Регуляция активности витагенов как новая антистрессовая стратегия в птицеводстве: обоснование и производственный опыт
Автор: Григорьева М.А., Величко О.А., Шабалдин С.В., Фисинин В.И., Сурай П.Ф.
Журнал: Сельскохозяйственная биология @agrobiology
Рубрика: Птицеводство: наука и технологии
Статья в выпуске: 4 т.52, 2017 года.
Бесплатный доступ
Применение промышленных технологий в птицеводстве сопряжено с различными стрессами, что ведет к снижению продуктивности и репродуктивных качеств у цыплят, родительского стада и несушек. В птицеводстве (и животноводстве в целом) различают четыре основных типа стрессов: технологические, средовые, кормленческие и внутренние/биологические. Растущий объем доказательств свидетельствует о том, что на клеточном уровне большинство стрессов птицы обусловлено окислительным стрессом вследствие избытка свободных радикалов или недостаточной антиоксидантной защиты. Концепция борьбы со стрессами на уровне витагенов, возникшая в медицине в результате исследований молекулярных механизмов стресса и адаптации, была успешно перенесена в птицеводство и свиноводство, где регулирование активности витагенов с помощью кормовых добавок по существу представляет собой инновационный подход к обеспечению полной реализации потенциала организма для адаптации к стрессу. Цель нашего исследования заключалась в выяснении того, может ли выпаивание витаген-регулирующей антиоксидантной смеси (Magic Antistress Mix/PerforMax, «Feed-Food Ltd», Великобритания) с питьевой водой улучшать продуктивность бройлеров в стрессовых условиях промышленного производства. Широкомасштабный производственный опыт проводили на птицефабрике в специальном корпусе, спроектированном и оборудованном для экспериментальных испытаний с еженедельным учетом параметров роста (АО «Продо Тюменский бройлер», Тюменская обл., Россия). Цыплят (12400 гол.) кросса Arbor Acres («Aviagen Group», США) в возрасте 1 сут разделили на две равные группы с четырьмя повторностями в каждой, посадили в птичник и выращивали до 35-суточного возраста. Полученные результаты подтвердили, что использование витаген-регулирующей смеси с питьевой водой может улучшить показатели роста и развития цыплят, в том числе достоверно - конверсию корма (соотношение его затрат к приросту живой массы - 1,50 против 1,56 в контроле), и повысить эффективность вакцинации, увеличив ее индекс на 40 %.
Витагены, стресс, кормление
Короткий адрес: https://sciup.org/142214064
IDR: 142214064 | УДК: 636.5:636.084:591.05 | DOI: 10.15389/agrobiology.2017.4.716rus
Текст научной статьи Регуляция активности витагенов как новая антистрессовая стратегия в птицеводстве: обоснование и производственный опыт
Различные стрессы в птицеводстве приводят к снижению продуктивных и репродуктивных характеристик растущих цыплят-бройлеров, кур родительского стада и промышленных несушек. Доместикация и генетический отбор на скорость роста, улучшение конверсии корма и повышение массы сделали домашнюю птицу (включая бройлеров и индеек) особенно восприимчивой к окислительному стрессу (1). Физиологически стресс связан с отклонением от оптимальных внутренних и внешних условий среды. За восстановление гомеостаза ответственны гипоталамо-гипофизарно-над-почечниковая ось, вегетативная нервная система и иммунная система. Таким образом, в организме задействован каскад регуляторных механизмов, приводящий к мобилизации энергии и изменению метаболизма, что отрицательно сказывается на росте птицы и эффективности использования корма (2). В современном промышленном птицеводстве метаболические заболевания, связанные с условиями кормления и окислительным стрессом (энцефаломаляция, экссудативный диатез, мышечная дистрофия и т.д.), практически не встречаются (3-4), но различные нарушения в системе антиоксидантной защиты по-прежнему вызывают существенные проблемы. Например, в рационе количество определенного питательного вещества может быть недостаточным для удовлетворения потребностей птицы или он может содержать компоненты, инактивирующие питательные вещества либо ингибирующие их всасывание и (или) использование, метаболизм мо-
∗ Исследования П.Ф. Сурая проводятся при поддержке мегагранта Правительства Российской Федерации (контракт ¹ 14.W03.31.0013).
жет быть нарушен взаимодействием пищевых и экологических факторов, провоцируя окислительный стресс (5).
Растущее число доказательств свидетельствует о том, что у птицы большая часть стрессов на клеточном уровне связана с окислительным стрессом из-за избытка образующихся свободных радикалов или недостаточной антиоксидантной защиты (3, 4), поэтому кормовые антиоксиданты считаются основным средством для борьбы с различными стрессами в птицеводстве (3, 4, 6-8). В последнее время концепция клеточной антиоксидантной защиты была пересмотрена с особым вниманием к сигнальным механизмам регуляции редокс-баланса в клетке. У животных редокс-сиг-нальные пути используют активные формы кислорода при передаче сигналов из разных источников в клеточное ядро для регулирования ряда функций, включая рост, дифференцировку, пролиферацию и апоптоз.
Концепция борьбы со стрессами с использованием витагенов возникла как новое направление в медицине. По V. Calabrese с соавт. (9), ви-тагены — это группа генов, которые вовлечены в сохранение клеточного гомеостаза и играют важную регуляторную роль в адаптации клеток и всего организма к различным стрессам. В семейство витагенов входят гены белков теплового шока (HSPs, Heme oxygenase-1 и HSP70), системы тиоредок-синов (Trx)/тиоредоксинредуктазы (TrxR), сиртуинов (9) и супероксиддис-мутазы (SOD) (10). Концепция витагенов применима в случае ряда заболеваний человека — при нейродегенеративных расстройствах (11), нейропротекции (12), шизофрении (13), сосудистой деменции (14), аутистических расстройствах (15), в связи со старением и долголетием (9, 16-18), при дерматологических заболеваниях (19), болезнях, связанных со свободными радикалами (20), остеопорозе и болезни Альцгеймера (21). Эта концепция была успешно перенесена из медицины в сельскохозяйственную науку и практику, в том числе в птицеводство и свиноводство (23, 25-28), и подробно обсуждалась нами ранее (23). Повышая активность витагенов (например, через систему Nrf2/ARE) и способность к адаптации, можно снизить негативные последствия различных стрессов при производстве домашней птицы и в животноводстве в целом. По существу регулирование витагенов с помощью кормовых добавок (10, 23, 27, 28) представляет собой новый подход в реализации потенциала организма птицы и сельскохозяйственных животных при адаптации к неблагоприятным условиям внешней среды. Такая модуляция эндогенных механизмов защиты клетки может быть инновационным подходом к решению проблем, связанных с промышленными стрессами в животноводстве, включая птицеводство.
В настоящей работе мы в широкомасштабном и строго контролируемом производственном опыте, организованном согласно нормам, принятым в большинстве стран с развитым птицеводством, убедительно показали, что витаген-активирующий антистрессовый препарат Magic Antistress Mix/PerforMax, выпаиваемый цыплятам-бройлерам кросса Arbor Acres с питьевой водой, снижает отрицательное воздействие стрессоров. Это приводит к улучшению конверсии корма и увеличению индекса вакцинации птицы.
Цель исследования заключалась в проверке возможности использовать разработанную витаген-регулирующую антиоксидантную смесь (Magic Antistress Mix/PerforMax) для повышения качества жизни и продуктивности бройлеров в промышленных условиях.
Методика. Производственный опыт проводился на птицефабрике в специальном корпусе, спроектированном и оборудованном для экспериментальных испытаний и еженедельной регистрации параметров роста и развития птицы (АО «Продо Тюменский бройлер», Тюменская обл., Рос- сия), на бройлерах кросса Arbor Acres («Aviagen Group», США). Из 12400 вылупившихся цыплят сформировали 2 равные группы (4 повторности в каждой), которые поместили в птичник. В опытной и контрольной груп- пах температурные, световые и другие условия содержания поддерживались в соответствии с рекомендациями для Arbor Acres, принятыми на птицефабрике. Рационы были сбалансированы по всем основным питательным веществам согласно тем же рекомендациям. Дополнительно в опыте птица получала с питьевой водой добавку Magic Antistress Mix/Per-forMax («Feed-Food Ltd», Великобритания) в дозе 1 мг/л в стрессовые периоды роста в соответствии с рекомендациями разработчика. Состав пре- парата, содержащего смесь витаминов, минералов, аминокислот, карнитина, бетаина, органических кислот и т.д., описан ранее (35).
Цыплят выращивали до 35-суточного возраста с еженедельным мониторингом показателей роста и развития. За три периода (15-21-е сут, 2228-е сут, 29-35-е сут) и в целом за все время выращивания (1-35-е сут) оценивали массу цыплят и ее ежесуточный прирост (г), падеж (%), суточное потребление (г) и конверсию корма, которую оценивали как отношение его затрат к приросту живой массы. Для сравнения результатов применяли европейский коэффициент эффективности производства (EPEF):
EPEF = (суточный прирост, г ½ сохранность, %)/(конверсия корма ½ 10).
По окончании опыта у цыплят определяли массу кишечника и про- водили патологоанатомическое исследование внутренних органов на наличие нефрита, энтерита и атонии кишечника.
1. Эффект выпаивания витаген-стимулирующей добавки Magic Antistress Mix/PerforMax по показателям роста и развития бройлеров кросса Arbor Acres ( M ±SEM, производственный опыт, АО «Продо Тюменский бройлер», Тюменская обл.)
Эффективность вакцинации против инфекционного бурсита кур оценивали в ELISA согласно рекомендациям производителя тест-системы («BioTek Instruments, Inc.», США). Индекс вакцинации рассчитывали как отношение среднего титра антител в ELISA к величине коэффициента его вариации ( Сv , %).
При математической обработке данных вычисляли средние ( M ) и их стандартные ошибки (±SEM). Достоверность различий с контролем оценивали по t -тесту с использованием однофакторного дисперсионного анализа (ANOVA), различия считали статистически значимыми при р < 0,05.
Результаты . Жизнеспособность цыплят (табл. 1) в обеих группах находилась в пределах стандартов, существующих на птице-
|
Показатель |
Контроль ( n = 6200) |
Опыт ( n = 6200) |
|
В возрасте |
15-21 сут |
|
|
Живая масса, г |
935,50±8,75 |
926,00±19,50 |
|
Суточный прирост живой массы, г |
68,60±2,40 |
68,30±1,60 |
|
Падеж, % |
0,40±0,04 |
0,30±0,05 |
|
Потребление корма, г/гол. |
101,40±1,80 |
99,50±1,90 |
|
Конверсия корма В возрасте |
1,48±0,03 22-28 сут |
1,46±0,03 ∗ |
|
Живая масса, г |
1457,50±6,50 |
1457,80±29,80 |
|
Суточный прирост живой массы, г |
74,60±0,60 |
76,10±3,10 |
|
Падеж, % |
0,50±0,05 |
0,30±0,08 ∗ |
|
Потребление корма, г/гол. |
119,60±1,40 |
116,50±3,70 |
|
Конверсия корма В возрасте |
1,61±0,02 29-35 сут |
1,54±0,04 ∗ |
|
Живая масса, г |
2019,00±7,50 |
2007,50±27,50 |
|
Суточный прирост живой массы, г |
80,20±0,80 |
78,60±2,80 |
|
Падеж, % |
1,90±1,30 |
0,90±0,20 |
|
Потребление корма, г/гол. |
132,40±3,30 |
126,70±2,70 |
|
Конверсия корма 1,65±0,04 В в о зр а ст е 1-35 сут |
1,62±0,04 |
|
|
Живая масса, г |
2019,00±7,50 |
2007,50±27,50 |
|
Суточный прирост живой массы, г |
56,50±0,20 |
56,20±0,80 |
|
Сохранность птицы, % |
96,20±1,20 |
97,70±0,50 ∗ |
|
Потребление корма, кг/гол. |
3,15±0,07 |
3,02±0,02 ∗ |
|
Конверсия корма Европейский коэффициент эффективности (European |
1,56±0,04 |
1,50±0,02 ∗ |
|
Production Efficiency Factor) |
356,80±10,80 |
372,8±9,6 ∗∗ |
|
∗ , ∗∗ Различия с контролем статистически значимы но при р < 0,05 и р < 0,01. |
соответствен- |
|
фабрике, но в контрольной группе падеж за 35 сут был значительно выше (3,8 %), чем в экспериментальной (2,3 %). В производственных масштабах такая разница может быть существенной. При применении Magic Antistress Mix/PerforMax отмечали значительное снижение частоты энтерита, нефрита и атонии кишечника, а также пододерматита (данные не приведены). В течение первых 2 нед ростовые показатели в контроле и опыте не различались (данные не приведены). В дальнейшем (см. табл. 1) в экспериментальной группе улучшалась конверсия корма, в результате чего снижалось его недельное потребление. Живая масса и ее ежесуточный прирост в опыте и контроле оставались практически одинаковыми. Следовательно, основное преимущество использования витаген-регулирующей композиции при выращивании бройлеров связано с конверсией корма, которая в опытной группе была достоверно лучше, чем в контрольной (1,50 против 1,56).
EPEF, наряду с европейским индексом бройлера (EBI), используется при сопоставлении итогов выращивания в разных стадах и регионах. Он стандартизирует технические результаты с учетом конверсии корма, падежа и среднесуточного прироста. С учетом данных о росте и развитии цыплят в течение 5 нед EPEF равнялся в контрольной группе 356,8, в опытной — 372,8, что сопоставимо с эффективностью производства бройлеров на лучших фермах в России и за рубежом.
Представляется важным и тот факт, что у получавших добавку цыплят на 35-е сут масса кишечника была на 5 % выше, чем у контрольной птицы (рис. 1, А).
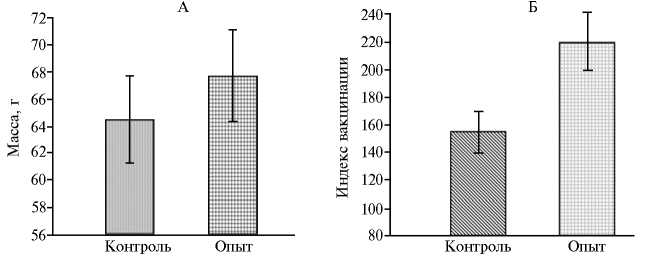
Рис. 1. Масса кишечника (А) и индекс вакцинации (Б) у 35-суточных бройлеров кросса Arbor Acres при выпаивании витаген-стимулирующей добавки Magic Antistress Mix/PerforMax (производственный опыт, АО «Продо Тюменский бройлер», Тюменская обл., Россия) .
При вакцинации против инфекционного бурсита у 35-суточных цыплят средняя величина, характеризующая уровень антител в ELISA-тесте, составила 4500 в контрольной группе и 5258 — в экспериментальной. В экспериментальной группе вариабельность ( Cv ) этого показателя (24 %) была ниже, чем в контрольной (29 %) и, как следствие, индекс вакцинации (титр ELISA/ Cv ) оказался значительно (на 40 %) выше контрольного (см. рис. 1, Б).
2. Экономические показатели (руб.) эффективности производства мяса бройлеров кросса Arbor Acres при выпаивании витаген-стимулирующей добавки Magic Antistress Mix/PerforMax (производственный опыт, АО «Продо Тюменский бройлер», Тюменская обл., Россия)
|
Показатель |
Контроль |
Опыт |
Разница |
|
Затраты на производство 1 кг мяса: заработная плата и налоги |
2,09 |
2,09 |
0,00 |
|
корма |
33,74 |
32,45 |
- 1,30 |
|
затраты на ветеринарные мероприятия |
1,99 |
2,19 |
0,20 |
|
другое |
8,21 |
8,13 |
- 0,08 |
|
Затраты на 1-суточного цыпленка в расчете на 1 кг произведенного мяса |
6,67 |
6,61 |
- 0,07 |
Затраты на 1 кг живой массы бройлеров
Затраты на 1 кг произведенного мяса
Затраты на убой и продажу в расчете на 1 кг произведенного мяса
Средняя отпускная цена за 1 кг мяса
Прибыль в расчете на 1 кг продукции
Продолжение таблицы 2
|
52,71 |
51,47 |
- 1,24 |
|
70,24 |
68,59 |
- 1,66 |
|
19,61 |
19,61 |
0,00 |
|
120,69 |
120,69 |
0,00 |
|
30,84 |
32,49 |
1,66 |
В результате улучшения конверсии корма при использовании питьевой воды с добавкой антистрессового препарата повысилась экономическая эффективность производства мяса бройлеров (табл. 2): увеличение прибыли в расчете на 1 кг продукции составило 1,66 руб.
Обсуждая полученные результаты, следует отметить, что в птицеводстве существуют четыре основных типа стрессов (25-27): технологические, средовые, кормовые и внутрение/биологические (28-33) (рис. 2).
Технологические стрессы
Посадка цыплят в корпус
Повышенная плотность посадки
Взвешивание, сортировка, формирование групп, отлов, транспортировка в промышленные помещения Длительное хранение яиц, транспортировка яиц, ненадлежащие условия хранения яиц, неправильные режимы инкубации
Стрессы, вызванные внешними условиями Ненадлежащая температура
Плохая вентиляция и высокая запыленность
Ненадлежащее освещение
Пищевые стрессы
Микотоксины
Окисленные жиры
Токсичные металлы (свинец, кадмий, ртуть и т.д.)
Несбалансированность рациона по минеральным веществам (Se, Zn, Cu, т.д.) и другим нутриетам Плохое качество воды
Применение кокцидостатиков и других лекарственных препаратов с кормом или водой Эндогенные стрессы
Вакцинация
Микробное или вирусное заражение
Кишечные дисбактерозы Наклев и вылупление
Рис. 2. Стрессы в птицеводстве (цит. по 30 с изменениями).
Стресс, связанный с посадкой птицы в корпус на ферме, — один из наиболее важных. Значимый фактор, определяющий прибыльность в птицеводстве, — это жизнеспособность цыплят. На нее влияет качество, условия хранения и инкубации яиц, а также условия хранения и транспортировки цыплят после вылупления (34). Доказано, что первые 24 ч жизни цыплят — наиболее критичный период (34-37). Считается, что после вылупления цыпленок как можно скорее должен получить доступ к корму и воде для стимуляции развития пищеварительной и иммунной систем. В период между вылуплением и размещением цыплят в птичнике они подвергаются стрессу из-за истощения запасов желточного мешка и обезвоживания. Вылупление, обработка, транспортировка и размещение цыпленка на ферме занимают 36-48 ч. За это время без корма и воды цыпленок быстро теряет в весе (38). Самые драматические изменения в тонком кишечнике происходят в первые 24 ч после вылупления (39). Фактически существует обратная зависимость между продолжительностью выдержки после инкубации и последующим ростом цыплят (34-37, 40, 41). Поэтому немедленное обеспечение кормом и водой помогает достичь увеличения массы тела у цыплят в возрасте 3 нед (42) или в возрасте убоя птицы (43). Кроме того, есть «окно» (24-36 ч), определяющее наличие поздних и ранних выводков, которое зависит от однородности инкубационных яиц по массе, связанной с возрастом родительской птицы и используемой технологией ее содержания (36, 37). Растянутость сроков вылупления увеличит число цыплят, до- полнительное время находившихся без доступа к корму и воде. Любая задержка в таком доступе (44, 45), обработка в инкубатории, например вакцинация, сексирование, и транспортировка на ферму могут привести к дополнительному стрессу (46). С длительным (36 ч) пребыванием в инкубаторе связано ослабление антиоксидантной защиты, что проявляется в снижении содержания витамина Е и коэнзима Q в тканях (47). Из-за относительно высокой температуры и влажности в инкубаторе цыпленок находится под хроническим окислительным стрессом в течение всего срока выдержки (23, 47). Поэтому антиоксидантная защита во время вывода молодняка считается важным фактором жизнеспособности цыплят в первые сутки после вылупления и в раннем постнатальном онтогенезе (3, 4850). При эмбриогенезе в тканях устанавливается антиоксидантный/проок-сидантный баланс, который поддерживает нормальное эмбриональное развитие и жизнеспособность цыплят после вылупления (48-51). Было высказано предположение о том, что накопление природных антиоксидантов, таких как витамины А, Е и каротиноиды, а также увеличение активности глутатионпероксидазы (GSH-Px) в печени эмбриона может иметь адаптивное значение и происходит для защиты ненасыщенных липидов от пероксидации, вызванной стрессом вывода молодняка (3, 49, 50). Следует подчеркнуть, что условия кормления в ранний постнатальный период считаются критическими для созревания многих систем, органов и формирования оптимальных физиологических функций. В научной литературе появляется все больше доказательств того, что окружающая среда, включая питание, в эти критические и чувствительные к стрессам периоды жизни может вызывать необратимые изменения во многих физиологических процессах, что известно как раннее программирование (52). Наши предыдущие исследования показали, что низкокачественное питание птенцов в ранний постанатальный период приводило к долгосрочному ухудшению способности ассимилировать антиоксиданты из рациона (53). Вероятно, раннее программирование, связанное с эпигенетическими механизмами, играет ключевую роль в развитии цыплят и в период их посадки в корпус для выращивания. Кроме того, накапливаются данные о существовании материнского программирования, когда изменения в организме могут передаваться потомству, не затрагивая последовательности ДНК (54). Трансгенерационные эффекты стресса потенциально опосредуются модуляцией гипоталамо-гипо-физарно-надпочечниковой оси, а также эпигенетическими механизмами, вызывая наследуемые изменения в экспрессии генов. Высказывалось предположение, что ранний опыт и (или) воздействие может формировать фенотип цыплят на длительный срок (55). Кроме упомянутых стрессов, цыплята подвергаются стрессам вылупления без материнского контакта, транспортировки и социальной изоляции. Так, стресс ранней социальной изоляции приводил к изменению концентрации кортикостерона у птиц и их мужского потомства. Кроме того, стрессоспецифические гены, такие как ген раннего ростового ответа 1 и ген рецептора кортиколиберина, активировались сразу после стресса изоляции (55).
В последние годы активно накапливаются данные и в поддержку гипотезы о том, что Е-витаминный статус цыплят и индюшат может быть неадекватным в первые недели после вылупления (56). Были использованы различные подходы для улучшения этого статуса сразу после вывода, включая применение добавок с высоким содержанием α-токоферола (3, 57), желчных солей (58) и жира (59), инъекций витамина E (60), а также изменение в обеспеченности рациона полиненасыщенными (омега-3 и омега-6) жирными кислотами (60). При добавлении D-α-токоферола в питьевую воду его содержание в тканях повышалось, а чувствительность эритроцитов к гемолизу снижалась (61). Когда цыплята, начиная с 1-суточного возраста, получали витамин Е с питьевой водой (3,25 мг•гол.-1•сут-1) в течение 2 нед, его количество в печени и плазме крови значительно повысилось по сравнению с контролем (62). Вероятно, выпаивание витамина Е и других жирорастворимых витаминов (А и D3) во время посадки может решить проблему их низкой доступности для cвежевылупившихся птенцов (23), ослабить стресс посадки и улучшить рост и развитие птицы.
Когда посадка цыплят в птичник происходит зимой при довольно низкой температуре, всегда возникает соблазн уменьшить вентиляцию, чтобы минимизировать энергопотребление. Тем не менее, для недавно вылупившихся птенцов очень важен теплый свежий воздух, который богат кислородом. Трахея цыпленка часто раздражена из-за его длительного пребывания в лотке вывода и коробке для транспортировки. Кроме того, цыплята могут подвергаться действию формальдегида и загрязненного воздуха при вылуплении (23, 50). Избыточные количества СО2 и аммиака вызывают депрессию, обезвоживание, истощение, а также проблемы с дыхательной системой (23, 50). Усиленное перекисное окисление липидов и снижение активности антиоксидантных ферментов у здоровых цыплят при нарушении микроклиматических условий (повышение температуры, влажности воздуха, концентрации аммиака, снижение освещенности) свидетельствуют об окислительном стрессе (63). Следует также упомянуть, что плохая вентиляция часто приводит к накоплению токсичного СО. Все это нарушает биологический баланс, приводя к необратимым физиологическим и биохимическим изменениям, которые не удается впоследствии восстановить (25, 26). Для борьбы с окислительным стрессом в период посадки в птичник существует несколько приемов. Рекомендуется добавлять в систему водоснабжения электролиты для поддержания их оптимального баланса у цыплят и увеличения потребления воды (23), вводить с питьевой водой жирорастворимые витаминные добавки для преодоления низкой эффективности усвоения витаминов из рациона (3, 23) и другие защитные питательные вещества (аскорбиновая кислота, Se, карнитин, бетаин, лизин, метионин и т.д.) для уменьшения окислительного стресса, связанного с переводом в птичник и адаптацией кишечника цыплят к новому типу корма (23, 28). Усиление антиоксидантной защиты в первые дни жизни способствует развитию иммунной системы в этот критический период (65, 66).
Как показали наши исследования, добавление витаген-регулиру-ющей композиции в питьевую воду помогает улучшить конверсию корма по сравнению с таковой у контрольной птицы. Таким образом, снизив стресс посадки, мы обеспечили цыплят более благоприятными стартовыми условиями, что впоследствии положительно отразилось на развитии кишечника и общем состоянии здоровья. Обнаруженная тенденция к увеличению массы кишечника может быть одним из проявлений обсуждаемых эффектов активности системы витагенов. Высказанная нами ранее идея об антиоксидантно-прооксидантном балансе в кишечнике (67, 68) недавно получила развитие применительно к стрессам при посадке птицы (51). Антиоксидантная защита в кишечнике основана на природных антиоксидантах, включая витамин Е и селен, и ферментативных системах (69), в том числе SOD, составляющих важную часть метаболической сети, контролируемой витагенами (10). Поэтому, используя с питьевой водой композицию, стимулирующую витагены, можно усилить антиоксидантную защиту кишечника, что будет способствовать улучшению его состояния и функции, обеспечивая более эффективную конверсию корма.
Еще одна возможность улучшения конверсии корма заключается в повышении иммунокомпетентности. Состояние иммунной системы — решающий фактор, определяющий здоровье цыплят, эффективность их роста и развития. Активация иммунной системы довольно затратна, и только оптимальный иммунный ответ дает надежную защиту от патогенов с минимальной нагрузкой на организм. В нашем опыте индекс вакцинации у экспериментальных цыплят повысился, что могло быть связано с иммуномодулирующими свойствами использованного препарата, способного активировать витагены. В предыдущих исследованиях мы ясно показали, что повышение активности витагенов обеспечивает высокую иммунокомпетентность птицы в промышленных условиях выращивания, при этом Antistress Mix/Per-forMax снижает стресс вакцинации (23, 66). Было высказано предположение, что ключом к высокой иммунокомпетентности служит связь между различными клетками иммунной системы (3, 4). Иммунная система объединяет триллионы лимфоцитов и миллиарды фагоцитов, и все эти клетки должны взаимодействовать друг с другом и с клетками других типов для реализации оптимальной стратегии иммунной защиты. За такие коммуникации, вероятно, ответственны рецепторы, расположенные на поверхности этих клеток, и при стрессе они могут повреждаться свободными радикалами. Кроме того, окислительный стресс способен влиять на функцию цитокинов и эйкозаноидов, вовлеченных в коммуникации. Следовательно, повышая активность витагенов, ответственных за синтез защитных молекул (белков теплового шока, тиоредоксина и тиоредоксинредуктазы, сиртуинов и супероксиддис-мутазы), можно обеспечить защиту рецепторов и поддерживать высокую иммунокомпетентность при стрессе. Ранее мы сообщали о положительном влиянии Magic Antistress Mix/PerforMax на конверсию корма (71, 72) и повышение иммунокомпетентности у уток (70). В настоящем исследовании предыдущие результаты подтвердились в промышленных условиях при выпаивании этой добавки с питьевой водой, обеспечив рост, развитие и конверсию корма, сопоставимые с лучшими примерами производства цыплят в России и за рубежом, а также дополнительную прибыль.
В последние годы все больше внимания уделяется микотоксинам как главным кормовым стрессорам в птицеводстве и животноводстве (7376). Окислительный стресс, вызываемый микотоксинами, — главный молекулярный механизм их действия (77-79). Это касается прежде всего охратоксина (80-83), Т-2 токсина (84-86), DON (87, 89), фумонизина (90-92) и афлатоксинов (93, 94). Избежать контаминации кормов микотоксинами очень трудно, поэтому для предотвращения их отрицательного действия с разным успехом применяют специальные адсорбенты (95-98). Однако активность связывания микотоксинов адсорбентами варьирует в широких пределах, из-за чего решить проблему микотоксикозов подобным способом не удается. Ключевая стратегия борьбы с микотоксикозами — использование различных комбинаций антиоксидантов в рационе для поддержания функции печени, где происходит детоксикация основных микотоксинов, и кишечника, микробиота которого способна в значительной степени обезвреживать DON (78, 99). В этой связи испытанный нами витаген-стимули-рующей препарат широкого спектра защитного действия может оказаться важнейшим инструментом в руках технологов птицеводства (22, 23).
Эффективность витаген-регулирующей добавки на птице яичного типа (Hy-Line, «Hy-Line International», США) была недавно изучена в промышленном и родительском стадах в условиях одной из крупнейших в России Боровской птицефабрики (Тюменская обл.) (28, 33, 100). Показано, что выпаивание PerforMax/Magic Antistress Mix в периоды повышенного 723
стресса может улучшить продуктивность родительского стада кур. Так, при выходе на пик яичной продуктивности показатели повысились на 2 % и сохранялись примерно на 50 сут дольше, чем у контрольной птицы. Интересно, что при этом яичная продуктивность, которая даже в контрольной группе (260,8 яйца) оказалось выше целевого показателя для линии по России (253,4 яйца), в экспериментальной группе увеличилась на 6 яиц, что сопровождалось увеличением массы яйцевода. Важно отметить, что показатель конверсии корма (затраты корма на откладку 10 яиц) тоже улучшался при использовании антистрессового препарата, превосходя целевой для этой птицы. У несушек, получавших добавку, яичная скорлупа в возрасте 26, 36 и 56 нед была крепче соответственно на 2,8; 5,6 и 5,6 %. Наиболее интересным представляется существенное увеличение содержания каротиноидов в яичном желтке в опытной группе. Поскольку каротиноиды не были включены в антистрессовый препарат, это может быть связано с улучшением всасывания питательных веществ под влиянием использованной антистрессовой добавки. Тем же может объясняться улучшение конверсии корма. У несушек из опытной группы также увеличилось количество витамина А в яичном желтке, вероятно, отражая его перенос из антистрессового препарата. Оплодотворенность в опытной группе тоже повысилась (на 2,5; 2,7; 2,8 и 3,7 % соответственно на 16-ю, 40-ю, 48-ю и 56-ю нед). В той же группе на 26-ю, 32-ю, 40-ю, 48-ю и 56-ю нед использования несушек число вылупившихся кондиционных цыплят возросло соответственно на 3,6; 2,1; 3.4; 4,9 и 4,3 % (28, 33, 100, 101). Применение антистрессовой добавки положительно сказалось на развитии семенников у 15-, 26- и 56-недельных петушков (28). Печень экспериментальной птицы разного возраста характеризовалась повышенным содержанием витамина А (28, 102). Было убедительно продемонстрировано, что Magic Antistress Mix/PerforMax увеличивает выход яиц и процент инкубационных яиц, а также выводимость. Это обеспечило рентабельность 29,3 % против 19,7 % в контроле (28). С помощью Magic Antistress Mix/PerforMax можно повысить яичную продуктивность родительского стада бройлеров (103). Кроме того, на птице Hy-Line показано (104), что однородность потомства, оцененная на 28-е сут, значительно возрастала относительно контроля при выпаивании антистрессовой добавки (1 г/л) родителям разного возраста, составив в 26 нед 81,3 против 67,3 %, в 32 нед — 85,5 против 76,8 %, в 40 нед — 83,2 против 68,8 %, в 48 нед — 75,5 против 68,0 % и в 56 нед — 73,7 против 62,0 %. Вероятно, изменения в составе яиц при обеспечении важными питательными веществами, включая доноры метильных групп (бетаин, метионин, витамин B12 и т.д.), могут иметь эпигенетические эффекты в потомстве (23, 104, 105).
Защитные свойства добавки доказаны также в свиноводстве (24, 51, 106). Кроме того, предварительные исследования свидетельствуют о том, что активация витагенов через коррекцию рациона — универсальный подход при борьбе со стрессами, поскольку витаген-регулирующая добавка на других видах птицы (индейка, утка, гусь, перепела), а также на телятах, ягнятах, жеребятах, кроликах, пушных и домашние животные, рыбе и т.д. весьма эффективна (П.Ф. Сурай, неопубликованные данные). Изучение взаимодействия витагенов и кишечной микробиоты открывает новую главу в понимании роли кишечного иммунитета и структурной целостности кишечника (107-110). Целевое воздействие на экспрессию витагенов в кишечнике позволит найти новые подходы к повышению резистентности как к микробиальным, так и вирусным патогенам. Однако этот вопрос до сих пор не освещался в мировой научной литературе.
Итак, новая концепция основана на идее, что обеспечение вита- ген-регулирующей комбинацией нутриентов через питьевую воду может помочь птице справляться со стрессом. В представленном исследовании подтверждено улучшение показателей роста и развития бройлеров (включая конверсию корма) и повышение индекса вакцинации. Эти эффекты обеспечиваются молекулярными механизмами, контролируемыми витаге-нами, которые подробно рассмотрены в недавно опубликованных обзорах, констатирующих важную регуляторную роль витагенов в адаптации клеток и всего организма к стрессам. Продукты экспрессии витагенов активно участвуют в распознавании и контроле разных форм стресса и повреждений клеток. Кроме того, по последним данным, возможна регуляция вита-генов нутриентами. В оптимальной комбинации витамины A, D, E, C, селен, карнитин и бетаин влияют на витагены и улучшают адаптивную способность организма, причем их защитные эффекты наиболее выражены в стрессовых условиях. Полученные нами результаты — первые шаги от разработки концепции витагенов к созданию коммерческого продукта для минимизации последствий экономически значимых стрессов в птицеводстве (и животноводстве в целом). Для понимания молекулярных механизмов регуляции экспрессии витагенов в клетке на уровне сигнальных систем и факторов транскрипции при адекватном адаптивном ответе требуются дальнейшие исследования, что даст возможность усовершенствовать используемый универсальный антистрессовый препарат и создать более специфические композиции для борьбы с дисфункцией печени у птицы при промышленном производстве мяса и яиц. Есть все основания полагать, что концепция витагенов также позволит решить другие проблемы современного птицеводства, в частности вызванные боем и насечкой яиц у несушек во второй половине продуктивного периода.
Список литературы Регуляция активности витагенов как новая антистрессовая стратегия в птицеводстве: обоснование и производственный опыт
- Soleimani A.F., Zulkifli I., Omar A.R., Raha A.R. Physiological responses of 3 chicken breeds to acute heat stress. Poultry Sci., 2011, 90: 1435-1440 ( ) DOI: 10.3382/ps.2011-01381
- Bureau C., Hennequet-Antier C., Couty M., Guemene D. Gene array analysis of adrenal glands in broiler chickens following ACTH treatment. BMC Genomics, 2009, 10: 430 ( ) DOI: 10.3201/eid1508.080772
- Surai P.F. Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, Nottingham, 2002.
- Surai P.F. Selenium in nutrition and health. Nottingham University Press, Nottingham, 2006.
- Mezes M., Surai P.F., Salyi G., Speake B.K., Gaal T., Maldjian A. Nutritional metabolic diseases in poultry and disorders of the biological antioxidant defence system. Acta Veterinaria Hungarica, 1997, 45: 349-360.
- Fellenberg M.A., Speisky H. Antioxidants: Their effects on broiler oxidative stress and its meat oxidative stability. World's Poultry Science Journal, 2006, 62: 53-70.
- Estevez M. Oxidative damage to poultry: from farm to fork. Poultry Sci., 2015, 94: 1368-1378 ( ) DOI: 10.3382/ps/pev094
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol., 2016, 7: 37 ( ) DOI: 10.1186/s40104-016-0097-5
- Calabrese V., Guagliano E., Sapienza M., Panebianco M., Calafato S., Puleo E., Pennisi G., Mancuso C., Butterfield D.A., Stella A.G. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem. Res., 2007, 32: 757-773.
- Surai P.F. Antioxidant systems in poultry biology: superoxide dismutase. Journal of Animal Research and Nutrition, 2015, 1: 1-17.
- Calabrese V., Boyd-Kimball D., Scapagnini G., Butterfield D.A. Nitric oxide and cellular stress response in brain aging and neurodegenerative disorders: the role of vitagenes. In Vivo, 2004, 18: 245-267.
- Calabrese V., Cornelius C., Mancuso C., Barone E., Calafato S., Bates T., Rizzarelli E., Kostova A.T. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Frontiers in Bioscience, 2009, 14: 376-397.
- Calabrese V., Giordano J., Crupi R., Di Paola R., Ruggieri M., Bianchini R., Ontario M.L., Cuzzocrea S., Calabrese E.J. Hormesis, cellular stress response and neuroinflammation in schizophrenia: Early onset versus late onset state. J. Neurosci. Res., 2017, 95(5): 1182-1193 ( ) DOI: 10.1002/jnr.23967
- Calabrese V., Giordano J., Signorile A., Laura Ontario M., Castorina S., De Pasquale C., Eckert G., Calabrese E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res., 2016, 94: 1588-1603 ( ) DOI: 10.1002/jnr.23925
- Calabrese V., Giordano J., Ruggieri M., Berritta D., Trovato A., Ontario M.L., Bianchini R., Calabrese E.J. Hormesis, cellular stress response, and redox homeostasis in autism spectrum disorders. J. Neurosci. Res., 2016, 94: 1488-1498 ( ) DOI: 10.1002/jnr.23893
- Calabrese V., Cornelius C., Cuzzocrea S., Iavicoli I., Rizzarelli E., Calabrese E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Molecular Aspects of Medicine, 2011, 32: 279-304 ( ) DOI: 10.1016/j.mam.2011.10.007
- Calabrese V., Cornelius C., Dinkova-Kostova A.T., Iavicoli I., Di Paola R., Koverech A., Cuzzocrea S., Rizzarelli E., Calabrese E.J. Cellular stress responses, hermetic phytochemicals and vitagenes in aging and longevity. BBA, 2012, 1822: 753-783 ( ) DOI: 10.1016/j.bbadis.2011.11.002
- Calabrese V., Scapagnini G., Davinelli S., Koverech G., Koverech A., De Pasquale C., Salinaro A.T., Scuto M., Calabrese E.J., Genazzani A.R. Sex hormonal regulation and hormesis in aging and longevity: role of vitagenes. Journal of Cell Communication and Signalling, 2014, 8: 369-384 ( ) DOI: 10.1007/s12079-014-0253-7
- Calabrese V., Calafato S., Puleo E., Cornelius C., Sapienza M., Morganti P., Mancuso C. Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: role of vitagenes. Clinics in Dermatology, 2008, 26: 358-363 ( ) DOI: 10.1016/j.clindermatol.2008.01.005
- Calabrese V., Cornelius C., Trovato A., Cavallaro M., Mancuso C., Di Rienzo L., Condorelli D., De Lorenzo A., Calabrese E.J. The hormetic role of dietary antioxidants in free radical-related diseases. Current Pharmaceutical Design, 2010, 16: 877-883.
- Cornelius C., Koverech G., Crupi R., Di Paola R., Koverech A., Lodato F., Scuto M., Salinaro A.T., Cuzzocrea S., Calabrese E.J., Calabrese V. Osteoporosis and Alzheimer pathology: Role of cellular stress response and hormetic redox signaling in aging and bone remodeling. Frontiers in Pharmacology, 2014, 5: 120 ( ) DOI: 10.3389/fphar.2014.00120
- Фисинин В.И., Сурай П.Ф. Эффективная защита от стрессов в птицеводстве: от витаминов к витагенам. Птица и птицепродукты, 2011: 5: 23-26.
- Surai P.F., Fisinin V.I. The modern anti-stress technologies in poultry production: from antioxidants to vitagenes. Sel'skokhozyaistvennaya Biologiya , 2012, 4: 3-13 ( ) DOI: 10.15389/agrobiology.2012.4.3eng
- Сурай П.Ф., Мельничук С.Д. Механизмы защиты от стрессов в свиноводстве. От витаминов к витагенам. Свиноводство Украины, 2012, 2: 10-15.
- Surai P.F., Fisinin V.I. Vitagenes in poultry production. Part 1. Technological and environmental stresses. World's Poultry Science Journal, 2016, 72, 721-733.
- Surai P.F., Fisinin V.I. Vitagenes in poultry production. Part 2. Nutritional and Internal stresses. World's Poultry Science Journal, 2016, 72: 761-772.
- Surai P.F., Fisinin V.I. Vitagenes in poultry production. Part 3. Vitagene concept development. World's Poultry Science Journal, 2016, 72: 793-804.
- Шацких Е.В., Латыпова Е.Н., Несвет У.Г., Кобурнеев И.В. Использование антистрессовых препаратов в яичном птицеводстве. Екатеринбург, 2016.
- Scanes C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poultry Sci., 2016, 95: 2208-2215 ( ) DOI: 10.3382/ps/pew137
- Gessner D.K., Ringseis R., Eder K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. (Berl.), 2017, 101: 605-628 ( ) DOI: 10.1111/jpn.12579
- Habibian M., Sadeghi G., Ghazi S., Moeini M.M. Selenium as a feed supplement for heat-stressed poultry: a review. Biol. Trace Elem. Res., 2015, 165: 183-193 ( ) DOI: 10.1007/s12011-015-0275-x
- Saeed M., Babazadeh D., Naveed M., Arain M.A., Hassan F.U., Chao S. Reconsidering betaine as a natural anti-heat stress agent in poultry industry: a review. Trop. Anim. Health Prod., 2017, First Online 21 July: 1-10 ( ) DOI: 10.1007/s11250-017-1355-z
- Shatskih E., Latipova E., Fisinin V., Denev S., Surai P. Molecular mechanisms and new strategies to fight stresses in egg-producing birds. Agric. Sci. Technol., 2015, 7: 3-10.
- Decuypere E., Tona K., Bruggeman V., Bamelis F. The day-old chick: a crucial hinge between breeders and broilers. World's Poultry Science Journal, 2001, 57: 127-138.
- Noy Y., Uni Z. Early nutrition strategy. World's Poultry Science Journal, 2010, 66: 639-646.
- Cherian G. Nutrition and metabolism in poultry: role of lipids in early diet. J. Anim. Sci. Biotechnol., 2015, 6(1): 28 ( ) DOI: 10.1186/s40104-015-0029-9
- Wang Y., Li Y., Willems E., Willemsen H., Franssens L., Koppenol A., Guo X., Tona K., Decuypere E., Buyse J., Everaert N. Spread of hatch and delayed feed access affect post hatch performance of female broiler chicks up to day 5. Animal, 2014, 8(4): 610-617.
- Noy Y., Sklan D. Different types of early feeding and performance in chicks and poults. The Journal of Applied Poultry Research, 1999, 8: 16-24.
- Geyra A., Uni Z., Sklan D. Enterocyte dynamics and mucosal development in the posthatch chick. Poultry Sci., 2001, 80: 776-782.
- Hager J.E., Beane W.L. Posthatch incubation time and early growth of broiler chickens. Poultry Sci., 1983, 62: 247-254.
- Pinchasov Y., Noy Y. Comparison of post-hatch holding time and subsequent early performance of broiler chicks and Turkey poults. British Poultry Science, 1993, 34: 111-120.
- Sklan D., Noy Y., Hoyzman A., Rozenboim I. Decreasing weight loss in the hatchery by feeding chicks and poults in hatching trays. The Journal of Applied Poultry Research, 2000, 9: 142-148.
- Viera S.L., Moran E.T., Jr. Effects of delayed placement and used litter on broiler yields. The Journal of Applied Poultry Research, 1999, 8: 75-81.
- Noy Y., Gyra A., Sklan D. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poultry Sci., 2001, 80: 912-919.
- Bigot K., Mignon-Grasteau P., Picard M., Tesseraud S. Effects of delayed feed intake on body, intestine and muscle development in neonate broilers. Poultry Sci., 2003, 85: 781-788.
- Geyra A., Uni Z., Sklan D. The effect of fasting at different ages on growth and tissue dynamics in the small intestine of the young chick. British Journal of Nutrition, 2001, 86: 53-61.
- Karadas F., Surai P.F., Sparks N.H. Changes in broiler chick tissue concentrations of lipid-soluble antioxidants immediately post-hatch. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 2011, 160: 68-71 ( ) DOI: 10.1016/j.cbpa.2011.05.006
- Surai P.F., Noble R.C., Speake B.K. Tissue-specific differences in antioxidant distribution and susceptibility to lipid peroxidation during development of the chick embryo. Biochimica et Biophysica Acta, 1996, 1304: 1-10.
- Surai P.F., Fisinin V.I., Karadas F. Antioxidant systems in chick embryo development. Part 1. Vitamin E, carotenoids and selenium. Animal Nutrition, 2016, 2: 1-11.
- Surai P.F., Fisinin V.I. Natural antioxidants in hen's embryogenesis and antioxidant defense in postnatal development. Sel'skokhozyaistvennaya Biologiya , 2013, 2: 3-18 ( , 10.15389/agrobiology.2013.2.3eng) DOI: 10.15389/agrobiology.2013.2.3rus
- Surai P.F., Fisinin V.I. Аntioxidant-prooxidant balance in the intestine: applications in chick placement and pig weaning. J. Veter. Sci. Med., 2015, 3(1): 16.
- Amarasekera M., Prescott S.L., Palmer D.J. Nutrition in early life, immunoprogramming and allergies: the role of epigenetics. Asian Pacific Journal of Allergy and Immunology, 2013, 31: 175-182.
- Blount J.D., Metcalfe N.B., Arnold K.E., Surai P.F., Devevey G.L., Monaghan P. Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proceedings of the Royal Society of London. Series B, Biological sciences, 2003, 270: 1691-1696.
- Champagne F.A., Rissman E.F. Behavioral epigenetics: a new frontier in the study of Hormones and Behavior. Hormones and Behavior, 2011, 59: 277-278 ( ) DOI: 10.1016/j.yhbeh.2011.02.011
- Goerlich V.C., Natt D., Eefwing M., Macdonald B., Jensen P. Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Hormones and Behavior, 2012, 61: 711-718 ( ) DOI: 10.1016/j.yhbeh.2012.03.006
- Sell J.L. Recent developments in vitamin E nutrition of turkeys. Animal Feed Science and Technology, 1996, 60: 229-240.
- Applegate T.J., Sell J.L. Effect of dietary linoleic to linolenic acid ratio and vitamin E supplementation on vitamin E status of poults. Poultry Sci., 1996, 75: 881-890.
- Marusich W.L., Deritter E., Ogrinz E.F., Keating J., Mitrovic M., Bunnell R.H. Effect of supplemental vitamin E on control of rancidity in poultry meat. Poultry Sci., 1975, 54: 831-844.
- Soto-Salanova M.F., Sell J.L. Influence of supplemental dietary fat on changes in vitamin E concentration in livers of poults. Poultry Sci., 1995, 74: 201-204.
- Soto-Salanova M.F., Sell J.L. Efficacy of dietary and injected vitamin E for poults. Poultry Sci., 1996, 75: 1393-1403.
- Soto-Salanova M.F. Vitamin E in young turkeys: а reassessment of the requirement. In: Retrospective Theses and Dissertations. Iowa State University, 1998. Режим доступа: http://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=11984&context=rtd. Без даты.
- Mezes M. Effect of vitamin E treatment on early postnatal changes of vitamin E status of chicken. Acta Veterinaria Hungarica, 1994, 42: 477-480.
- Georgieva N.V., Stoyanchev K., Bozakova N., Jotova I. Combined effects of muscular dystrophy, ecological stress, and selenium on blood antioxidant status in broiler chickens. Biol. Trace Elem. Res., 2011, 142: 532-545 ( ) DOI: 10.1007/s12011-010-8782-2
- Balnave D., Gorman I. A role for sodium bicarbonate supplements for growing broilers at high temperatures. World's Poultry Science Journal, 1993, 49: 236-241.
- Gore A.B., Qureshi M.A. Enhancement of humoral and cellular immunity by vitamin E after embryonic exposure. Poultry Sci., 1997, 76: 984-991.
- Fisinin V.I., Surai P.F. Gut immunity in birds: facts and reflections. Sel'skokhozyaistvennaya Biologiya , 2013, 4: 3-25 ( , 10.15389/agrobiology.2013.4.3rus) DOI: 10.15389/agrobiology.2013.4.3eng
- Surai K.P., Surai P.F., Speake B.K., Sparks N.H.C. Antioxidant-prooxidant balance in the intestine: Food for thought. 1. Prooxidants. Nutritional Genomics and Functional Foods, 2003, 1: 51-70.
- Surai K.P., Surai P.F., Speake B.K., Sparks N.H.C. Antioxidant-prooxidant balance in the intestine: Food for thought. 2. Antioxidants. Current Topics in Neutraceutical Research, 2004, 2: 27-46.
- McLean J.A., Karadas F., Surai P.F., Speake B.K., McDevitt R.M., Sparks N.H.C. Lipid-soluble and water-soluble antioxidant activities of the avian intestinal mucosa at different sites along the intestinal tract. Comparative Biochemistry and Physiology, 2005, 141(B): 366-372.
- Сурай П.Ф., Фотина А.А., Фотина Т.И. Влияние препарата Фид-Фуд Антистресс Микс на естественную резистентность утят. Вестник Сумского национального аграрного университета, 2012, 7: 58-61.
- Величко О.А., Шабалдин С.А., Сурай П.Ф. Практические вопросы использования концепции витагенов в птицеводстве. Птица и птицепродукты, 2013, 4: 42-45.
- Velichko O.A., Surai P.F. Effect of an antistress composition supplied with water on chick growth and development. Proc. XIV European Poultry Conference. EPC, Stavanger, Norway, 2014: 551.
- Guerre P. Worldwide mycotoxins exposure in pig and poultry feed formulations. Toxins (Basel), 2016, 8(12): E350.
- Murugesan G.R., Ledoux D.R., Naehrer K., Berthiller F., Applegate T.J., Grenier B., Phillips T.D., Schatzmayr G. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult. Sci., 2015, 94: 1298-1315 ( ) DOI: 10.3382/ps/pev075
- Escrivá L., Font G., Manyes L. In vivo toxicity studies of fusarium mycotoxins in the last decade: a review. Food Chem. Toxicol., 2015, 78: 185-206 ( ) DOI: 10.1016/j.fct.2015.02.005
- Ghareeb K., Awad W.A., Böhm J., Zebeli Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. J. Appl. Toxicol., 2015, 35: 327-337 ( ) DOI: 10.1002/jat.3083
- Wu Q.H., Wang X., Yang W., Nüssler A.K., Xiong L.Y., Kuča K., Dohnal V., Zhang X.J., Yuan Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update. Arch. Toxicol., 2014, 88: 1309-1326 ( ) DOI: 10.1007/s00204-014-1280-0
- Sorrenti V., Di Giacomo C., Acquaviva R., Barbagallo I., Bognanno M., Galvano F. Toxicity of ochratoxin a and its modulation by antioxidants: a review. Toxins (Basel), 2013, 5: 1742-1766 ( ) DOI: 10.3390/toxins5101742
- Doi K., Uetsuka K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int. J. Mol. Sci., 2011, 12: 5213-5237 ( ) DOI: 10.3390/ijms12085213
- Sheu M.L., Shen C.C., Chen Y.S., Chiang C.K. Ochratoxin A induces ER stress and apoptosis in mesangial cells via a NADPH oxidase-derived reactive oxygen species-mediated calpain activation pathway. Oncotarget, 2017, 8: 19376-19388 ( ) DOI: 10.18632/oncotarget.14270
- Malir F., Ostry V., Pfohl-Leszkowicz A., Malir J., Toman J. Ochratoxin A: 50 years of research. Toxins (Basel), 2016, 8(7): E191 ( ) DOI: 10.3390/toxins8070191
- Kőszegi T., Poór M. Ochratoxin A: molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins (Basel), 2016, 8(4): 111 ( ) DOI: 10.3390/toxins8040111
- Vettorazzi A, van Delft J, López de Cerain A. A review on ochratoxin A transcriptomic studies. Food Chem. Toxicol., 2013, 59: 766-783 ( ) DOI: 10.1016/j.fct.2013.05.043
- Yang L., Yu Z., Hou J., Deng Y., Zhou Z., Zhao Z., Cui J. Toxicity and oxidative stress induced by T-2 toxin and HT-2 toxin in broilers and broiler hepatocytes. Food Chem. Toxicol., 2016, 87: 128-137 ( ) DOI: 10.1016/j.fct.2015.12.003
- Wu Q.H., Wang X., Yang W., Nüssler A.K., Xiong L.Y., Kuča K., Dohnal V., Zhang X.J., Yuan Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update. Arch. Toxicol., 2014, 88: 1309-1326 ( ) DOI: 10.1007/s00204-014-1280-0
- Rezar V., Frankic T., Narat M., Levart A., Salobir J. Dose-dependent effects ofT-2 toxin on performance, lipid peroxidation, and genotoxicity in broiler chickens. Poultry Sci., 2007, 86: 1155-1160.
- Mishra S., Dwivedi P.D., Pandey H.P., Das M. Role of oxidative stress in deoxynivalenol induced toxicity. Food Chem. Toxicol., 2014, 72: 20-29 ( ) DOI: 10.1016/j.fct.2014.06.027
- Li D., Ye Y., Lin S., Deng L., Fan X., Zhang Y., Deng X, Li Y, Yan H., Ma Y. Evaluation of deoxynivalenol-induced toxic effects on DF-1 cells in vitro: cell-cycle arrest, oxidative stress, and apoptosis. Environmental Toxicology and Pharmacology, 2014, 37(1): 141-149 ( ) DOI: 10.1016/j.etap.2013.11.015
- Osselaere A., Santos R., Hautekiet V., De Backer P., Chiers K., Ducatelle R., Croubels S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE, 2013, 8(7): e69014 ( ) DOI: 10.1371/journal.pone.0069014
- Wang X., Wu Q., Wan D., Liu Q., Chen D., Liu Z., Martínez-Larrañaga M.R., Martínez M.A., Anadón A., Yuan Z. Fumonisins: oxidative stress-mediated toxicity and metabolism in vivo and in vitro. Arch. Toxicol., 2016, 90: 81-101 ( ) DOI: 10.1007/s00204-015-1604-8
- Garbetta A., Debellis L., De Girolamo A., Schena R., Visconti A., Minervini F. Dose-dependent lipid peroxidation induction on ex vivo intestine tracts exposed to chyme samples from fumonisins contaminated corn samples. Toxicology in Vitro, 2015, 29: 1140-1145 ( ) DOI: 10.1016/j.tiv.2015.04.018
- Stockmann-Juvala H., Savolainen K. A review of the toxic effects and mechanisms of action of fumonisin B1. Human & Experimental Toxicology, 2008, 27(11): 799-809 ( ) DOI: 10.1177/0960327108099525
- Liu Y., Wang W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Animal Science Journal, 2016, 87(12): 1490-1500 ( ) DOI: 10.1111/asj.12550
- Ma Q., Li Y., Fan Y., Zhao L., Wei H., Ji C., Zhang J. Molecular mechanisms of lipoic acid protection against Aflatoxin B1-induced liver oxidative damage and inflammatory responses in broilers. Toxins (Basel), 2015, 7: 5435-5447 ( ) DOI: 10.3390/toxins7124879
- Shannon T.A., Ledoux D.R., Rottinghaus G.E., Shaw D.P., Dakovic A., Markovic M. The efficacy of raw and concentrated bentonite clay in reducing the toxic effects of aflatoxin in broiler chicks. Poultry Sci., 2017, 96: 1651-1658 ( ) DOI: 10.3382/ps/pew408
- Zadeh M.H., Shahdadi H. Nanocellulose coated with various free fatty acids can adsorb fumonisin B1, and decrease its toxicity. Colloids Surf B Biointerfaces, 2015, 134: 26-30 ( ) DOI: 10.1016/j.colsurfb.2015.06.037
- Nedeljković-Trailović J., Trailović S., Resanović R., Milićević D., Jovanovic M., Vasiljevic M. Comparative investigation of the efficacy of three different adsorbents against OTA-induced toxicity in broiler chickens. Toxins (Basel), 2015, 7: 1174-1191.
- Hahn I., Kunz-Vekiru E., Twarużek M., Grajewski J., Krska R., Berthiller F. Aerobic and anaerobic in vitro testing of feed additives claiming to detoxify deoxynivalenol and zearalenone. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess., 2015, 32(6): 922-933 ( ) DOI: 10.1080/19440049.2015.1023741
- Alpsoy L., Yalvac M.E. Key roles of vitamins A, C, and E in aflatoxin B1-induced oxidative stress. Vitam. Horm., 2011, 86: 287-305.
- Латыпова Е.Н. Эффективность использования антистрессовых препаратов «Витаминоцид» и «Меджик Антистресс Микс» в яичном птицеводстве. Автореф. канд. дис. Оренбург, 2014. Режим доступа: http://www.feedfood.com.ua/download/Latipova_PhD.pdf. Без даты.
- Latipova E.N., Shatskikh E.V., Surai P.F. Effect of an antistress dietary supplement on the reproductive performance of layer breeders. Proc. XXV World's Poultry Congress. Beijing, China, 2016: 57.
- Shatskikh E.V., Latipova E.N., Surai P.F. Supplying an antistress composition with water to decrease negative consequences of commercially-relevant stresses in rearing birds. Proc. XXV World's Poultry Congress. Beijing, China, 2016: 58.
- Сурай П.Ф., Фисинин В.И., Шацких Е.В., Латыпова Е.Н. Современные методы борьбы со стрессами в птицеводстве и свиноводстве: Концепция витагенов в действии. Сфера: технологии, корма, ветеринария, 2016, 5(2): 40-43.
- Shatskikh E.V., Latipova E.N., Fisinin V.I., Surai P.F. Epigenetic effects of an antioxidant composition in layer breeder diet. Proc. XXV World's Poultry Congress. Beijing, China, 2016, p. 58.
- Фисинин В.И., Шацких Е.В., Латыпова Е.Н., Сурай П.Ф. Материнский эффект в птицеводстве -от витаминов к витагенам и эпигенетике. Птица и птицепродукты, 2016, 1: 29-33.
- Гапонов И.В., Фотина Т.И., Сурай П.Ф. Физиологические и технологические стрессы при отьеме поросят. Защитный эффект антистрессового препарат. Свиноводство Украины, 2012, 13: 6-9.
- Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev., 2014, 94: 329-354.
- Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci., 2009, 87(14 Suppl): E101-108 ( ) DOI: 10.2527/jas.2008-1339
- Clark A, Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front. Physiol., 2017, 8: 319 ( ) DOI: 10.3389/fphys.2017.00319
- Gyuraszova M, Kovalcikova A, Gardlik R. Association between oxidative status and the composition of intestinal microbiota along the gastrointestinal tract. Med. Hypotheses, 2017, 103: 81-85 ( ) DOI: 10.1016/j.mehy.2017.04.011


