Responses of Auxin Derivatives on Rooting and Sprouting Behavior of Excoecaria agallocha L. Stem Cuttings
Автор: G. Roseline Jebapriya, Ramamurthy Somasundaram
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.17, 2021 года.
Бесплатный доступ
In our study, the auxin effect on rooting and sprouting behavior of stem cuttings of Excoecaria agallocha L. has been studied. Initially stem cuttings were pretreated to remove the phenol content of cutting and then the stem cuttings that were devoid from phenols were subjected to hormonal treatment with auxins including indole-3-butyric acid (IBA) 2000ppm, indole-3-pyruvic acid (IPA) 2000ppm, naphthalene acetic acid (NAA) 2000 ppm and IBA+NAA combination 2000 ppm. The root length, root number, rooting and sprouting percentage, number of leaves per cutting, leaf area and photosynthetic pigments were analyzed on 40, 50 and 60 days after planting. Besides this, peroxidase isoenzyme pattern of root and leaf was also analyzed. All the growth attributes have shown an increasing trend at all stages of growth with auxin treatments. Among the auxin treatments, IBA 2000ppm vastly enhances rooting and sprouting behaviour of blinding eye mangrove. The isoenzyme analysis for peroxidase clearly showed that peroxidase (POX) highly supported root initiation and root elongation processes in Excoecaria agallocha.
Auxin derivatives, Excoecaria agallocha L., mangrove, peroxidase isoenzyme, stem cutting
Короткий адрес: https://sciup.org/143173882
IDR: 143173882
Текст научной статьи Responses of Auxin Derivatives on Rooting and Sprouting Behavior of Excoecaria agallocha L. Stem Cuttings
Mangroves are one among the world’s productive ecosystems, widely distributed in the intertidal zone of the tropical and subtropical regions of the globe (Tomlinson, 1986; Hogarth, 1999). Mangroves are generally treated as even-aged forests, primarily developing after an eruption or establishment of mudflats and such an uneven-aged mangrove forest is represented by the area covered (diameter distribution) by both trees and saplings (Saenger, 2002; Trettin et al. , 2016). Taxonomically, mangroves create a varied flowering plants (angiosperms) that exhibits a set of distinct physiological adaptations (Tomlinson, 1986); conserving the coastal line from natural calamities including cyclones, tidal thrust, and tsunamis, and also providing a wide variety of goods and services used by coastal people (Zhang et al. , 2006). The mangroves significance for coastal communities and humans has been well documented throughout the tropics (Sandilyan and Kathiresan, 2012).
The mangroves counteract encroachment by the seas by monitoring soil erosion and thereby stabilizing the sea shoreline. Mangrove habitat has been under serve destruction globally at alarming levels and reclamation (Kathiresan and Bingham, 2001). However, Mangrove ecosystems being unique habitats of the coastal wetland areas are facing intense pressure due to improper destruction by humans for various developmental purposes. Such levels of eradication and habitat fragmentation raise concerns about the conservation of mangrove diversity. The site of work done was pichavaram mangrove situated on the southeast coast of Tamil Nadu, India. The mangroves of pichavaram are threatened largely due to many natural and anthropogenic pressures. Grazing is the one reasons for the degradation of mangroves in the peripheral area of Pichavaram (Selvam et al. , 2004).
Excoecaria agallocha L. (Euphorbiaceae) known as “milk mangrove” grows in sandy soil or hard sandy mud near the terrestrial fringers of mangrove vegetation. All plant parts secrete white latex, which causes temporary blindness if enters in the eyes and hence, called as “blinding tree”. It is traditionally used to healing sores and the smoke of its bark has property of cure leprosy (Ghani, 2003). Clinical trials carried out on this plant have shown its potentiality against anticancer, antibacterial anti-HIV, and other antiviral properties (Peter and Sivasothi, 1999). The vegetative reproduction of E. agallocha is necessary because, this species is threatened by human intervention i.e. encroachment upon the land for cultivation and shrimp culture, for the exploitation of timber, fuel, and fodder and other uses. Generally, E. agallocha reproduces through seeds. Sudden depletion of the growing stock, and post-dispersal predation on seeds by crabs, poor flowering and seed setting necessitate asexual method like vegetative propagation of such species for reestablishment in degraded forest areas (Das et al., 1997a, b).
In Excoecaria agallocha adventitious root formation is extremely difficult. But it can be achieved with the help of phytohormones application. Among various plant growth substances, auxin plays main role in root formation (Davis et al. , 1986; de Klerk et al. , 1999). They promote root initials (Nordstrom et al. , 1991; Nag et al. , 2001) and affect the newly formed roots in the expressive phase of root development (Bellamine et al. , 1998). Further, auxin along with peroxidase plays a major role in root initiation and elongation. Several studies have suggested a central role of peroxidase on adventitious root formation. The role of peroxidase in growth can be clearly studied by isoenzyme pattern. Numerous papers have reported auxin-induced peroxidase isoenzyme changes during adventitious rooting (Chen et al. , 2002a).
Keeping all these in view the auxin derivatives, Indole-3-butyric acid (IBA), Napthylacetic acid (NAA) and Indole-3-pyruvic acid (IPA) have been selected for the present study. The present studies dealt with the action of auxins (IBA, NAA and IPA) on rooting and sprouting of the stem cuttings of E. agallocha. Moreover, the isoenzyme pattern of peroxidase in both leaf and root of E. agallocha was also studied.
MATERIALS AND METHODS
Plant materials and hormonal treatment
Healthy and uniform stem cuttings (10-15cm in long) Excoecaria agallocha were assembled from Pichavaram in Chidambaram, Cuddalore district, Tamilnadu, India.
Initially, these cuttings were pretreated with sodium carbonate and sodium tungstate for 5-10 mins and treated cuttings were washed two to three times with distilled water. The stem cutting that was devoid from phenol was dipped for 30mins in IBA 2000 ppm, IPA 2000 ppm, NAA 2000 ppm and IBA+NAA combination 2000 ppm. After hormonal treatment plants were transferred to a plastic tray containing coarse sand and soil in 1:1 ratio and tray was kept in the mist chamber at 32/26°C (maximum and minimum) temperature and relative humidity ( H) was between 60-75% during the experimental period. The observations on the number of cuttings rooted, number of roots and sprouts formed on each cutting and their length, biomass were recorded on 40, 50 and 60 (DAP) respectively, for each treatment.
Pigment composition
Chlorophyll was extracted from leaves and determined according to the protocol previously given by Arnon (1949). Xanthophyll contents were quantified by the protocol explained by Neogy et al. (2001). The results were expressed in mg g-1 fresh weight (FW).
Extraction for enzyme activity
Fresh plant tissue, one gram weighed was homogenized in 1ml of an ice-cold solution containing 100mM phosphate buffer (pH7.8), 1mM EDTA, 0.5% (v/ v) Triton X–100. Later, the homogenate collected was centrifuged for 30mins at 18,000 rpm. The eluent was stored at -20˚C for analysis of peroxidase isoenzyme.
Isoenzyme analysis
Isoenzymes were separated using 7.5% separating and 5% stacking polyacrylamide native gels. Electrophoresis was performed at 4°C under nondenaturing conditions as described by Laemmli (1970). Following electrophoretic separation, the gel was stained for peroxidase isoenzymes. Subsequently, gel was incubated in the staining solution for few minutes to get clear bands. Once the clear bands were appeared, the gel was washed with sterile water and photographed immediately. The staining solution was made ready by dissolving 500mg benzidine in 0.5ml of ethanol and 5ml of acetic acid, and 95ml of distilled water were added to it. The contents were mixed thoroughly and filtered using filter paper. 250µl of hydrogen peroxide was added before staining.
Statistical analysis
Each treatment data was analyzed statistically by using SPSS software (version 16.0, SPSS Inc., USA), with at least four replicates. A mean value (n=4) was calculated and (±) represents standard deviation (SD) of four replicates.
RESULTS
The results showed that the application of IBA, IPA, NAA and IBA+NAA combination resulted in the best root and shoot growth in stem cutting. (Table 1 and 2) show significant differences with respect to percent rooting, root number, root length, percent sprouting, number of leaves and leaf area in auxin treatments at different concentrations.
The root length of Excoecaria agallocha increased with the age of the plant. The root length was increased significantly to a large extent than the control in all the treatments. IBA 2000ppm treatment highly enhanced the root length when compared to IPA 2000ppm; IBA+NAA 2000ppm combination and NAA 2000ppm treated plant. IBA 2000ppm treatment highly increased the number of roots also when compared to the other treatment and control. The highest rooting rate (80%) on 60th day was noted in the cuttings treated with IBA 2000ppm followed by IPA 2000ppm and other treatments. The results revealed a profound influence of auxin derivatives on increasing the rooting and sprouting of E. agallocha (Fig.1).
Different sprouting rates were obtained for the cuttings exposed with different auxins (Tab 2). The cuttings subjected to IBA at 2000ppm had a sprouting rate of (80%) which was significantly higher when compared with control and other treatments. IBA treated plants shows an increased number of leaves per plant followed by IPA, IBA+NAA, and NAA. The leaf area increased with the age in the control and treated plants. IBA 2000ppm treated plant highly increased the leaf area, relative to other treatments and control.
The biomass of root and leaves per cutting shows an increasing trend with the age of treated and control cuttings (Tab.3). Among different auxin treated plants, IBA significantly increased fresh weight and dry weight of both roots and leaves when compared with other treatments and control.
Auxin treated plants typically appear dark greener and this has been correlated with an enhancement of the chlorophyll content in Excoecaria agallocha . Hormonal treatment increased the xanthophyll content to a larger extent than the control one. Among different auxin treatments, the IBA concentration has shown increased photosynthetic pigments in all the stages of growth (Fig.2). The hormonal treatment significantly enhanced the chlorophyll and xanthophyll contents in E. agallocha . Among different auxin treatments, IBA shows more increased content of photosynthetic pigments.
The isoenzyme pattern of peroxidase showed a single peroxidase band in leaves treated with IBA, IPA, NAA and IBA+NAA combination, exhibiting a high intensity of band in NAA treatment with relative mobility value ( m) of 0.62. While in the root two peroxidase bands were found in all auxin treatments as well as in control, in contrast, the NAA treated root alone showed a significantly increased intensity of band with m value 0.57(Fig.3). In auxin treated E. agallocha stem cutting the isoenzyme pattern of peroxidase showed a single peroxidase band in leaves. Whereas root has shown two peroxidase bands in all auxin treatments and control (Fig.3).

Control IBA (2000)
IPA(2000) NAA(2000) IBA+NAA (2000)
Figure 1. Effect of auxin on growth of Excoecaria agallocha
о c о u
Ф
Total chlorophyll and Xanthophyll
170 _
120 ^^^^^^^^^
110 —^^^ - - о
|
40 DAP 50 DAP 60 DAP Total chlorophyll |
40 DAP ) |
50 DAP (anthophyl |
60 DAP 1 |
|||
|
-----IBA |
112.7 |
140 |
165.6 |
103.8 |
104.1 |
110.2 |
|
IRA |
100.3 |
103.4 |
106.2 |
101.5 |
100.3 |
100.8 |
|
---NAA |
100.3 |
121.9 |
122.1 |
101.4 |
101.2 |
103.5 |
|
----IBA+NAA |
100.8 |
130.8 |
155.3 |
102.4 |
104.4 |
104.1 |
Figure 2. Effect of auxin on total chlorophyll and xanthophyll of Excoecaria agallocha
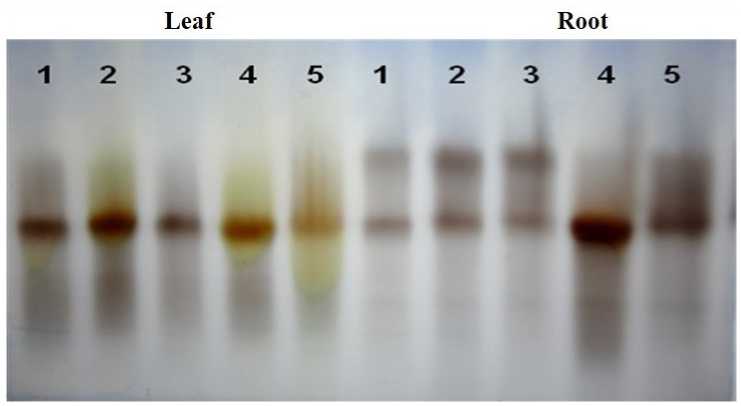
Figure 3. Effect of auxin on isoenzyme pattern of peroxidase. The lanes represent (1) Control (2) IBA (3) IPA (4) NAA and (5) IBA + NAA.
DISCUSSION
Auxin showed to regulate different aspects of plant growth performance by affecting numerous physiological processes like cell division, cell elongation, and cell differentiation (Woodward and Bartel, 2005). Although, exogenous auxin application induced rooting has previously been reported by many workers in other plant species (Davies, 1996; Blakely et al. , 1988). Exogenous application of IBA to the base of cuttings positively impacted rooting percentage, and other root related traits in our study. A similar result was obtained in Cynometra iripa, Heritiera fomes (Basak et al. , 1995). The rooting in the cutting of another mangrove Avicennia alba shows a similar type of response ( eddy et al. , 1994). In soybean hypocotyls auxin (IBA, IAA, and NAA) effectively promotes the rooting (Chou et al. , 2010). The differential root regenerating ability of auxins either alone or blend might depend on their respective capability to synthesize proteins necessary for the regeneration and elongation of roots (Ghosh, 1974; Basak et al. , 1999).
Our finding also suggests the pretreatment of IBA could be very effective in enhancing the sprouting of Excoecaria agallocha. Similar results were also observed in Jatropha curcas (Kochhar et al. , 2008). Different concentrations of auxin significant difference over control with regard to the number of leaves developed per cutting. The results reported by
Chalapathi et al. (1997) in Stevia followed a similar trend. The possible reason for such an increase may be due to the activation of shoot growth by counteracting ABA levels of buds with probably increased the number of nodes that lead to the development of more number of leaves. Generally, auxin plays a significant role in the elongation of petiole, midrib and major lateral veins of the leaves. The leaf production was increased in auxin treated plants. Similar results were reported in Cotinus coggygria (Pacholczak et al. , 2005). The interesting observation is that the shoots are formed much earlier than roots in E. agallocha . This earlier Shoot formation is a result of reserved carbohydrates and shoots start producing auxin, which moves basipetally and accumulates in the lower portion of the cuttings. When the concentration reached a threshold, endogenous auxins at the extreme basal end start becoming metabolized and signal the process of root initiation.
The increased fresh and dry mass of root and leaf per cutting was recorded in the cuttings treated with IBA followed by other treatments. A similar effect has been observed in Psoralea corylifolia (Faisal and Anis, 2006), Azadirachta indica and Pongamia pinnata (Palanisamy et al. 1998).
IBA increased the vegetative growth and pigment concentration in maize (Kaya et al., 2006). A similar result was also noticed in grapevine cuttings. In our study increased photosynthetic contents of leaves was more in IBA treated cuttings, and this might have altered the synthesis and translocation of assimilates (Kaur et al., 2002). Moreover, the exogenous IBA serves as a balanced storage form of IAA, since IBA can be converted back to IAA and thus can be gradually released when required by the plant (Normanly, 2010; Woodward and Bartel, 2005).
egarding POX activity, similar result was noticed in Jatropha curcas cutting, where it shows two peroxidase bands at the phase of root elongation in auxin treatment (Kochhar et al. , 2008). In soybean hypocotyls, the activity of both anionic POX and cationic POX was significantly repressed by exogenous auxins at the inductive phase. The anionic POXs are most significantly elevated in IBA-treated tissues when compared with control (Chou et al. , 2010). The role of peroxidase in the rooting of poplar cuttings has been pointed out earlier by Gunes (2000). The present studies suggest that peroxidase helps in auxin catabolism and in prompting the root initiation process. A similar role can be done by IAA-oxidase also but IAA oxidase plays a part only for stimulating and initiating the roots/root primordial; peroxidase is involved in both the processes i.e., root initiation and elongation.
CONCLUSIONS
Our study revealed that auxin derivative IBA alone showed a profound influence in increasing rooting and sprouting of E. agallocha stem cuttings, relative to other treatments and control. Moreover, increasing number isoenzyme peroxidase bands formed in roots clearly showed the role of peroxidase during rooting initiation and elongation. Taken together, the study chosen might help in understanding mechanisms by which auxin derivatives promoting plant growth performance under in vitro or in vivo conditions.
ACKNOWLEDGEMENT
Список литературы Responses of Auxin Derivatives on Rooting and Sprouting Behavior of Excoecaria agallocha L. Stem Cuttings
- Arnon D. I. (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. J. Plant Physiol. 24(1):1.
- Basak U. C., Das A. B. and Das P. (1995) Metabolic changes during rooting in stem cuttings of five mangrove species. J. Plant Growth Regul., 17(2), 141-148.
- Bellamine J., Penel C., Greppin H. and Gaspar T. (1998) Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. J. Plant Growth Regul., 26(3), 191-194.
- Blakely L. M., Durham M., Evans T. A. and Blakely R. M. (1982) Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Botanical Gazette, 143(3), 341-352.
- Bose T. K., Bhattacharjee S. K., Das P. and Basak U. C. (1999) Orchids of India (No. Ed. 2). Naya Prokash.
- Chalapathi M. V., Thimmegowda S., Sridhara S., Ramakrishna Parama V. R. and Prasad T. G. (1997) Natural non-calorie sweetener stevia (Stevia rebaudiana Bertoni)-A future crop of India. CROP RESEARCH-HISAR-, 14, 347-350.
- Chen L. M., Cheng J. T., Chen E. L., Yiu T. J. and Liu Z. H. (2002a). Naphthaleneacetic acid suppresses peroxidase activity during the induction of adventitious roots in soybean hypocotyls. J. Plant Physiol. 159(12), 1349-1354.
- Chou C. H., Huang Y. C. and Liu Z. H. (2010) Peroxidase genes differentially respond to auxin during the formation of adventitious roots in soybean hypocotyl. J. Plant Growth Regul., 60(2), 151-161.
- Das P., Basak U. C. and Das A. B. (1997a) Metabolic changes during rooting in pre-girdled stem cuttings and air-layers of Heritiera. Bot. Bull. Acad. Sin. (TAIPEI), 38, 91-96.
- Das P., Basak U. C. and Das A. B. (1997b) Restoration of the mangrove vegetation in the Mahanadi delta, Orissa, India. Mangroves and Salt marshes, 1(3), 155-161.
- Davies P.J. (1996) Plant hormones and their role in plant growth and development. Dordrecht, etherlands: Kluwer Academic Publishers.
- Davis T. D., Sankhla N. and Upadhyaya A. (1986) Paclobutrazol: A promising plant growth regulator. Hormonal regulation of plant growth and development, Agro Botanical Publishers, Bikaner, India 3, 311-331.
- de Klerk G. J., van der Krieken W. and de Jong J. C. (1999) Review the formation of adventitious roots: new concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant, 35(3), 189-199.
- Faisal M. and Anis, M. (2006) Thidiazuron induced high frequency axillary shoot multiplication in soralea corylifolia. Biol. Plant., 50(3), 437-440.
- Ghani A. (2003) Medicinal Plants of Bangladesh, Asiatic Society of Bangladesh. Dhaka, Bangladesh, 500-504.
- Ghosh S. K. and Basu R. N. (1974) Metabolic changes during the regeneration of roots on cuttings. Indian J. Exp. Biol., 12: 166-168.
- GÜNEŞ T. (2000) Peroxidase and IAA-oxidase activities during rooting in cuttings of three poplar species. Turk J Botany, 24(2), 97-102.
- Hogarth P. J. (1999) The biology of mangroves. Oxford University Press (OUP).
- Kathiresan K. and Bingham B. L. (2001) Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol., 40, 84-254.
- Kaur S., Cheema S.S., Chhabra B.R. and Talwar K.K. (2002) Chemical induction of physiological changes during adventitious root formation and bud break in grapevine cuttings. J. Plant Growth Regul., 37(1), 63-68.
- Kaya, C., Tuna, A. L. and Alfredo A. A. (2006) Gibberellic acid improves water deficit tolerance in maize plants. Acta Physiol. Plant, 28(4), 331-337.
- Kochhar, S., Singh, S. P. and Kochhar V. K. (2008) Effect of auxins and associated biochemical changes during clonal propagation of the biofuel plant—Jatropha curcas. Biomass Bioenergy, 32(12), 1136-1143.
- Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature, 227(5259), 680-685.
- Nag, S., Saha, K. and Choudhuri M. A. (2001) Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J. Plant Growth Regul., 20(2), 182-194.
- Neogy, M., Datta, J. K., Mukherji S. and Roy A. K. (2001) Effect of aluminium on pigment content, Hill activity and seed yield in mungbean. Indian J Plant Physiol., 6(4), 381-385.
- Nordström, A. C., Jacobs, F. A. and Eliasson L. (1991) Effect of exogenous indole-3-acetic acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic acid during adventitious root formation in pea cuttings. Plant physiol., 96(3), 856-861.
- Normanly J. (2010) Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harb. Perspect. Biol., 2(1), a001594.
- Pacholczak, A., Szydło W. and Łukaszewska A. (2005) The effect of etiolation and shading of stock plants on rhizogenesis in stem cuttings of Cotinus coggygria. Acta Physiol. Plant., 27(4), 417-428.
- Palanisamy, K., Ansari, S. A., Kumar P. and Gupta B. N. (1998) Adventitious rooting in shoot cuttings of Azadirachta indica and Pongamia pinnata. New Forests, 16(1), 81-88.
- Ng, P. K., Sivasothi N. and Morgany T. (1999) Guide to the mangroves of Singapore I: The ecosystem and plant diversity. Singapore Science Centre 111-112.
- Reddy, K. K., Venkaiah K. and Bramaramba B. (1994) Rooting of Stem Cuttings of Avicennia officinalis. Linn. And Avicennia alba BL.: a Tool for Afforestation of Blanks in Mangrove Forests. Indian Forester, 120(2), 158-161.
- Saenger P. (2002) Mangrove ecology, silviculture and conservation. Springer Science & Business Media.
- Sandilyan, S. and Kathiresan K. (2012) Mangrove conservation: a global perspective. Biodivers. Conserv., 21(14), 3523-3542.
- Selvam, V., Eganathan, P., Karunagaran, V. M., Ravishankar T. and Ramasubramanian R. (2004) Mangrove Plants of Tamil Nadu. MS Swaminathan Research Foundation, Chennai, India, 56pp.
- Tomlinson P. B. (1986) The Botany of Mangroves Cambridge University Press London, 419.
- Trettin, C. C., Stringer C. E. and Zarnoch S. J. (2016) Composition, biomass and structure of mangroves within the Zambezi River Delta. Wetl Ecol Manag, 24(2), 173-186.
- Woodward A. W. and Bartel B. (2005) Auxin: regulation, action, and interaction. Ann. Bot., 95(5), 707-735.
- Zhang, Y., Wang, W., Wu, Q., Fang B. and Lin P. (2006) The growth of Kandelia candel seedlings in mangrove habitats of the Zhang jiang estuary in Fujian, China. Sheng Tai Xue Bao, 26(6), 1648-1655.


