Responses of carthamus tinctorius at two development stages to low light intensity (LLI): changes on phenolic metabolites and related antioxidant activities
Автор: Wasli Hanen, Zaouia Sonia, Saada Mariem, Chaabania Ghaya, Ksouri Riadh, Lachal Mokhtar, Karray-Bouraoui Najoua
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.18, 2022 года.
Бесплатный доступ
We investigated the contributions of low light intensity (LLI) and development stage on growth status, nutrient uptake, pigment contents, bioactive molecule contents and biological activities on carthamus tinctorius plants at two growth periods: vegetative and flowering periods in order to optimize natural culture conditions required to improve leaf antioxidant accumulation; while maintaining acceptable biomass production under LL conditions. For this purpose, an open field culture experiment was conducted on safflower leaves subjected to optimal light (250 µmol m-2 s-1) or LLI condition (125 µmol m-2 s-1). Shade constraint affected extensively the growth in terms of dry weight, RGR and water content particularly at vegetative stage. MDA and EL levels had a noteworthy increase particularly at flowering stage S reaching +34%in comparison to sun-exposed leaves. In addition TPC, TFC and TCT were mostly enhanced at full flowering stage than vegetative one. In the same line, the antioxidant activities were found to be enhanced at the FS stage as compared to the vegetative one. These results strongly indicate that LL induces the accumulation of secondary metabolites in C. tinctorius leaves by altering the phenolic synthesis pools, as well as for the up-regulation of antioxidant molecules defense
Low light intensity, vegetative stage, floral stage, phenolic pools, antioxidant activities
Короткий адрес: https://sciup.org/143178809
IDR: 143178809
Текст научной статьи Responses of carthamus tinctorius at two development stages to low light intensity (LLI): changes on phenolic metabolites and related antioxidant activities
Light is a major environmental factor that plays an important role in plant development and metabolism (Berenschot and Quecini, 2014). In fact, light is crucial for photosynthesis and photo-morphogenesis. Low light (LL) constraint, as abiotic stress, can result from light blocking by horticulture facilities, clouds and eventually snow. It was shown to substantially affect plant agronomic traits and hinders physiological metabolic processes, including photosynthesis and antioxidant production, as well as carbon and nitrogen fixation (Zhu et al. , 2017). LL stress usually inhibits plant growth and productivity by affecting gas exchange (Zhan et al. 2002). In fact, it affects negatively leaf thikness and stomatal size (Huang et al. , 2004; Wei et al. , 2005;
regoriou et al. , 2007), Many studies showed that light stress was dependent on plant developmental stage (Deng et al. , 2009; Liu et al. , 2009).
The chloroplast lumen in plants at LL becomes acidic in nature, and excitation energy accumulates within chloroplasts; which results in generation of singlet and triplet forms of singlet oxygen Due to the excess light absorption, according to Ali et al. (2005),. This will be accompanied by ROS generation such as superoxide radical (O 2 •-) and hydrogen peroxide (H 2 O 2 ).Low light causes oxidative stress in plants and induces ROS accumulation. In general, ROS over generation harm cell membranes and functions (Zhang et al. , 2011).
In order to alleviate ROS induced oxidative damage plants induce different mechanisms blocking ROS-generating chain reactions propagation (Jelali et al. 2017). Polyphenols are among the most active secondary metabolites implicated in response to low light intensity. These metabolites are considered potential molecules owing to their chemical structure. Indeed, due to their particular basic structure they exhibit important antioxidant properties (Huang et al. 2005; Wasli et al., 2018).These bioactive metabolites are synthesized in different plant parts and contribute in preventing ROS accumulation) at toxic levels during abiotic/biotic stresses (Naczk and Shahidi, 2006). In plants, polyphenol biosynthesis and accumulation are generally stimulated in response to biotic/abiotic constraints (Naczk and Shahidi, 2004). Thus, shade- stressed plants might represent potential sources of polyphenols, by increasing tissues polyphenol accumulation. Optimal polyphenol yield would be obtained using stress-tolerant species (Bettaieb et al. 2012)
Tunisia has a wide array ofaromatic and medicinal plants used mainly for culinary and for therapeutic purposes, thus representing potential sources of active biomolecules. Among them, carthamus tinctorius i safflower (Asteraceae family), is well cultivated crop in Mediterranean regions, Europe and central Southern Asia and frequently used for flavoring and seasoning meals(Karray-Bouraoui et al. , 2011; Zaoui et al. 2016).Safflower is actually cultivated for its edible oil and as a birdseed ( yulai 1996). This oriental herb is cultivated mainly in arid and semi-arid environments due to its ability to tolerate abiotic stresses this criterion is linked to its rich pool of antioxidants, including phenolic compounds which are important in protecting biological molecules against oxidative damage (Karray-Bouraoui et al. , 2010; Karray-Bouraoui et al. , 2011).
Safflower responses to salinity and drought along with other abiotic stress have been intensively studied using physiological, biochemical and metabolic traits (Ben Abdallah et al. , 2013; Salem et al. , 2014; Zaoui et al. , 2016) but data relative to its secondary metabolism under low light intensity particularly in open-field conditions still scarce. For this reason, the present study was carried out to assess shade condition effects on growth activity, photosynthetic performance and production of phenolic compounds in Carthamus tinctorius L., this study was conducted firstly to define culture conditions optimizing biomass and biomolecules production and secondly to estimate polyphenols and antioxidant activity variations depending in leaf stage. This study will be useful for crop breeders and growers to produce safflower with high levels of natural antioxidants even in shade conditions. These findings would be a useful tool in determining plant growth requirements for quality enhancement of medicinal plants.
MATERIALS AND METHODS
Plant culture, growth conditions and harvest
Experimental fields were conducted in Djedeida provenance 36°51 '02 "Nord 9° 56' 10" East, Tunisia (Figure 1); which is characterised by a semi-arid climate with mean annual rainfall ranging from 0.4 to 87 mm. The soil type at the experimental site was loam classified as Typic Haplustalfs Machacha (2011) , with a pH of 7.2, and electrical conductivity (EC) of 442 µS/cm. In the field, the soil was basally dressed using a commercial fertiliser (NPK ratio of 2:3:2 (22%)) at 19.8 kg/ha N. After germination, plants were watered daily at dawn until the 4th true leaf stage, to maintain soil moisture near field capacity until the soil surface underneath the plants was covered with water for approximately 2 s.
Seeds were sown in soil and then irrigated for 60 days with distilled water until germination was occurred. Seedlings were watered daily with a nutrient solution of Hoagland and Arnon (1950)[1.25 mm Ca (NO 3 ) 2 , 1.25 mM KNO 3 , 0.5 mM MgSO 4 , 0.25 mM KH 2 PO 4 and 10 μm H 3 BO 3 , 1 μM MnSO 4 , 0.5 μm ZnSO 4 , 0.05 μm (NH 4 ) 6 Mo 7 O 24 and 0.4 μM CuSO 4 ]. Safflower plants were grown either in shade conditions by disposing the plants on a sloping slope whichretains50% of light (shade treatment) or exposed to direct sunlight (control treatment). During experimental period light intensity was measured with a quantum sensor light meter with separate sensor (QMSS) from germination to harvesting for data analysis. Each reading was expressed as µmol m-2 s-1. C. tinctorius leaves were harvested at two growth stages: vegetative stage and full-flowering stage (Figure 2). Twenty-five plants were harvested for every growth stage; only leaves were used in experiments and divided to three groups: the first was dried and used for growth parameters evaluation, the second was kept fresh and used for biochemical parameters and the third was dried and used for polyphenols extraction and determination.
Plant growth evaluation and mineral analysis
After 3 days of oven drying at 60° C, leaves dry weight (DW) was determinate, and their water content was calculated as (FW - DW)/FW, where FW and DW represent the fresh and dry weight, respectively. Relative growth rate (R R) values, based on leaf dry weight, were determined as follow: (R R, day-1) = ln W2-ln W1/105 or ln W2 - ln W1/135), whereW1andW2aredry weights at the beginning and the end of the treatment period, and 105and 135are the treatment duration in days. An amount of 20 mg from grounded oven-dried leaves at both growth stages was digested with 25 mL of nitric acid solution (HNO3 0.5 %). After 15 days, the mixtures were filtered through a Whattman filter paper, and K+ and Ca2+ concentrations in the digests were determined with a flame emission photometry (Jenway PFP7) after calibration with standard solutions (Karray-Bouraoui et al., 2010).
Measurement of photosynthetic parameters
The variation of pigment concentrations was assessed by measuring absorption spectra of frond extracts using a U spectrophotometer (Spectro.U - IS Dual Beam 8 Auto cell UUS-2700). Two hundred (200) mg of safflower plant material frozen in liquid N 2 was ground to a fine powder (on ice) and immediately immersed in 5 mL of 80% acetone solution. Then the total extraction took place after 72 hrs in darkness, at 4°C and absorbance of extracts was measured in the supernatants after 3 days at 663, 645 and 470 nm respectively for ChLa, ChlL b and carotenoids (Lichtenthaler and Buschmann, 2001).
Lipid peroxidation and electrolyte leakage
The level of lipid peroxidation was measured as 2-thiobarbituric acid-reactive substances (mainly malondialdehyde (MDA) according to (Jelali et al., 2014). Frozen samples (1g of fresh material) were homogenized with a pre-chilled mortar and pestle with 10 mL of (0, 1%; p/v) trichloroacetic acid and centrifuged at 10000 g for 10 min and at 25°C. 1ml of the upper liquid layer (supernatant) was added to 4 ml thiobarbituric acid (TBA) (0,5 %; p/v). After centrifugation at 1000 g for 10 min, the supernatant absorbance was read (532 nm) and values corresponding to non specific absorption (600 nm) were subtracted. MDA concentration was calculated using its molar extinction coefficient (155 mM-1·cm-1) according to the following formula:
MDA (nm/ gFW) = [( OD 532 – OD 600 )*vs/ 0.155 ] x FW
Electrolyte leakage parameter was determined on excised leaves of safflower (Mao et al., 2007). After being rinsed for 2–3 min with deionized water, 5 pieces were immersed in 20 mL of deionized water in test tubes and shaken every 5 min for 30 min.. Total conductivity was accoured after keeping the test tubes boiling for 15 min ; then results was expressed as percentage of total conductivity.
Determination of total phenolic contents (TPC)
The total phenolic content (TPh) of safflower extract from each treatment was assessed using a colorimetric assay based on the Folin-Ciocalteu reagent. A volume of 125 µL of SE was added to 60 µL of H 2 O and 15 µL of the Folin-Ciocalteu reagent (0.2 mol. L-1). After shaking 150 µL of Na 2 CO 3 (7%) was added. After incubation for 1 hour at room temperature, the optical density (OD) at 750 nm was determined. Results were expressed as mg of gallic acid equivalent per g of dry weight ( AE g-1 DW) using a calibration curve (Dewanto et al., 2002).
Determination of total flavonoid contents (TFC)
For total flavonoid content (TFC), 250 µL of safflower extract was mixed with 75 µL NaNO 2 (5%; w/v). Then 150 µL of AlCl 3 /6H 2 O (10%; w/v) and 500 µL of NaOH (1 M) were added after 6 min of incubation. After adjusting the volume to 2500 µL with H 2 O, the absorbance was determined at 510 nm. TFC were expressed as mg (+)-catechin equivalent/g DW (mg CE. g-1 DW) (Dewanto et al., 2002).
Determination of condensed tannins contents (TCT)
Proanthocyanidin were revealed according to vanillin-H 2 SO 4 method (Saada et al., 2014). Fifty µL of safflower extract from each treatment were pipetted out into a test tube. Then, 3 mL of 4% methanolic-vanillin solution and 1.5mL of concentrated H 2 SO 4 were added and vortexed. Tubes were stand for 15 min. The absorbance was measured at 500 nm. The amount of condensed tannins were expressed as mg catechin equivalent/ g DW (mg CE. g-1 DW).
Total antioxidant capacity (TAC)
Total antioxidant capacity was assessed according (Saada et al., 2014). Briefly, 100 µL of SE was combined to 1 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The mixture was allowed to cool, after incubation at 95°C for 90 min. The absorbance was measured at 695 nm and TAC was expressed as mg gallic acid equivalent / g DW (mg AE. g-1 DW).
ABTS scavenging activity
Antiradical capacity of safflower extract (SE) against ABTS radical was assessed according to Wasli et al. (2018) method. Briefly, 250 µL of stable radical ABTS solution (prepared by reacting the stock solution of ABTS (7 mM) with potassium persulfate (2.45 mM) in a ratio of 1:1) was added to 50 µL of increasing concentrations of SE. After 6 min of incubation at room temperature, the absorbance was read against a blank at 734 nm using an ELX800 microplate reader. ABTS scavenging ability was expressed as IC 50 (mg/ mL) which is the inhibiting concentration of 50% of the synthetic radical.
The inhibition percentage (IP %) of ABTS radical was calculated using the following formula:
IP (%) = [( A control - A sample )/ A control ] x 100
Iron reducing power
Ferric reducing antioxidant power (FRAP) was focused on the reduction of the trivalent iron produced by the FeCl 3 (Wasli et al., 2018). The intensity of the blue-green color was measured at 700 nm. alues were expressed as EC 50 (mg/mL): the effective concentration of SE corresponding to an OD = 0.5.
β-carotene linoleic acid model system
Initially, β -carotene (20 mg) was suspended in 10 mL chloroform; linoleic acid (50 mg) and Tween 80 (1g) were added to 1 mL of this solution (Wasli et al., 2018). Chloroform was removed using a vacuum at 40°C, and 100 mL oxygenated water added.The obtained β-carotene/linoleic acid emulsion was vigorously shaken before 250 µL was added to each well of 96-well microliter plates alongwith 50 µL of test samples.The initial absorbance at 470 nm was recorded.
The emulsion system with two controls [one containing BHA as a positive control (Figure 1) andthe other with the same volume of distilled water instead of the extracts]was incubated at 50C for 120 min and the absorbance at 470 nmread using a model ELX800 microplate reader. Readings for all samples were performed immediately and after 120 min of incubation. The antioxidant activity of the extracts was evaluated in terms of blanching inhibition of the β-carotene as follows:
((Сг=о — Cf=2) — №=0 — Et=i)
% of inhibition = —----- —-----—-------x 100
(.4=0 ~ 4=2)
Statistical analyses
Data were analyzed using one-way ANO A followed by Tukey’s post-hoc test was performed. The statistical tests were applied using raph Pad Prism, version 6 and the significance level was p < 0.05. Multivariate data analysis was carried out using principal component analysis (PCA). The PCA type used is Pearson’s correlation and it was done using XLSTAT, considering variables centered on their means and normalized with a standard deviation of 1.
RESULTS
Plant growth parameters
The effects of LLI and growth phases on dry weight, hydration and relative growth rate are shown in Table 1. Results showed a clear depressive effect of shade condition on leaf dry weight (DW) for both development stages; with a higher effect in vegetative than flowering stage. Indeed, during the vegetative period the decrease, was about -94%; contrawise, -80% during the reproductive one as compared to the control. Relative growth rate, exhibited also the same pattern observed for (DW) where the reduction was about -44% and -25% respectively in vegetative and flowering stages. As well, a relevant decline in water content was occurred in response to LLI with a more pronounced effect in floral stage (-27%) than the vegetative period (-18%) (Table 1).
Potassium (K+) and calcium (Ca2+) contents; were distinctly reduced in flowering periods (-29% and -24% as compared to control) with a slight decrease at vegetative stage (Table 1).
Photosynthetic pigments
For both physiological stages, LLI had a regressed effect on leaf chlorophyll contents. In fact, as compared to control plants, a reduction in ChL a , ChL b and T ChL contents was noted predominantly in flowering stage (66%, -33% and -60% and -24% respectively) as compared to control leaves ( Table 1).
Lipid peroxidation and electrolytes leakage
Shade conditions had a noteworthy effect on leaf MDA content in C.tinctorius leaves during vegetative and flowering stage; where the relevant increase was especially detected at full flowering stage (+34%) in comparison to sun-exposed leaves. Similarly electrolytes leakage was significantly correlated with lipid peroxidation results (Table 2). Indeed, EL was incremented at both periods, and was more superior in flowering period than in the vegetative one as compared to the normal plants.
Change in phenolic pools as function of physiological development stage
Results of quantitative estimation of total phenols flavonoїd and tannins contents of C. tinctorius leaves during different growth stages and LLI conditions are presented in Table 3. LLI induced a noteworthy accumulation of polyphenols at both physiological stages predominantly in floral stage; where it increased in twice from 19 mg/g AE DW) (shaded leaves) to (41 mg AE/g DW). With similar tendency to phenol flavonoid content was remarkably higher in shadingplants; where the highest amounts waere detected during the flowering period (23.74 mg AE/g DW of the plant. Concerning the condensed tannins these compounds seem were also incremented at both periods (from 13.61 to 16.50 and from 15.12 to 21.44 respectively for vegetative and flowering stage) (Table 2).
Change in antioxidant as function of physiological development stage
TAA of the two developmental stages was extremely different (Figure 3). Indeed, this ability was 1.5-fold higher in the flowering stage (45 mg AE/g DW) than that in vegetative one (29.7 AE/g DW). The exposure to LLI, the antioxidant activity increased in the leaves of C. tinctorius and was in proportion with the total phenols. The magnitude of ABTS· radicals quenching activity seemed to be related to the physiological stage and LLI too, as IC50 values largely differed between the two periods (Figure 3). The highest antiradical activity against ABTS was recorded for the plants exposed to LLI at both stages and it reached 411 µg/mL (vegetative period) and 207 µg/mL(flowering period) The trend for ferric reducing activity of the different growth periods showed a similar tendency as compared to their ABTS radical scavenging activities, when a comparison between EC50 and IC50 propensities is made. In fact flowering stage showed relatively the stronger FRAP activity reflected by low EC50 values, as compared to the vegetative stage ones. For β-carotene/linoleate system shoots displayed the highest capacity of inhibiting the lipid peroxides as compared to different treatment with the lowest IC50 value (3.7 mg/mL) from floral stage.
Figure 1. Geographical localization of Djedeida provenance( overnorateof Manouba)

Figure 2. Carthamus tinctorius at vegetative (A) and floral (B) periods
Table 1. Effects of LL treatment on growth parameters, photosynthetic pigments and nutrient uptake in leaves of C. tinctorius plants. alues are means of six replicates and standard deviation. alues with different superscripts (a-d) are significantly different at P < 0.05.
|
FLI |
LLI |
|||
|
egetative period |
Flowering period |
egetative period |
Flowering period |
|
|
DW (g/ plant ) |
1.37±0.240A |
0.30±0.01B |
0.078±0.01C |
0.059±0.01D |
|
R R (day-1 ) |
0.19 ± 0.024A |
0.094±0.07B |
0.008 ± 0.00C |
0.006 ± 0.002D |
|
Water content (mL/ g DW) |
3.58±0.019A |
2.93±0.34B |
2.71±0.89bC |
1.98±0.03D |
|
K+ (mg/g DW) |
1.25±0.45 A |
1.19±0.21 A |
1.13±0.19 A |
0.85±0.29 A |
|
Ca2+ (mg/g DW) |
0.98±0.11 A |
0.76±0.09 A |
0.85±0.05 A |
0.58±0.17 A |
|
ChL a (mg/gFW) |
0.79±0.05A |
0.67±0.02B |
0.36±0.06C |
0.23±0.03D |
|
ChL b (mg/gFW) |
0.21±0.04A |
0.15±0.09B |
0.15±0.02C |
0.10±0.00D |
|
ChL a +b (mg/g FW) |
1.00±0.09A |
0.82±0.04B |
0.51±0.08C |
0.33±0.04D |
|
CAR (mg/g FW) |
0.125±0.01A |
0.133±0.01B |
0.157±0.12C |
0.187±0.09D |
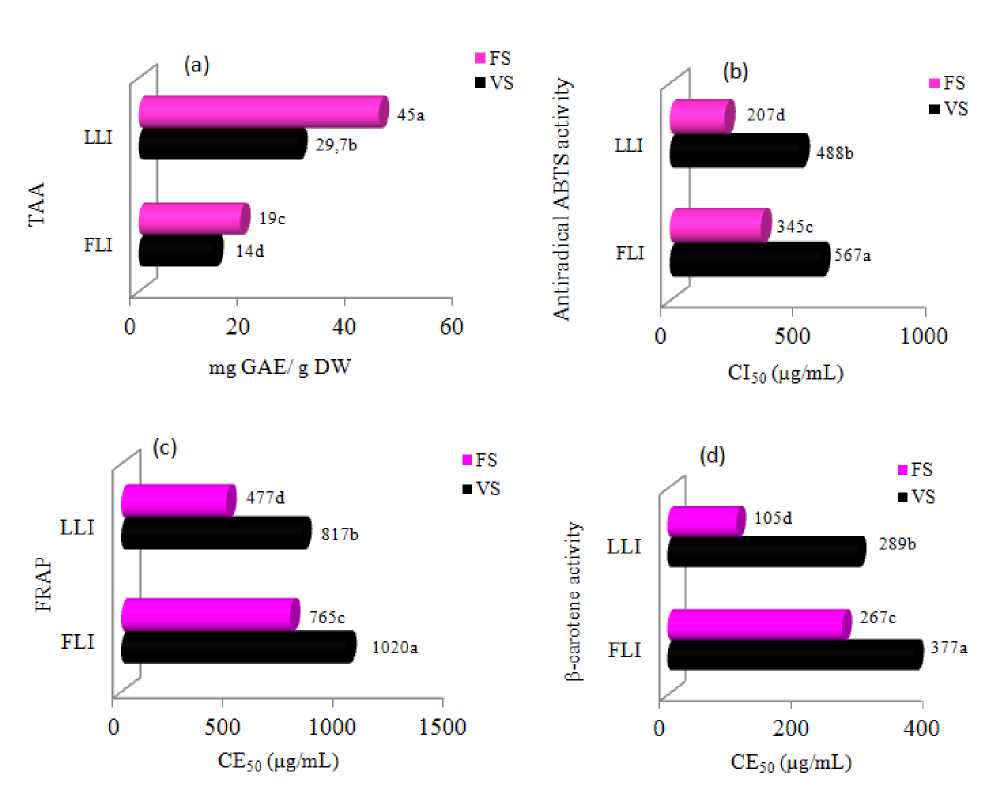
Figure 3. Changes in (a): Total antioxidant activity, (b): ABTS radical scavenging activities, (c): ferric reducing antioxidant power and (d): β-Carotene bleaching test cultivated under LLI conditions.
ABTS, 2 2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid; IC 50 , the concentration of the extract causing 50% inhibition; EC 50 , the effective concentration at which the absorbance was 0.5; Values are means of three replicates and standard deviation. Values with different superscripts (a-d) are significantly different at P < 0.05.
Table 2. Changes in MDA, EL, total polyphenols, flavonoids and tannins in the leaves of C. tinctorius plants cultivated under LLI conditions. AE, gallic acid equivalent; CE, catechin equivalent; MDA, malondialdehyde; TCT, total condensed tannin, TFC, total flavonoids; TPC, total polyphenol
DISCUSSION
Low light was shown to substantially distress the agronomic traits of plants and restrain physiological metabolic processes, including photosynthesis and antioxidant characteristics, as well as carbon and nitrogen fixation; it can influence plant growth and development by altering the plant niche and its most visual effect is plant morphological change (Wang et al. 2012). Previous studies have suggested that shaded plants preferentially supplied photosynthetic products to leaves that were beneficial to their growth, which could partially compensate for the decrease in growth rate due to reduced light energy; additionally, shade could also decrease the plant canopy and distribute assimilated carbon to the vertical growth to furthest capture light energy (Zhu et al. , 2017). These observations were all confirmed by our present study when subjecting C. tinctorius to LLI resulted in a severe reduction of LDW in both growth stage phases (-94 and -80% respectively fo
S and FS ).
In Echinochloa colona a reduction of -50% and -75% in LI led to a decrease of about 28% and 69% of the LDW. According to Zavala and Ravetta (2001), plants cultivated in shade supplied photosynthetic products to leaves in order to promote their growth; thus offsetting the reduction of plant growth induced by light deficiency.
Another factor that affects plant growth is the intensity of light that varies hugely depending on the season. Indeed, photosynthetic process depends deeply on the quantity of light received by plants which affects directly plant metabolism. In fact, photosynthetic activity generated essential organic compounds for plant growth, as described by Brüggemannet al. (2011). Thus the reduction of plant growth under light deficiency could be explained by an insufficient production of ATP essential for carbon fixation and carbohydrate biosynthesis.
A decrease in ChL content with LLI has been detetcted in many species, such as the cape gooseberry (Aldana et al., 2014), tomatoes black beans (Bansal et al. , 2019 ), and cabbage (Casierra-Posada and Cutler 2017), which is manifested by foliar yellowing, followed by wilting, affecting photosynthesis (Wu et al., 2015); thus reducing the biomass of plants, such as with a decrease in leaf area and root volume (Cardona et al., 2016). LL stress causes different types and levels of damage to plant cells. One type involves leaf blade cells membrane destruction, which leads to increased cell permeability and intracellular conductivity. MDA, which is produced during lipid peroxidation, is an important index of cell damage under stress (Zhu et al., 2017).
In the current study, MDA levels in safflower leaves was lower in shaded leaves at vegetative stage than in sun-exposed, which might be correlated with plant adaptive physiological regulation toshade which did not seem to harm C. tinctorius leaves at such stage. Although an increase in MDA contents at full flowering stage of low light-leaf development could help to dissipate excess excitation energy to a certain extent (Zhou et al., 2004), it is also likely to result in an increase in excitation pressure of PS II and induce membrane-lipid peroxidation is related to the duration of low light treatment and the degree of shaded light (Asada and Takahashi 1987), which is the peroxidation of unsaturated fatty acids in the membrane triggered by free radicals and thereby the production of peroxide toxic to cells, eventually might do some damage to photosynthetic apparatus. A remarkable increase of malondialdehyde (MDA), a product of membrane lipid peroxidation, was observed in cherry leaves when treated with just low light, indicating more serious peroxidation in the membrane (Huang et al., 2002).
Phenolics play multiple chemical and biological functions in plants mainly related with adaptation to environmental changes since they represent a clear example of metabolic plasticity as plants are able to respond to external stresses rapidly inducing their biosynthesis in a reversible way (Wasli et al., 2018). In this context, monitoring of total phenolic, flavonoїd and condensed tannin contents were assessed. As shown in the results, phenols, flavonoid and tannin levels were superior in shading leaves than to those exposed to full sunlight for both developmental stages. Such findings were in agreement with the resource allocation hypothesis proposed by Coley et al. (1985) that assumed that the production of flavonoids and phenolics would be up-regulated under low light conditions in L. pumila. It was suggested that increasing phenolic and flavonoid components in shading plants are related to lower temperatures under LL conditions. Chan et al. (2011) reported much greater concentrations of flavones and flavonols in leaves of vegetables that are exposed to shade. This finding is in agreement with Bergquist (2007), who showed that low irradiance use is important for the production of baby spinach with high in flavonoid concentration and composition.
On other hand, physiological stages can also affect polyphenols content and composition. We found that safflower at the flowering stage had a higher level of phenol than the vegetative stage. Similarly, Ichiho et al. (2013) accounted that phenol content of six crops cultivated in Japan was strongly affected by the growing season, Bano et al. (2013) also reported that secondary metabolites distribution may alter during plant development which may be related to the harsh climatic conditions of the plant’s usual habitat which stimulate secondary metabolites biosynthesis. Phenolic content of a plant depends on a number of intrinsic (genetic extracting solvent) and extrinsic(environmental, handling and development stage) factors (Medini et al., 2013).
Hamrouni et al. (2009) concluded phenolic acids predominance during the early vegetative stage whereas flavonoids predominated during other growth stages O. majorana . In another study, Ayan et al. (2007) reported that total phenol content reached the highest level at floral budding in Hypericum hyssopifolium and Hypericum scabrum and at full-flowering in Hypericum pruinatum . The findings of erma and Kasera (2007) designated that peak concentration of phenols was observed in flowering stage in Boerhavia diffusa and Sidacordifolia except in Asparagus racemosus that showed maximal accumulation of phenols in the vegetative stage.
The antioxidant activities displayed a noteworthy difference from many studies. Indeed, Ben Mansour et al. (2018), found that leaves of Cakile maritima have an important antioxidant activity in the vegetative stage. This might suggest that these compounds play different roles depending on the state of the plant which could lead us to believe that, during flowering, these molecules are moving more toward a physiological role. While during the vegetative stage, they act preferentially as protector of plants by acting primarily as antioxidants.
CONCLUSION
No-enzymatic antioxidants and biological activities depend on several factors, mainly environmental conditions, and their light-tolerance are influenced by endogenous (ie, physiological development stage) and exogenous factors (i.e low light intensity). Our results demonstrated the influence of LL conditions and period of growth on dry weight and nutrient uptake content evolution of phenolic pools and antioxidant activities of C. tinctorius. The stimulation of phenol amounts and antioxidant capacities confirmed the stimulation of their synthesis in absence of light and their role as protectors of plant structures against the oxidative stress. Besides floral stage proved an important activity in real food systems relative to commercially used, antioxidant extracts and economic feasibility of practical applications due to high phenolic contents. Therefore, the favored harvest of safflower could be in F.S., which supports the utilization of this plant in a large field of application including cosmetic, pharmaceutical, agro alimentary and biological defense.
ACKNOWLEDGMENTS
We the authors of this article do firstly thank the Tunisian Ministry of Scientific Research and Technology for its support. We do also thank Ahlem Wasli harb and Safa Béjaoui for their technical support during the experiments and theoric parts.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Responses of carthamus tinctorius at two development stages to low light intensity (LLI): changes on phenolic metabolites and related antioxidant activities
- Aldana, F., García, P., Fischer, G., (2014). Effect of waterlogging stress on the growth, development and symptomatology of cape gooseberry (Physalis peruviana L.) plants. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 38, 393-400
- Ali, M.B., Hahn, E.J., Paek, K.Y., (2005). Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environnemental Experimental Botany54, 109-120.
- Anderson, J.M., Chow, W.S., Park, Y.I., (1995). The grand design of photosynthesis: acclimation ofthe photosynthetic apparatus to environmental cues. Photosynthesis Research 46, 129-139.
- Anderson, J.M., Park, Y.I., Chow, W.S., (1997). Photo inactivation and photoprotection of photosystem II in nature. Physiology Plantarum 100, 214-223.
- Asada, K., Takahashi, M., 1987.Photoinhibition. In: Kyle, D.J., Osmond, C.B., Arntzen, C.J. (Eds.), Production and Scavenging of Active Oxygen in Photosynthesis. Elsevier, Amsterdam, pp. 227-287
- Baas, W.J. Secondary Plant Compounds, Their Ecological Significance and Consequences for the Carbon Budget.Introduction of the Carbon/Nutrient Cycle Theory. In Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plant; Lambers, H., Cambridge, M., Konings, H., Pons, T.L., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1989; pp. 313-340.
- Bano, M.J., Lorente, J., Casstillo, J., Benavente, G.O., Rio, J.A.,Ortuno, A.,Quirin, K.W., Gerard, D., (2003).Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development ofleaves, flowers, stems and roots of Rosmarinus officinalis antioxidant activity. Journal of Agricultural and Food Chemistry 51, 4247-4253.
- Bansal, R., Sharma, S., Tripathi, K., Gayacharan, C., Kumar, A., (2019). Waterlogging tolerance in black gram [Vigna mungo (L.) Hepper] is associated with chlorophyll content and membrane integrity. Indian Journal of Biochemistry and Biophysics 56, 81-85.
- Bell, GE., Danneberger, T.K., (1999). Temporal shade on creeping bentgrass turf. Crop Science 39, 11421146
- Bergquist, S., Gertsson, U., Nordmark, L.Y., Olsson, M.E., (2007). Effects of shade nettings, sowing time and storage on baby spinach flavonoids. Journal of Science and Food Agriculture 87, 2464-2471.
- Cardona, W.A., Bautista, L.G., Flórez-Velasco, N., Fischer, G., (2016). Desarrollo de la biomasa y raíz en plantas de lulo (Solanum quitoense var. septentrionale) en respuesta al sombrío y anegamiento. Revista Colombiana de Ciencias Hortícolas 10, 53-65
- Casierra-Posada, C., Cutler, J., (2017). Photosystem II fluorescence and growth in cabbage plants (Brassica oleracea var. capitata) grown under waterlogging stress. Revista UDCA Actualidad & Divulgación Científica 20, 321-328.
- Coley, P.D., Bryant, J.P.,Chapin, F.S., (1985). Resource availability and plant anti-herbivore defense. Science 230, 895-899.
- Dewanto, V., Wu, X., Adom, K.K., Liu, R.H., (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agriculture and. Food Chemistry 50, 3010-3014.
- Gajula, D., Verghese, M., Boateng, J., Walker, L.T., Shackelford, J., Mentreddy, S.R., Cedric, S., (2009). Determination of total phenolics, flavonoids and antioxidant chemopreventive potential of basil (Ocimum basilicum L. and Ocimum tenuiflorumL.) I.J.C.R.5, 130-143.
- Gitz, D.C., Lui-Gitz, L., McClure, J.W., Huerta, A.J., (2004). Effects of PAL inhibitor on phenol accumulation and UV-B tolerance in Spirodelain termedia (Koch.). Journal of Experimental. Botany. 55, 919-927.
- Hernández, J.A., Escobar, C., Creissen, G., Mullineaux, P.M., (2004). Role of hydrogen peroxide and the redox state of ascorbate in the induction of antioxidant enzymes in pea leaves under excess light stress. Funct. Plant Biology.31, 359-368.
- Karray-Bouraoui, N., Hamrouni-Maa'zoul, H., Rabhi, M., Harbaoui, F., Attia, H., Oueslati, S., Ksouri, R., Lachaa'l, M., (2010). Enzymatic and non-enzymatic antioxidant responses of two Mentha pulegium provenances to salt stress. Journal of Medicinal Plants Research 4, 2518-2524.
- Karray-Bouraoui, N., Harbaoui, F., Rabhi, M., Jallali, I., Ksouri, R., Attia, H., Msilini, N., Lachaa'l, M., (2011). Different antioxidant responses to salt stress in two different provenances of Carthamus tinctorius L. Acta Physiolgia Plantarum 33,14351444
- Ksouri, R., Megdiche, W., Debez, A., Falleh, H., Grignon, C., Abdelly, C., (2007). Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima .Plant Physiology and Biochemistry 45, 244-249.
- Ichiho, M.K., Shiro, M., Keijiro, N., T. Yumico, Y. Minako, S. Tetsuo, W.Jun, H. Akihiro., (2013). Antioxidant capacity and polyphenols contents of extracts from crops cultivated in Japan and the effect of the cultivation, Environ. Food Science and Technolgy Research 19, 69-79.
- Medini, F., Fellah, H., Ksouri, R., Abdelly, C., (2014). Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. Journal of Taibah University for Science 8, 216-224.
- Naczk, M., Shahidi, F., (2004). Extraction and analysis of phenolics in food. Journal of Chromatography A 105,95-111.
- Powles, S.B., (1984). Photoinhibition of photosynthesis induced by visible light.Annu. Revue of Plant Physiology, 35, 15-44.
- Salem, N., Msaada, K., Dhifi, W., Limam, F., Marzouk B., (2014). Effect of salinity on plant growth and biological activities of Carthamus tinctorius L. extracts at two flowering stages . Acta Physiologia Plantarum36, 433-445
- Savikin, K., Mikulic-Petkovsek, M., Djordjevic, B., Zdunic, G., Jankovic, T., Djurovic, D., & Veberic, R. (2013). Influence of shading net on polyphenol profile and radical scavenging activity in different varieties of black currant berries. Scientia horticulturae, 160, 20-28.
- Sielewiesiuk, J., (2002). Why there are photodamages to photosystem II at low light intensities. Acta Physiologia Plantarum 24, 399-406
- Singh VP, Dey SK, Murty KS (1988) Effect of low light stress on growth and yield of rice. Indian J Plant Physi 31: 84-91
- Saada, M., Falleh, H., Jellali, I., Snoussi, M., Ksouri, R., (2014). Phenolic profile, biological activities and fraction analysis of the medicinal halophyte Retama raetam. South AfrIcain Journal of Botany 94, 114-121.
- Verma, V., Kasera , P.K., (2007). Variation in secondary metabolites in some medicinal plants in relation to season and plant growth. Indian Journal of Plant Physiology 12, 203-206
- Wang, L., Deng, F., Ren, W.J, Yang, W.Y. (2012). Effects of shading on starch pasting characteristics of indica hybrid rice (Oryza sativa L.). PLoS One 8, e68220.
- Wasli, H., Jelali, N., Silva, A.M.S., Ksouri, R., Cardoso S.M., (2018). Variation of polyphenolic composition, antioxidants and physiological characteristics of dill (Anethum graveolens L.) as affected by bicarbonate-induced iron deficiency conditions. Industrial Crops Products. 126, 466-467
- Wu, L., Gao, Y., Huang, W., Saig, H., (2011). Effects of EU3+ on anthocyanin content and PAL activity in potted Photiniafraseri under different light intensity. Journal of Chinese Rare Earth Sociecty 29, 217223.
- Zaoui, Sonia., H Gautier, H., Bancel, D., Chaabani, G., Wasli, H., Mokhtar Lachaal, M., Karray-Bouraoui, K., (2016). Antioxidant pool optimization in Carthamus tinctorius L. leaves under different NaCl levels and treatment durations . Acta Physiolgia Plantarum 38,187
- Zavala, J.A., Ravetta, D.A, (2001). Allocation of photoassimilates to biomass, resin and carbohydrates in Grindelia chiloensis as affected by light intensity. Field Crop Research 69,143-149.


