according to generally accepted methods.
The pot culture of the regenerants was established under controlled conditions in a light room with a 16h photoperiod and a temperature of 23°С.
Results and discussion
Sterilizing the P. hybrida seeds with a 0.5% solution ofNaOCl for 15 minutes was highly effective – none of the explants introduced into the culture was infected. The first leaves appeared on the 2nd-3rd day. The seeds germinated well on an MS medium without plant hormones – the first roots were observed on the 4th-5th day. The initiation of axillary bud development occurred simultaneously. The root length increased considerably and ranged from 7 to 21 mm on the 11th day of cultivation (Figure 1). H. Abu-Qaoud et al. [18] obtained analogous results in an experiment, where a 0.52% solution of NaClO was used as a sterilizing agent for P. hybrida seeds, which were later inoculated on a hormone-free MS medium. The germination rate was 100%. R.A. Dixon et al. [24] also reported that a high germination rate ofpetunia seeds was achieved on an agar-based medium without plant growth regulators. In our research, the germination rate of the seeds cultured onto a hormone-free nutrient medium was 73.9%. This might be linked first of all to their initial qualitative characteristics because a preliminary sowing in pots with a soil substrate showed a 68.5% germination rate of the garden petunia.
On the 21 st day of cultivation, P. hybrida microshoots of dif-

Figure 1. Developmentof am icroshooton aprimary explantof Petuniahybrida(the 11 th day of cultivation)
ferent length were forming, axillary buds continued to develop in their leaf axils (Figure 2, Figure 3a).
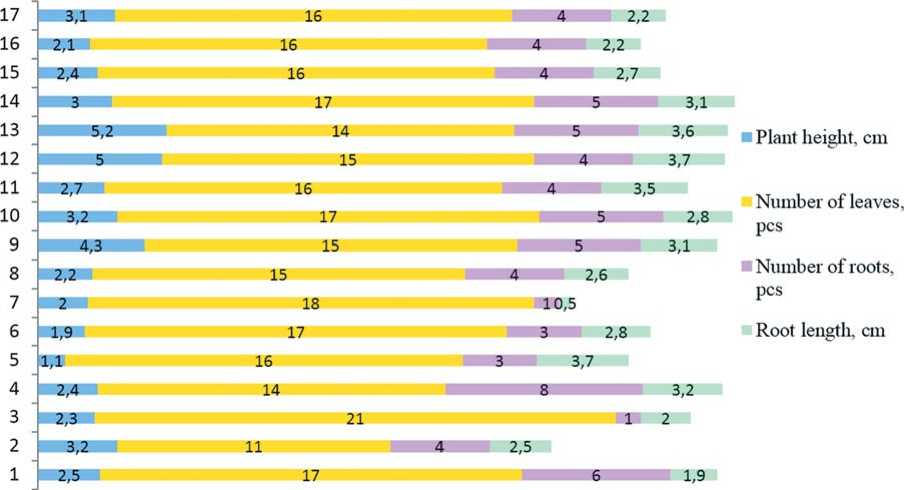
The plant height ranged from 1.1 to 5.2 cm and was 2.86 cm on average. The number of leaves was 11-18 per plant (15.9 leaves on average). Rhizogenesis was considered to be the most important parameter characterizing the adaptability of the plants. In our experiments, the 21-day-old regenerants had up to eight roots (4.1 roots on average) and the average оо
и
8 с
£ g
|
Statistical parameters |
Plant height, cm |
Number of leaves, pcs |
Number of roots, pcs |
Root length, cm |
|
MEAN case 1-17 |
2.9 |
15.9 |
4.1 |
2.7 |
|
MEDIAN case 1-17 |
2.5 |
16 |
4 |
2.8 |
|
SD case 1-17 |
1.09 |
2.08 |
1.65 |
0.81 |
|
MIN case 1-17 |
1.1 |
11 |
1 |
0.5 |
|
MAX case 1-17 |
5.2 |
21 |
8 |
3.7 |
|
25th% case 1-17 |
2.2 |
15 |
4 |
2.2 |
|
75th% case 1-17 |
3.2 |
17 |
5 |
3.2 |
Figure 2. Characteristics of the growth and developmentof Petuniahybrida in vitro on the 21 st day of cultivation

Figure 3. Petuniahybridain vitro:
а – the 21 st day of cultivation,b – the 40 th day of cultivation
root length was 2.7 cm. Similar results were obtained for P. hybrida Vilm. Cv. “Bravo” when it was grown on an MS medium supplemented with BA and IBA [14].
It should be noted that the regenerants had a high growth and development rate on the employed nutrient medium (an MS without growth regulators). On the 40 th day of cultivation, the plants were 4.7-12.8 cm in height and had a great number of internodes. Reproductive buds started to form on a few specimens (Figure 3b). At the end of the passage (on the 60 th day of cultivation) the plant height ranged from 17.3 to 25.9 cm. The plants were characterized by good leaf coverage and rhizogenesis. As the result, the reproductive rate of the garden petunia was 8.77 on average.
In contrast, R.R. Habas et. all [15] suggested a protocol for

Figure 4. Cuttings of Petuniahybrida on an MS medium (the 7 th day of cultivation)
the in vitro regeneration of the garden petunia from seeds (an 8%solution of NaOCl was used as a sterilizing agent, the exposure time was10 minutes) with a subsequent intermediate and final passage of explants from shoot tips and nodes obtained from the seedlings onto an MS medium supplemented with plant hormones. The goal of the experiment was to obtain regenerants with roots. A maximum shoot length of 5.8 cm was recorded on the 21 st day, the survival rate was 70%. R.R. Habas et. all also noted that the disease rate among the inoculated seeds was 30-80% when the working solution had lower concentrations of NaOCl (4-6%). Higher concentrations (10%) resulted in the absence of germination. Our experiments showed that an increase of seed exposure time to 15 minutes allowed us to decrease the concentration of NaOCl. This reduced the toxic impact on the embryos and simultaneously made the sterilization of the primary explants more effective.
Some researchers suggested protocols for the micropropagation of the garden petunia in vitro via callus culture [14, 2527]. The authors use different methods with various primary explants (shoot tips, segments of nodes and leaves, flower explants) and a stepwise application of sterilizing agents, for example, 0.02% fungicide (carbendazim), 0.1% HgCl 2 , and 70% ethanol. A new nutrient medium with a certain proportion of plant growth regulators is required for every mandatory stage of the experiment – callus formation, regeneration, and organogenesis. The number of successfully acclimatized micropropagated plants does not exceeds 20 per one explant. We believe that this approach might not be cost- and time-efficient enough for the production of Petunia double planting material. However, it is generally accepted as an effective

Figure 5. Establishmentof the potculture of P. hybridaregenerants
method for obtaining somaclonal variations in plant breeding.
The regenerated plants with well-developed roots acclimatized successfully and were transferred to pots with a soil substrate where they continued to grow under laboratory conditions. The soil substrate for root formation consisted of soil and vermiculite in the proportion 4:1. For more successful acclimatization, the regenerants were covered with plastic hoods (Figure 5).
The plants were kept under the plastic hoods for 14 days, and then the hoods were removed. The acclimatized plants grew and developed normally. The survival rate was high; the percentage of dead plants was 3.5%. Subsequently, the Petunia hybrida plants were transferred to flower beds in vivo, where they bloomed abundantly until the first frosts in autumn.
Conclusions
-
1. The optimal method for sterilizing the Petunia hybrida seeds used as the primary explants for the introduction into the in vitro culture was to treat them with bleacher ACE diluted
-
2. The Petunia hybrida regenerants showed a high growth and development rate on the hormone-free MS medium. The first leaves appeared on the 2nd-3rd day of cultivation. The initiation of axillary bud development and the formation of the first roots occurred on the 4 th -5 th day.
-
3. Petunia hybrida maintained a high regeneration rate for the whole passage. The obtained regenerants were characterized by good leaf coverage and rhizogenesis as well as a high reproductive rate (8.77).
-
4. The plant cuttings 7-10 mm in length with an axillary bud and two-three leaves developed successfully on the hormone-free MS medium. The survival rate was 100%. This method for micropropagating Petunia hybrida was proved to be effective.
-
5. The test tube plants of Petunia hybrida had a highsurvival rate on the soil substrate (the percentage of dead plants is 3.5%). This demonstrates the high plasticity of the culture.
with distilled water in the proportion 1:9 (the working solution contained 0.50% NaOCl) for 15 minute.
Об авторах:
Лаборатория селекционно-генетических исследований полевых культур, автор для переписки, ,
Наталия Геннадьевна Богинская – младший научный сотрудник,
Лаборатория селекционно-генетических исследований полевых культур, ,
Aboutthe Authors:
Svetlana A. Borovaya – Postgraduate Student, Researcher, the Laboratory of Breeding and Genetic Research on Field Crops, Corresponding Author, ,
Natalia G. Boginskaya – Junior Researcher, the Laboratory of Breeding and Genetic Research on Field Crops, ,
-
• Литература / References
-
1. Hill K., Schaller G.E. Enhancing plant regeneration in tissue culture. Plant Signaling & Behavior. 2013;(8):10.
-
-
2. Ikeuchi M., Ogawa Y., Iwase A., Sugimoto K. Plant regeneration: Cellular origins and molecular mechanisms. Development. 2016;(143):1442–1451. 3. Melnyk C.W., Meyerowitz E.M. Plant grafting. Curr. Biol. 2015;(25):183–188.
-
4. Zhang H., Zhang T.T., Liu H., Shi D.Y., Wang M., Bie X.M., Li X.G., Zhang X.S. Thioredoxin mediated ROS homeostasis explains natural variation in plant regeneration. Plant Physiol. 2018;(176):2231-2250.
-
5. Gomaa S.E., Esmaiel N.M. In vitro Preliminary Study on Petunia hybri-da breeding under Sodium Chloride Stress Conditions. Middle East Journal of Agriculture Research. 2015;4(4):867-872.
-
6. Solano R., Nieto C., Avila J., Canas L., Diza I., Paz-Ares J. Dual DNA binding specificity of a petal epidermis – specific MYB transcription factor from Petunia hybrida. EMBO J. 1995;(14):1773-1784.
-
7. Davies K.M., Bloor S.J., Spiller G.B., Deroles S.C. Production of yellow color in flowers: redirection of flavonoid biosynthesis in petunia. PLANT J. 1998;(13):259-266.
-
8. Козлова Е.А. Совершенствование технологий выращивания, размножения и оценка декоративных качеств линий петунии гибридной ( Petunia x Hibrida Vilm.). Москва, 2016. 26 с. [Kozlova E.A. Improvement of technologies for growing, reproduction and evaluation of the decorative qualities of hybrid petunia lines ( Petunia x Hibrida Vilm.). Moscow, 2016. 26 p. (In Russ.)]
-
9. Gerats T., Strommer J. Petunia. Evolutionary, developmental and physiological genetics. Second Edition. 2009. 435 р.
-
10. Angenent G.C., Franken J., Busscher M., Colombo L and van Tunen А.J. Petal and stamen formation in Petunia is regulated by the homeotic gene fbp1. Plant J. 1993;(4):101-112.
-
11. Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;(126):485-493.
-
12. Souer E., Houwelingen A., Kloos D., Moland J, Koes R. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordial boundaries. Cell. 1996;(85):159-170.
-
13. Farshad T., Hinkley C.S and Ramprashad N. A Comparison of DNA Extraction Methods using Petunia hybrida Tissues. J. Biomolecular Techniques. 2013;(24):113-118.
-
14. Farooq I., Qadri Z.A., Rather Z.A., Nazki A.T., Banday N., Rafiq S., Masoodi K., Alotaibi S., Mansoor S. Optimization of an improved, efficient
and rapid in vitro micropropagation protocol for Petunia hybrida Vilm. Cv. “Bravo”. Saudi Journal of Biological Sciences. 2021.
-
15. Habas R.R., Turker M., Ozdemir F.A. In vitro Multiple Shoot Regeneration from Petunia hybridа. Turkish Journal of Agriculture - Food Science and Technology. 2019;7(10):1554-1560.
-
16. Begum S.S., Arundhati A. Study Of Phytohormone Autonomy In In Vitro In Petunia Hybrida. International Journal of Advanced Biotechnology and Research. 2016;7(2):536-539. http://www.bipublica-tion.com .
-
17. Sharma A.K., Mitra G.C. In vitro culture of shoot apical meristem of Petunia hybrida for mass production of plants. Indian journal of experimental biology. 1976. Р.348-350.
-
18. Abu-Qaoud H., Abu-Rayya A., Yaish S. In vitro regeneration and somaclonal variation of Petunia hybridа. Journal of Fruit and Ornamental Plant Research. 2010;18(1):71-81.
-
19. Rao P.S., Handro W., Harada H. Bud formation and embryo differentiation in in vitro cultures of Petunia. Zeitschrift für Pflanzenphysiologie. 1973;69(1):87-90. https://orcid.org/10.1016/SOO44-328X(73)80156-4
-
20. Dash S.N., Singhsamant P.K. Induction of plantlets and callus from shoot-tips of Petunia hybrida cultured in vitro. Orissa Journal of Horticulture. 1990;18(1-2):65-69.
-
21. Beck M.J., Camper N.D. Shoot regeneration from petunia leaf discs as a function of explant size, configuration and benzyladenine exposure. Plant Cell, Tissue and Organ Culture. 1991;26(2):101-106. https://orcid.org/10.1007/BF00036113
-
22. Kulpa D., Nowak N. In vitro flowering of Petunia atkinsiana D. Don. Folia Hort. 2011;23(2):125-129.
-
23. Murashige T.; Skoog F. A revised medium for rapid growth and bio assays with tobacco tussue cultures. Physiol. Plant. 1962;(15):473-497. https://orcid.org/10.1098/rstb.2000.0713
-
24. Dixon R.A., Gonzales R.A. (eds). Plant cell culture: a practical approach. Oxford University Press, New York Published by British Library Cataloguing in Publication. 1995. 250 р.
-
25. Mishra A., Panday R.K., Sharma J.P., Kumar J. In vitro propagation of petunia ( Petunia hybrida ) var “Cascade Burgundy” through multiple shoot culture. Environment and Ecology. 2006;24S(1):109-111.
-
26. Li F., Li C., Li M., Yu M., Fang C., Shenghua W. In vitro culture of Petunia hybrida microspores and Agrobacterium -mediated transient expression of β -glucuronidase (GUS) reporter gene. Int. J. Agric. Biol. 2013;(15):1098‒1104.
-
27. Burbulis N., Blinstrubiene A., Jonytiene V. In vitro regeneration from leaf explants of Petunia hybrida L. Propagation of Ornamental Plants. 2015;15(2):47-52.
Список литературы Regenerative ability and micropropagation of Petunia hybrida in vitro
- Hill K., Schaller G.E. Enhancing plant regeneration in tissue culture. Plant Signaling & Behavior. 2013;(8):10. https://orcid.org/10.4161/psb.25709
- Ikeuchi M., Ogawa Y., Iwase A., Sugimoto K. Plant regeneration: Cellular origins and molecular mechanisms. Development. 2016;(143):1442-1451.
- Melnyk C.W., Meyerowitz E.M. Plant grafting. Curr. Biol. 2015;(25):183-188.
- Zhang H., Zhang T.T., Liu H., Shi D.Y., Wang M., Bie X.M., Li X.G., Zhang X.S. Thioredoxin mediated ROS homeostasis explains natural variation in plant regeneration. Plant Physiol. 2018;(176):2231-2250.
- Gomaa S.E., Esmaiel N.M. In vitro Preliminary Study on Petunia hybrida breeding under Sodium Chloride Stress Conditions. Middle East Journal of Agriculture Research. 2015;4(4):867-872.
- Solano R., Nieto C., Avila J., Canas L., Diza I., Paz-Ares J. Dual DNA binding specificity of a petal epidermis - specific MYB transcription factor from Petunia hybrida. EMBO J. 1995;(14):1773-1784.
- Davies K.M., Bloor S.J., Spiller G.B., Deroles S.C. Production of yellow color in flowers: redirection of flavonoid biosynthesis in petunia. PLANT J. 1998;(13):259-266.
- Козлова Е.А. Совершенствование технологий выращивания, размножения и оценка декоративных качеств линий петунии гибридной (Petunia x Hibrida Vilm.). Москва, 2016. 26 с.
- Gerats T., Strommer J. Petunia. Evolutionary, developmental and physiological genetics. Second Edition. 2009. 435 р. https://orcid.org/10.1007/978-0-387-84796-2
- Angenent G.C., Franken J., Busscher M., Colombo L and van Tunen А.J. Petal and stamen formation in Petunia is regulated by the homeotic gene fbp1. Plant J. 1993;(4):101-112.
- Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;(126):485-493.
- Souer E., Houwelingen A., Kloos D., Moland J, Koes R. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordial boundaries. Cell. 1996;(85):159-170.
- Farshad T., Hinkley C.S and Ramprashad N. A Comparison of DNA Extraction Methods using Petunia hybrida Tissues. J. Biomolecular Techniques. 2013;(24):113-118.
- Farooq I., Qadri Z.A., Rather Z.A., Nazki A.T., Banday N., Rafiq S., Masoodi K., Alotaibi S., Mansoor S. Optimization of an improved, efficient and rapid in vitro micropropagation protocol for Petunia hybrida Vilm. Cv. “Bravo”. Saudi Journal of Biological Sciences. 2021. https://doi.org/10.1016/j.sjbs.2021.05.018.
- Habas R.R., Turker M., Ozdemir F.A. In vitro Multiple Shoot Regeneration from Petunia hybridа. Turkish Journal of Agriculture - Food Science and Technology. 2019;7(10):1554-1560. https://doi.org/10.24925/turjaf.v7i10.1554-1560.2570
- Begum S.S., Arundhati A. Study Of Phytohormone Autonomy In In Vitro In Petunia Hybrida. International Journal of Advanced Biotechnology and Research. 2016;7(2):536-539. http://www.bipublication.com.
- Sharma A.K., Mitra G.C. In vitro culture of shoot apical meristem of Petunia hybrida for mass production of plants. Indian journal of experimental biology. 1976. Р.348-350.
- Abu-Qaoud H., Abu-Rayya A., Yaish S. In vitro regeneration and somaclonal variation of Petunia hybridа. Journal of Fruit and Ornamental Plant Research. 2010;18(1):71-81.
- Rao P.S., Handro W., Harada H. Bud formation and embryo differentiation in in vitro cultures of Petunia. Zeitschrift für Pflanzenphysiologie. 1973;69(1):87-90. https://orcid.org/10.1016/SOO44-328X(73)80156-4
- Dash S.N., Singhsamant P.K. Induction of plantlets and callus from shoot-tips of Petunia hybrida cultured in vitro. Orissa Journal of Horticulture. 1990;18(1-2):65-69.
- Beck M.J., Camper N.D. Shoot regeneration from petunia leaf discs as a function of explant size, configuration and benzyladenine exposure. Plant Cell, Tissue and Organ Culture. 1991;26(2):101-106. https://orcid.org/10.1007/BF00036113
- Kulpa D., Nowak N. In vitro flowering of Petunia atkinsiana D. Don. Folia Hort. 2011;23(2):125-129.
- Murashige T.; Skoog F. A revised medium for rapid growth and bio assays with tobacco tussue cultures. Physiol. Plant. 1962;(15):473-497. https://orcid.org/10.1098/rstb.2000.0713
- Dixon R.A., Gonzales R.A. (eds). Plant cell culture: a practical approach. Oxford University Press, New York Published by British Library Cataloguing in Publication. 1995. 250 р.
- Mishra A., Panday R.K., Sharma J.P., Kumar J. In vitro propagation of petunia (Petunia hybrida) var “Cascade Burgundy” through multiple shoot culture. Environment and Ecology. 2006;24S(1):109-111.
- Li F., Li C., Li M., Yu M., Fang C., Shenghua W. In vitro culture of Petunia hybrida microspores and Agrobacterium-mediated transient expression of β-glucuronidase (GUS) reporter gene. Int. J. Agric. Biol. 2013;(15):1098‒1104.
- Burbulis N., Blinstrubiene A., Jonytiene V. In vitro regeneration from leaf explants of Petunia hybrida L. Propagation of Ornamental Plants. 2015;15(2):47-52.


