Regulation of NPR1 Under Salinity and Osmotic Stress in Soybean (Glycine max L.) Leaves
Автор: Burcu Seckin Dinler, Eda Tasci
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.16, 2020 года.
Бесплатный доступ
Aim of study: Non expressor of pathogenesis related gene (NPR1) is a key regulator of the SA dependent systemic acquired resistance (SAR) in plants. Although NPR1 is a well known important regulator of salicylic acid to biotic stress, researching on abiotic stress have not yet been well founded. Materials and methods: With this aim, soybean (Glycine max L.) SA88 plants were grown with Hoagland solution for two weeks. Seedlings were treated with 200 mM NaCl, 10 % PEG 6000 and 200 mM NaCl + 10 % PEG 6000 and harvested at 2h, 4h, 6h (short term) and 7 day and 14 day (long term) of treatment. Main results: The results showed that plants treated with NaCl showed a better defense response in physiological parameters than PEG. Additionally, PEG stress lead to more oxidative damage at long term, while combined stress at short term in soybean leaves. Otherwise, the highest accumulation of ABA, SA and proline level was with PEG treatment at both short term and long term of treatment. However, GmNPR1 gene expressions were upregulated with PEG stress significantly at 7 day and combined stress at 14 day. Discussion: Considering the short term effects on GmNPR1 transcript levels, combined stress were more pronounced compared to NaCl and PEG stress alone. Research highligts: Consequently, this work firstly determined that osmotic stress may role as a potential signal but not salt stress for the regulation of NPR1 in soybean leaves.
NPR1, osmotic stress, salt stress, soybean
Короткий адрес: https://sciup.org/143173854
IDR: 143173854
Текст научной статьи Regulation of NPR1 Under Salinity and Osmotic Stress in Soybean (Glycine max L.) Leaves
Salinity and osmotic stress are regarded as two important abiotic stress factors reduces plant growth and development. Under salt stress, plants accumulate excess Na+ and Clˉ, which leads to limited water and nutrient uptake, altered metabolic and photosynthetic activity, and increased lipid peroxidation (Deinlein et al. 20 4; Liang et al. 20 8). Osmotic stress induces detrimental effects on crop productivity by arresting numerous plant metabolic processes such as loss of turgor, carbon assimilation rate, leaf gas exchange and increased oxidative damage. Although they have similar effects on plant metabolism, it has been suggested that the plants suffers from osmotic stress firstly then later affected by salinity (Munns et al 2002). They have to cope with two these detrimental factors at the same time and consume more energy to survive.
Both stress increases the production of various forms of reactive oxygen species (ROS) such as superoxide, singlet oxygen, hydrogen peroxide and hydroxyl radical in plants. The excessive ROS generation is found in the stressed plants and induces decomposition of membranes, inactivation of enzymes and alteration in gene expression leading to cell death (Singh et al. 20 9). The ROS are scavenged by enzymatic and non enzymatic antioxidants to protect plants from stress. Also it has been known that ROS are regulated by the hormonal (SA, ABA, JA) signaling pathway (Herrera-Vasquez et al. 20 5).
The phytohormone salicylic acid (SA) is a signaling molecule in regulating plant growth and development. Also it plays role in defence responses against biotic and abiotic stress factors such as pathogen infection, salinity, drought, heavy metals and chilling. In recent years, it was determined that exogenous addition of SA can improve photosynthethic capacity, enhance antioxidant enzymes, increase accumulation of soluble contents and maintain ion balance (K+/Na+) in many plants species (Hayat et al. 20 0). Beside this, SA stimulates the root development and stomata closure as well as induces the expression of defense genes and secondary metabolites (Miura and Tada, 20 4).
Non expressor of pathogenesis-related genes (NPR1) was detected firstly in the study of Arabidopsis mutants against pathogens (Cao et al. 997). NPR1 is a key regulator of systemic acquired resistance (SAR) and regulates the salicylic acid (SA) dependent genes. NPR is an important regulator of responses downstream of SA (Mou et al. 2003; Zhang et al. 2003). During SA mediated plant defense responses, oligomeric NPR in cytoplasm is reduced to monomeric NPR and translocated to nucleus by thioredoxins (TRX) (Herrara Vaquez et al. 20 5). SA is essential for NPR redox modificiation but this is still not well explained. Given that, SA involves in the regulation of salt tolerance mechanisms by the activation of plant defense responses, NPR might play role in mediating defence responses, induction antioxidant defence system, redox signaling and maintaining hormone level.
Although the role of NPR has been determined under pathogen stress, some studies suggested that NPR is also effective on abiotic stress such as salinity, drought and heavy metal in plants. Only a few researchers underlined the importance of NPR but there are contraversial results in literature (Cao et al.
997; Zhang et al. 20 3, Jayakannan et al. 20 5; Liu et al. 20 7; Lee et al. 20 9). To understand the functions of “NPR ” will be guide for analyse in plant stress tolerance. With this aim, in this work, the comparative analysis of NPR under two different stress in soybean was studied which has never been addressed until now.
MATERIALS AND METHODS
Experimental design and plant material
Soybean ( Glycine max L. Merr.) SA88 seeds were obtained from a commercial provider (Agrova, Adana, TR). The seeds were sown in plastic trays ( 0 cm× 4 cm) filled with soil under dark conditions. After germination, seedlings were placed into a growth chamber at 25 °C with 6 h/8 h day/night photoperiod and light intensity of 500 µmol m–2 s– with Hoagland solution for 2 weeks. Then, seedlings were treated with 200 mM NaCl, 0 % PEG 6000, 200 mM NaCl + 0 % PEG 6000 in Hoagland solution. After stress treatment, leaves were harvested at hours (2h, 4h, 6h), days (7 th and 4 th) and stored at -80 °C until further analysis.
Physiological parameters
Relative growth rate
The relative growth rate (RGR) of shoot was calculated from the dry mass data taken at initial and final harvests, using the formula given by (Hunt et al. 2002). For dry weight (DW) calculations, shoots and roots were dried in the oven at 70 °C for 48 hours and then weight.
RGR = (ln (DW2 ) – ln (DW )/(t2 – t ) where DW = dry mass (g) at time ; DW2 = dry mass (g) at time 2; and t and t2 = initial harvest time and final harvest time 2 in days.
Relative water content
The RWC was calculated using the following formula The relative water content (RWC) was calculated by (Smart and Bingham, 974). Six leaf discs were obtained from plants in every group on hours, 2h, 4h and 6h and days 7 and 4 of salinity and PEG treatment. After FW of these discs were determined, they were floated on deionized water for 4h under low irradiance. Two turgid tissues were then quickly blotted dried prior to determining turgid weight. DW was then determined after oven drying at 70°C for 72h, the time point at which a constant weight was reached.
RWC (%) = [(FW - DW)/(TW - DW)] × 00
Relative electrolyte leakage
The relative electrolyte leakage (REL) was determined according to Singh et al. (2008). Leaf tissue was vibrated for 30 min in deionised water followed by measurement of conductivity of bathing medium (C ). Boiled the samples for 5 min and again measured the conductivity (C 2 ) was measured. Percent relative electrolyte leakage (REL) was determined using the following formula:
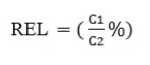
Biochemical analysis
Proline content
The proline content of the leaves was determined according to Claussen (2005). The absorbance of the reaction mixture was determined at 546 nm. The proline concentration was determined from a standard curve and calculated on fresh weight basis (μg proline g- FW).
Hydrogen peroxide content
The H 2 O 2 content was determined according to Velikova et al. (2000). Fresh leaves (0. g) were homogenized in 5ml of 0. % trichloroacetic acid (TCA) and centrifuged at 2.000 rpm for 5 minutes. The supernatant (0.5 ml) was then mixed with 0.5 ml of buffer ( 0 mM potassium phosphate, pH 7) and ml of
M KI. The absorbance reading was taken at 390 nm.
Malondialdehyde content
The level of lipid peroxidation in leaf samples was determined in terms of the malondialdehyde (MDA) content according to the method specified by Madhava Rao and Sresty (2000). The MDA content, an end product of lipid peroxidation, was determined by using the thiobarbituric acid reaction. The MDA concentration was calculated from the absorbance at 532 nm, and measurements were corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. An extinction coefficient of 55 mM- cm- was used to determine the MDA concentration.
Determination of hormone levels
SA and ABA level will be determined by using AOAC Official Method 2007. 0 Quechers exctraction method with LC-MS/MS. 5 g sample will be weight into 50 ml Ouechers extraction centrifuge tupes containing 6 g Mg sulfate, .5 g Na acetate. Then, 5 ml acetonitrile containing % acetic acid will be put into tupe. Then, this will be shaken vigorously for minute. It will be centrifuged for 5 minute with 4000 cycle. 8 ml will be taken from upper phase. Then, it will be put into 5 ml teflon centrifuge tupe containing 0.2 g primer seconder amine, 0.6 g magnesium sulfate. Then it will be shaken vigorously for minute. It will be centrifuged for 5 minute with 4000 cycle. The upper phase will be taken into vials. The kit containing 0.04 g active carbon will be used for second phase. Calibration graphic will be prepared with -5- 0-25-50- 00-200 μg/kg concentration (matrix-match). The residue concentration in sample will be calculated calibration graphic. The results will be presented by multiplied with dilution factor.
Gene expression analysis
Non-expressor of pathogen related gene expression
Relative quantification of gene expression and statistical analysis of all qRT-PCR data (pair wise fixed reallocation randomisation test) were performed using the REST software according to Pfaffl et al . (2002).
RNA Isolation cDNA Synthesis and Real-time RT-PCR Assay
RNA extraction was performed using Tripure reagent (Roche) according to the manufacturer’s instructions. The integrity of total RNA was checked spectrophotometrically using a NanoDrop Spectrophotometer ND-2000 (Labtech International), followed by gel electrophoresis. cDNA synthesis was performed from 4 g total RNA using a Transcriptor st strand cDNA synthesis kit (Roche) according to the manufacturer’s instructions and cDNAs of independent biological replicates (n = 3) from same treatments were pooled into single samples. Subsequently, transcript levels were analyzed in a LightCycler 480II real-time PCR cycler (Roche) using a FastStart Essential DNA Probes Master kit (Roche) according to the manufacturer’s instructions. Reaction conditions were 95 ◦C for 600s, followed by 45 cycles of 95 ◦C for 0 s, 56 ◦C for 0 s, and 72 ◦C for s. Relative quantification of gene expression and statistical analysis of all qRT-PCR data (pairwise fixed reallocation randomization test) were performed using the REST software according to Pfaffl et al. (2002). GmNPR -specific products were obtained using the following primers: forward primer, 5-TCAGATGATGTTGAGCTTGTTAAAC-3; reverse primer. ACCAAGTACCTCAGAAACAACCTT. Actin beta gene was used as reference gene. GmAct F; GAGCTATGAATTGCCTGATGG, GmAct R;
CGTTTCATGAATTCCAGTAGC, Probe upl 6 (roche). Primer design was designed by using Acs number: XM, Acs number NM, NCBI and ensemble gene banks by us.
Statistical analysis
The experiment was conducted in a completely randomizeddesign and measurements were performed with 6 replicates (n = 6). Statistical variance analysis of the data was performed using ANOVA and differences among treatments were compared usingTukey’s post-hoc analysis with least significant differences at the 5% level.
RESULTS
Relative water content is an indicator of water stress in plants. In this study, the results showed that short and long term of stress reduced RWC values in soybean leaves (Table , 2). Especially, alone PEG treatment induced damage in water status was more pronounced according to NaCl stress. This content was reduced by 7.76 % at 7 day, 20.89 % at 4 day comparing with controls. Otherwise, the reduction was 52.96 % at 2h and 36.53 % at 4h and 49. 5 % at 6h in short term application with PEG stress. By the way, combined treatment lead the highest reduction in both short and long term of stress as compared to control groups.
Relative growth rate is generally reduced under stress conditions in plants. In our results, PEG treatment lead a more reduction in RGR with PEG treatment (60.7 % at 7 day, 60.66 % at 4 day) compared to salinity in soybean plants (Table ). Considering the short term effects of stress, the results showed us that salinity or PEG stress had a similar effects on plants at 2 h, 4 h and 6 h while combined stress reduced RGR content by 48.57 % , 43.75 % and 69.69 % respectively (Table 2).
Relative electrolyte leakage is induced with salinity in plants. Long term of stress increased REL levels by salinity 66.20 % and 2 fold at 7 and 4 day (Table ). Otherwise, combined stress increased this level under all treatment while it was 2 fold under PEG and salinity. Short term of salinity also induced REL levels but the highest increase was at 4h under all treatments. Proline is a compatible osmolyte in plants to tolerate salinity in plants. In results, PEG induced stress increased the proline content by 5.34 % and 37.52 % at 7 and 4 day. Also, PEG was more effective than NaCl stress at 4h and 6 h (46.47 % and 46.62 %) (Table 2).
Malondialdehyde content is an indicator for stress damage. Stress treatments induced MDA content under all treatment but it was increased more by 26.06 % and 9.80 % at 7 and 4 day under long term effects of PEG stress as compared to salinity. Neverthless, PEG and salinity lead to highest increase in MDA content by 28.28 % at 2h, 40.82 % at 4h and 2. 6 fold at 6h according to control treatments (Fig. ). Hydrogen peroxide is produced via superoxide dismutase enzyme from superoxide anion radical in cells. PEG stress caused a higher increase in hydrogen peroxide level compared to salinity in both short and long term of stress. Beside this, PEG and salinity induced by 87.55 %, 3.98 and 5.60 fold at 2h, 4h and 6h as compared to control groups (Fig 2).
Abscisic acid levels was checked in this study to determine the effect of stress on soybean leaves. In results, all treatment induced ABA levels but PEG stress induced significiantly by 45.99 % and 4 .22 % at 7 and
-
4 day. Otherwise, considering the short term of stress, PEG treatment also induced ABA level (54.53 %, 7 .42
%, 76.4 %) at 2h, 4h and 6h compared to salt stress but combined stress showed similar results to control treatments (Fig.3). Salicylic acid levels were determined with treated soybean leaves. In parallel with the results of ABA, PEG stress induced more SA levels according to salt stress. At 7 and 4 day of stress, 33. 9 % and 3.77 fold increase was reported significiantly (Fig.4). Similarly, short term of application of PEG stress induced SA levels in this plant but not as well as long term effect.
Non expresor of pathogen related gene ( NPR1 ) was upregulated with combined stress according to salt and PEG treatment alone at 4h. There was also upregulation in salinity or PEG stress but they were not significiant. However, salinity induced NPR1 gene expressions at 7 day of treatment, while it was also upregulated with combined stress at 4 day (Fig. 5).
Table 1. Long term (7 d and 4 d) effects of salt and osmotic stress on relative growth rate (RGR), relative water content (RWC), relative electrolyte leakage (REL), proline (PRO) of soybean ( Glycine max L.) seedlings. Control (C), Osmotic Stress (PEG), Salt stress (NaCl), PEG+NaCl (Osmotic and Salt stress). Columns with different letters represent significantly different (P < 0.05) values
|
RGR (%) |
RWC (%) |
REL (%) |
PRO |
|||||
|
7 DAY |
14 DAY |
7 DAY |
14 DAY |
7 DAY |
14 DAY |
7 DAY |
14 DAY |
|
|
C |
0.28±0.05a |
2.39±0. a |
86.69±2.4a |
77.78± .6a |
22.4±2.2a |
32.08± .9a |
535.30±3.4a |
573.2 ±7.4a |
|
PEG |
0. ±0.03b |
0.94±0.07b |
7 .29± .9b |
6 .5 ± .8b |
26.6±2.6b |
36.08±0.3b |
632.67±0.5b |
788.75±8.05b |
|
NaCl |
0. 6±0.008c |
.33±0.04c |
74.84±2.05b |
73.52± .7c |
37.2±0.8c |
64.8±0.2c |
549.05±3.2a |
58 .98± 3.7a |
|
PEG+NaCl |
0. 4±0.004c |
.2±0.04c |
65.54±2.5c |
54.08±3. d |
46.48±2.8d |
55.8± .6d |
567.43±3.4c |
468.9±7.5c |
Table 2. Short term (2h, 4h, 6h) effects of salt and osmotic stress on relative growth rate (RGR), relative water content (RWC), relative electrolyte leakage (REL), proline (PRO) of soybean ( Glycine max L.) seedlings. Control (C), Osmotic Stress (PEG), Salt stress (NaCl), PEG+NaCl (Osmotic and Salt stress). Columns with different letters represent significantly different (P < 0.05) values.
|
RGR (%) |
RWC (%) |
REL (%) |
PRO |
|||||||||
|
2h |
4h |
6h |
2h |
4h |
6h |
2h |
4h |
6h |
2h |
4h |
6h |
|
|
C |
0. 75±0.0 a |
0.08±0. a |
0.33±0.5a |
65. 4±6. a |
69.42±3.7a |
79.05±3.7a |
29.07±7.4a |
29.85±2.9a |
36.3 ±6.4a |
409.33±3.2a |
3 2. 3± .2a |
356.2± a |
|
PEG |
0. 8±0.06b |
0.06±0.03a |
0.23±0.06b |
30.64±8.4b |
44.06±9.5b |
40. 9±5.2b |
49.39±8.8b |
64.52±7.4b |
40.62± 0.9b |
4 3.42±2.2a |
388. 6± .9b |
350.8±2.6a |
|
NaCl |
0. 5±0.03b |
0.06±0.04a |
0.25±0. b |
45.92±5.4c |
46.39±3.9b |
58.08±4.2c |
37.83±3.7c |
8.5 ±7.8c |
6 .57±4.5c |
435.05± 2.2b457.38± .2c |
522.2±3. b |
|
|
PEG+NaCl |
0.09±0.05c |
0.04±0.07c |
0. 0±0. c |
22.40±4.8d |
35.02±5.7c |
26.94±6.4d |
36.56±8.5c |
59.63±6.9d |
49.43±0. d |
40 .49±2 a |
378.22±0.7d |
367.9± .8c |
Table 3. Genes used in this study. Gene identification number (gene ID number), forward (OR), reverse and primer sequences, probe number and expected amplicon length (Bp).
Gene Gene ID No Foward Primer Reverse Primer Probe
Number Bp
NPR NM_00 25 745. TCAGATGATGTTGAGCTTGTTAAAC ACCAAGTACCTCAGAAACAACCTT 39 22
GmAct XM_003547534.3 GAGCTATGAATTGCCTGATGG CGTTTCATGAATTCCAGTAGC 6 8
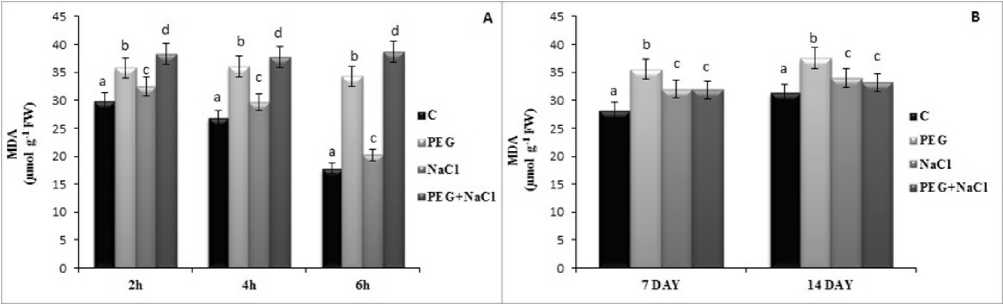
Figure 1. Short (A) and long term (B) effects of salt and osmotic stress on maliondaildehyde (MDA) level of soybean ( Glycine max L.) seedlings. Control (C), Osmotic Stress (PEG), Salt stress (NaCl), PEG+NaCl (Osmotic and Salt stress). Columns with different letters represent significantly different (P < 0.05) values.
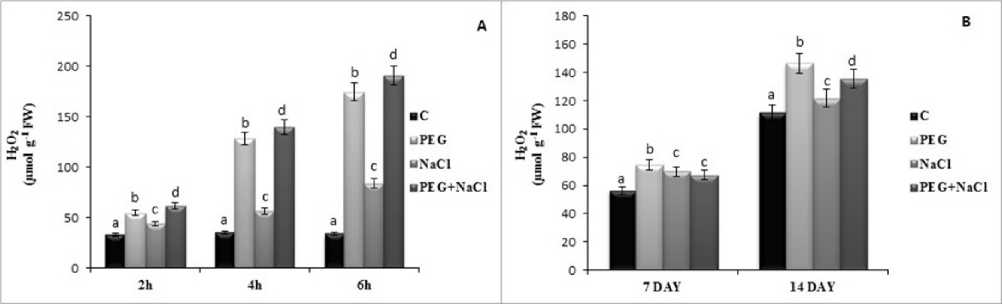
Figure 2. Short (A) and long term (B) effects of salt and osmotic stress on hydrogen peroxide (H 2 O 2 ) level of soybean ( Glycine max L.) seedlings. Control (C), Osmotic Stress (PEG), Salt stress (NaCl), PEG+NaCl (Osmotic and Salt stress). Columns with different letters represent significantly different (P < 0.05) values.
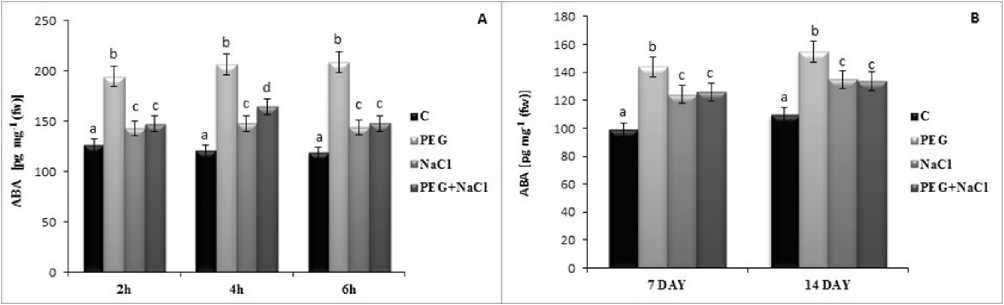
Figure 3 Short (A) and long term (B) effects of salt and osmotic stress on abscisic acid (ABA) content of soybean ( Glycine max L.) seedlings. Control (C), Osmotic Stress (PEG), Salt stress (NaCl), PEG+NaCl (Osmotic and Salt stress). Columns with different letters represent significantly different (P < 0.05) values.
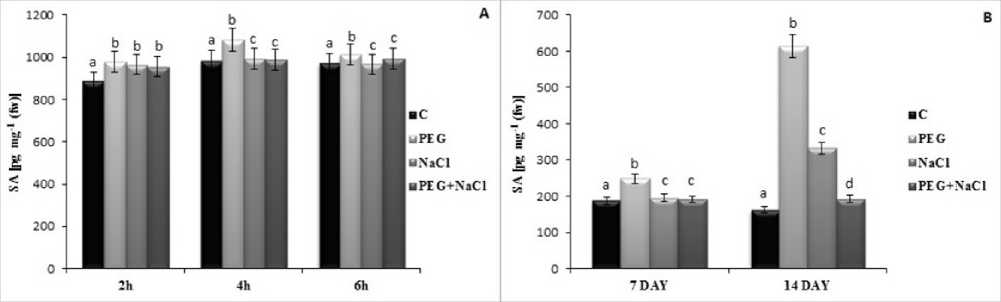
Figure 4 Short (A) and long term (B) effects of salt and osmotic stress on salicylic acid (SA) content of soybean ( Glycine max L.) seedlings. Control (C), Osmotic Stress (PEG), Salt stress (NaCl), PEG+NaCl (Osmotic and Salt stress). Columns with different letters represent significantly different (P < 0.05) values.
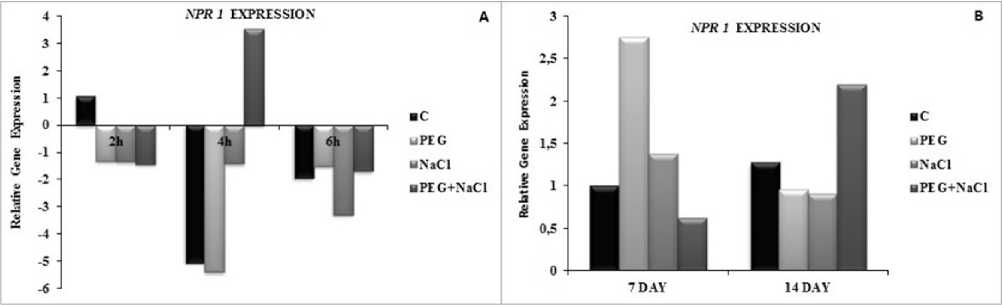
Figure 5 Short and long term effects of salt and osmotic stress on non expressor of pathogen related (NPR ) gene expressions of soybean ( Glycine max L.) seedlings. Control (C), Osmotic Stress (PEG), Salt stress (NaCl), PEG+NaCl (Salt and Osmotic stress). Columns with different letters represent significantly different (P < 0.05) values
DISCUSSION
PEG is non ionic and non toxic osmoticum to study the water status of plants while NaCl is an ionic and is well known to produce specific ion toxicities (Handa et al. 982). Although salinity and osmotic stress have common effects on plant metabolism, there are many various results in literature (Perz-Alfocea, 993; Slama et al. 2007; Lokhande et al. 20 0). In the present work, plants treated with NaCl showed a better defense response in physiological parameters than PEG. This may be due to maintain of higher succulence under salt than PEG induced stress and higher viscosity traits of PEG as suggested by Chazen et al. ( 995). In results, RWC content was decreased under all treatment but the highest inhibition was by combined stress comparing with NaCl or PEG treatment alone. Similarly, Bai et al. (20 9) determined that water content and cell turgor of soybean under drought and salinity were higher than those of drought alone. Given the duration of stress, short term treatment was more pronounced with a reduction of over 50 % with PEG stress in RWC values. This could be explained by rapid effect of osmotic stress within hours. Similarly, PEG treatment caused a more pronounced decrease in relative growth rate, when compared with NaCl or combined treatment, consistent with the findings of Silva et al. (20 0). However, the most inhibition in growth was under combined stress of 6h in short term application different from long term. This finding could be explained with the maintain leaf water status, integrity of photochemical activity and loss of growth depending on time of the exposure of stress. These results are also agreement with the proline content. Liang et al. (20 3) reported that proline is an osmolyte which acts as chelator, reactive oxygen scavenger and signal molecule under stress. There was a slight increase in proline accumulation under stress in soybean leaves according to controls. In parallel to this results, previous studies determined that stress induces proline level in soybean to increase stress adaptation mechanisms (Sarısoy et al. 20 8). In the present study, proline content was higher in PEG induced stress as compared to NaCl at both short and long term of stress. This results are also agreement with the reports of Ahmad et al. (2007). Totally, it could be suggested that proline accumulation is the first response of plants exposed to osmotic stress and may be related to a higher mobilization of this metabolite synthesis as suggested by Grzesiak et al. (20 3).
Relative electrolyte leakage is an important indicator for membrane damage or deterioration. Electrolyte leakage was enhanced with increasing salinity levels as compared to the control plants in many reports (Tang et al. 20 9). In our results, plants treated with NaCl had a higher REL values than treated with PEG at 7 and 4 day, while combined stress increased REL level more than the other treatments at all hours but the most at 4h. This results are also in agreement with the reports of Filek et al. (20 2) and Patade et al. (20 2).
Under abiotic and biotic stress conditions, plants produce reactive oxygen species include superoxide anion radical (O 2 -.), hydrogen peroxide (H 2 O 2 ), hydroxyl radical (OH) and singlet oxygen ( O 2 ). These molecules are harmful for the proteins, lipids and DNA. One of the ROS radical, “hydrogen peroxide” is produced via superoxide dismutase enzyme from superoxide radical in plants Beside this, H 2 O 2 plays acts as a messenger molecule involved in acclimatory signalling, and it orchestrates programmed cell death at high concentrations (De Azevedo Neto et al. 2005). In results, hydrogen peroxide is induced more by PEG stress as compared to NaCl at 7 and 4 day, while combined stress showed the highest increase at short term of treatment.
Malondialdehyde is a lipid peroxidation product of membranes in plants which is known as and indicator. Increase of accumulation of ROS content in plants lead to increase oxidative damage. It’s well known that tolerant plants have low MDA content under stress conditions. Similar to hydrogen peroxide, PEG treated plants showed higher MDA content according to NaCl at 7d and 4 day. However, short term application combined lead to much damage with higher MDA content as compared to other groups. This findings are also consistent with the RWC, RGR and REL values. Totally, this result made us think that soybean leaves were more sensitive to PEG induced stress condition than NaCl but this can be changed with the duration of stress.
Salicylic acid is known as a signalling molecule that modifies plant responses to stress factors. In this work, SA level was increased by PEG treatment in the long and short term of stress but it was more pronounced with daily practise (7 and 4 d). Several studies have indicated that exogenous application of SA improved stress tolerance and increase in SA accumulation is an important response to stress in plants. It could be suggested that the significant increase in SA with PEG stress was an evidence that soybean leaves were more effected from PEG. This results are also agreement with the report of Lee et al. (20 9), who reported that the induce SA level in Brassica napus under drought stress. To the best of our knowledge, this is the first report of the comparative analysis of SA change under PEG and salinity in soybean.
ABA plays essential roles in seed maturation and germination, maintaining plant water status, stomatal closure, and stress-responsive gene expression under osmotic stress conditions (Yoshida et al. 20 9). In soybean leaves, NaCl induced ABA level but the most induction was under PEG induced stress at 7 day. In parallel to our results, 0 % PEG treated Brassica plant for 96 h, showed an induced ABA level (Sahay et al. 20 9). This findings are also consistent with the physiological parameters and MDA content. Given the results of SA, it could be suggested that two important phytohormone was responsible to struggle with water deficit and damage in soybean leaves.
The main trends observed were the significant induction of GmNPR1 in PEG treated samples at 7 day, while combined stress treated leaves also showed significantly induced GmNPR1 transcript levels at 4
day. This result was consistent with the endogenous SA accumulation during PEG treatment. In parallel to this finding, SA and ABA treatment leads to changes in NPR1 gene expression (Ding et al. 20 6). Nevertheless, GmNPR1 gene expressions were down regulated by short term of stress application but only the significant increase was under combined stress according to PEG or NaCl alone at 4h. As previously mentioned, salicylic acid is essential for NPR redox modification but it is not well documented. Oxidative stress also induces NPR monomerization but it has not been explained yet. Our results shows that PEG induced osmotic stress was more effective to induce GmNPR1 upregulation. Similarly, Jayakannan et al. (20 5) reported that NPR dependent SA signaling is important to the salt stress tolerance in Arabidopsis. In parallel to our results, Liu et al . (20 7) determined that GmNPR1 was upregulated under Al stress with increase SA level in soybean roots. Beside this, Sarısoy et al . (20 8) showed an upregulation of GmNPR1 trancript levels in soybean leaves under salinity. Interestingly, Zhang et al. (20 3) determined that overexpressions of NPR increased salt tolerance but no effect on drought tolerance in Arabidopsis. However, Arabidopsis npr1 mutant showed enhanced growth during salt stress (Hao et al. 20 2). Collectively, it could be said that the role of NPR is variable and is altered depending of stress type and time.
CONCLUSION
In conclusion, considering the short term effects on physiological parameters, REL values were remarkable with combined stress at 4h according to NaCl and PEG stress alone. From this result, it could be said that membrane damage of soybean leaves were higher at this situation. Beside this, the highest damage was at 6h according to MDA content. This paradox could be explained by the ion movement such as K+ with PEG stress. It is difficult to allege that there is a connection between REL values and GmNPR gene expressions under combined stress but it is clear that PEG stress may role as a potential signal but not salt stress for the regulation of NPR in soybean leaves. The present work firstly intends to present the change of NPR gene expressions with comparative analysis of PEG induced osmotic stress and salinity or combined effects in soybean leaves. In future, NPR could be used as a defense material, based on this results to improve resistant against to environmental stress factors in plants.
ACKNOWLEDGEMENTS
We gratefully to thank Prof. Dr. İsmail TURKAN, Ege University, for his support with improve manuscript. COMPETING INTERESTS
The authors declare no competing interests.
AUTHOR’S CONTRIBUTIONS
Список литературы Regulation of NPR1 Under Salinity and Osmotic Stress in Soybean (Glycine max L.) Leaves
- Ahmad M.S.A., Javed F., Ashraf M. (2007) Iso-osmotic effect of NaCl and PEG on growth, cations and free proline accumulation in callus tissue of two indica rice (Oryza sativa L.) genotypes. Plant Growth Regul., 53, 53.
- Bai X., Dai L., Sun H., Chen M., Sun Y. (2019) Effects of moderate soil salinity on osmotic adjustment and energy strategy in soybean under drought stress. Plant Physiol Biochem., 139, 307-313.
- Cao H., Glazebrook J., Clarke J.D., Volko S., Dong X. (1997) The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell, 88, 57-63.
- Chazen O., Hartung W., Neumann P.M. (1995) The different effects of PEG 6000 and NaCI on leaf development are associated with differential inhibition of root water transport. Plant Cell Environ., 18, 727-735.
- Claussen W. (2005) Proline as a measure of stress in tomato plants. Plant Sci., 168, 241-248.
- De Azevedo Neto A.D., Prisco J.T., Enéas-Filho J., Medeiros J.V.R., Gomes-Filho E. (2005) Hydrogen peroxide pre-treatment induces salt-stress acclimation in maize plants. J Plant Physiol., 162, 1114-1122.
- Deinlein U., Stephan A.B., Horie T., Luo W., Xu G., Schroeder J.I. (2014) Plant salt-tolerance mechanisms. Trends Plant Sci., 19, 371-379.
- Ding Y., Dommel M., Mou Z. (2016) Abscisic acid promotes proteasome‐mediated degradation of the transcription coactivator NPR 1 in Arabidopsis thaliana. The Plant J., 86, 20-34.
- Filek M., Walas S., Mrowiec H., Rudolphy-Skórska E., Sieprawska A., Biesaga-Kościelniak J. (2012) Membrane permeability and micro-and macroelement accumulation in spring wheat cultivars during the short-term effect of salinityand PEG-induced water stress. Acta Physiol Plant., 34, 985-995.
- Grzesiak M., Filek M., Barbasz A., Kreczmer B., Hartikainen H. (2013) Relationships between polyamines, ethylene, osmoprotectants and antioxidant enzymes activities in wheat seedlings after short-term PEG-and NaCl-induced stresses. Plant Growth Regul., 69, 177-189.
- Handa A.K., Bressan R.A., Handa S., Hasegawa P.M. (1982) Characteristics of cultured tomato cells after prolonged exposure to medium containing polyethylene glycol. Plant Physiol., 69, 514-521.
- Hao L., Zhao Y., Jin D., Zhang L., Bi X., Chen H., Li G. (2012) Salicylic acid-altering Arabidopsis mutants response to salt stress. Plant and Soil., 354, 81-95.
- Hayat Q., Hayat S., Alyemeni M.N., Ahmad A. (2012) Salicylic acid mediated changes in growth, photosynthesis, nitrogen metabolism and antioxidant defense system in Cicer arietinum L. Plant Soil Environ., 58, 417-423.
- Herrera-Vásquez A., Salinas P., Holuigue L. (2015) Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front Plant Sci., 6, 171.
- Hunt R., Causton D.R., Shipley B., Askew A.P. (2002) A modern tool for classical plant growth analysis. Ann Bot., 90, 485-488.
- Jayakannan M., Bose J., Babourina O., Shabala S., Massart A., Poschenrieder C., Rengel Z. (2015) The NPR1-dependent salicylic acid signaling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis. J Exp Bot., 66, 1865-1875.
- Lee B.R., Islam M.T., Park S.H., Jung H.I., Bae D.W., Kim T.H. (2019) Characterization of salicylic cidmediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ Exp Bot., 157, 1-10.
- Liang W., Ma X., Wan P., Liu L. (2018) Plant salttolerance mechanism: A review. Biochem Biophys Res., 495, 286-291.
- Liang X., Zhang L., Natarajan S.K., Becker D.. (2013) Proline mechanisms of stress survival. Antioxid Redox Sig., 19, 998-1011.
- Liu N., Song F., Zhu X., You J., Yang Z., Li X. (2017) Salicylic acid alleviates aluminum toxicity in soybean roots through modulation of reactive oxygen species metabolism. Front Chem., 5, 96.
- Lokhande V.H., Nikam T.D., Penna S. (2010) Biochemical, physiological and growth changes in response to salinity in callus cultures of Sesuvium portulacastrum L. Plant Cell Tiss and Org., 102, 17-25.
- Madhava R., Sresty T.V. (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L. millspaugh) in response to Zn and Ni stresses. Plant Sci., 157, 113-128.
- Miura K., Tada Y. (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci., 5, 4.
- Mou Z., Fan W., Dong X. (2003) Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell, 113, 935-944.
- Munns R. (2002) Salinity, growth and phytohormones. In Salinity: Environ Plants Mol., 271-290. Springer, Dordrecht.
- Patade V.Y., Bhargava S., Suprasanna P. (2012) Effects of NaCl and iso-osmotic PEG stress on growth, osmolytes accumulation and antioxidant defense in cultured sugarcane cells. Plant Cell Tiss and Org., 108, 279-286.
- Pfaffl M.W., Horgan G.W., Dempfle L. (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res., 30, e36-e36.
- Pérez‐Alfocea F., Estan M.T., Caro M., Guerrier G. (1993) Osmotic adjustment in Lycopersicon esculentum and L. pennellii under NaCl and polyethylene glycol 6000 iso–osmotic stresses. Physiol Plant., 87, 493-498.
- Sahay S., Khan E., Gupta M. (2019) Nitric oxide and abscisic acid protects against PEG-induced drought stress differentially in Brassica genotypes by combining the role of stress modulators, markers and antioxidants. Nitric Oxide, 89, 81-92.
- Sarısoy U., Dinler B.S., Tascı E. (2018) The Effects of NPR1 Dependent Salicylic Acid Change in Increasing Salt Tolerance of Soybean Leaves by Acclimation. Not Bot Horti Agrobot Cluj., 46, 356-364.
- Silva E.D., Ribeiro R.V., Ferreira-Silva S.L., Viégas R.A., Silveira J.A.G. (2010) Comparative effects of salinity and water stress on photosynthesis, water relations and growth of Jatropha curcas plants. J Arid Environ., 74, 1130-1137.
- Singh A., Kumar A., Yadav S., Singh I.K. (2019) Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene, 18, 100173.
- Singh A., Kumar J., Kumar P. (2008) Effects of plant growth regulators and sucrose on post harvest physiology, membrane stability and vase life of cut spikes of gladiolus. Plant Growth Regul., 55, 221.
- Slama I., Ghnaya T., Hessini K., Messedi D., Savouré A., Abdelly C. (2007) Comparative study of the effects of mannitol and PEG osmotic stress on growth and solute accumulation in Sesuvium portulacastrum. Environ Exp Bot., 61, 10-17.
- Smart R.E., Bingham G.E. (1974) Rapid estimates of relative water content. Plant Physiol., 53, 258-260.
- Tang Y., Bao X., Zhi Y., Wu Q., Yin X., Zeng L., Wang Q. (2019) Overexpression of a MYB family gene, OsMYB6, increases drought and salinity stress tolerance in transgenic rice. Front. Plant Sci., 10, 168.
- Velikova V., Yordanov I., Edreva A. (2000) Oxidative stress and some antioxidant systems in acid raintreated bean plants protective roles of exogenous polyamines. Plant Sci., 151, 59-66.
- Yoshida T., Obata T., Feil R., Lunn J.E., Fujita Y., Yamaguchi-Shinozaki K., Fernie A.R. (2019) The role of abscisic acid signaling in maintaining the metabolic balance required for Arabidopsis growth under nonstress conditions. Plant Cell., 31, 84-105.
- Zhang Y., Goritschnig S., Dong X., Li X. (2003) A gainof-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. The Plant Cell., 15, 2636-2646.
- Zhang Y.M., Ni X.L., Qiu W. (2013) Characterization of NPR1 genes from Norton and Cabernet sauvignon grapevine. J Integr Agr., 12, 1152-1161.


