Rhythmical changes of a level nitric oxide (NO) in roots etiolated seedlings of pea ( Pisum sativum L.) and influence of exogenous calcium
Автор: Glyanko A.K., Ischenko A.A.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.10, 2014 года.
Бесплатный доступ
Studied time dynamics (during 60 mines) a level oxide nitric (NO) in cross cuts of roots 2 - day etiolated seedlings of pea sowing ( Pisum sativum L.) by use of fluorescent probe DAF-2DA and a fluorescent microscope depending on action exogenous calcium (Ca 2+). During an exposition of seedlings on water, solution CaCl 2 are shown fluctuation in level NO in roots - his increase and decrease that testifies to the certain rhythm in generation NO. Exogenous factors (Ca 2+) change time dynamics of level NO in comparison with variant “water”. Ca 2+chelate EGTA removes action exogenous calcium on rhythmical change of a level NO in roots. Results are discussed in aspect of close interference of signaling systems and molecules (Ca 2+, NO, Н 2О 2).
Pisum sativum l, calcium (ca2+), calcium chelate (egta), fluorescent probe, nitric oxide (no)
Короткий адрес: https://sciup.org/14323911
IDR: 14323911
Текст научной статьи Rhythmical changes of a level nitric oxide (NO) in roots etiolated seedlings of pea ( Pisum sativum L.) and influence of exogenous calcium
В данном случае, речь может идти об автоколебательных процессах в химических реакциях, когда эти колебания включаются в цепь взаимодействий между собой и регулируются, по-видимому, геномом (Detari, Karcagi, 1984). О тесном взаимодействии сигнальных молекул, в частности, Са2+, NO и Н 2 О 2 , в растительных клетках в литературе известно (Jeandroz et al ., 2013; Steinhorst, Kudla, 2013). Однако механизмы, управляющие этими взаимодействиями, до конца не выяснены. Наиболее изученными в этом плане являются механизмы колебаний (осцилляций) Са2+ в цитоплазме, образующиеся за счет притока этого элемента из внеклеточного пространства и внутриклеточных органелл и последующим его уменьшением (оттоком) из цитоплазмы (Denisenko,
Kuz’mina, 2013).
В задачу наших исследований входило изучение динамики уровня NO в корнях этиолированных проростков гороха с целью выяснения характера изменений NO в зависимости от экзогенного кальция и оценке генерации NO как автоколебательного процесса, зависимого от других процессов и регулируемого, вероятно, единым механизмом.
MATERIALS AND METHODS
Объектом исследований служили проростки гороха посевного ( Pisum sativum L.), сорт Ямальский, выращенные в пластмассовых кюветах на влажной фильтровальной бумаге при 22оС. Перед замачиванием семена промывали теплой проточной водой с мылом и обеззараживали 3 % раствором перекиси водорода в течение 15 мин. После этого семена заливали прокипяченной водопроводной водой (60оС) и помещали в термостат для прорастания при 22оС в течение 48 ч. Для исследований отбирали однородные проростки с длиной корней 25-30 мм.
В экспериментах с действием экзогенного кальция опытные проростки экспонировали на растворе СаСl 2 в концентрации 100 мкМ. Обработку проростков кальцием и ЭГТА (100 мкМ) проводили перед началом опыта. Содержание NO в тканях корня определяли с использованием флуоресцентного зонда 4,5-диаминофлуоресцеин диацетата (DAF - 2DA). Это соединение проникает через клеточную мембрану и деацетилирует с помощью внутриклеточных эстераз в 4,5-диаминофлуоресцеин (DAF-2), который образует с
NO флуоресцирующее соединение – диаминотриазолфлуоресцеин триазол (DAF-2T) (Nakatsuboa et al., 1998). Перед окрашиванием исходные корни прединкубировали на соответствующих варианту опыта средах (Н 2 О, СаСl 2 , СаСl 2 +ЭГТА) в течение необходимого времени: 10, 20, 30 и так далее минут. Затем отрезки корней (участки 5 – 15 мм от апекса) инкубировали в среде для окрашивания, содержащей 10 мкM DAF-2DA и 10 мM трис-HCl (pH 7.4), в течение 20 мин на качалке, при 26оС. Интенсивность флуоресценции оценивали на поперечных срезах (толщина 100 – 150 мкм) из окрашенных отрезков корней с использованием флуоресцентного микроскопа Axio Observer Zl (Германия) с цифровой монохромной камерой Axio Cam MRm3 и пакетом программного обеспечения для захвата и анализа изображений Axio Vision Rel.4.6. Блок фильтров № 10, длина волны возбуждения 450-490 нм, эмиссия 515-565 нм. Основное флуоресцентное свечение в срезах корней в наших опытах зафиксировано в эпидермальных клетках корня (Glyan’ko, 2013). Результаты анализа срезов корней представлены в относительных единицах интенсивности флуоресцентного свечения, выраженные в % от нулевой точки (0 мин), значение которой принималось за 100%.
Содержание общего кальция в корнях проростков определяли на атомно-абсорбционном спектрометре модели 403 фирмы Perkin Elmer (CША) в пламени ацетилен-воздух (Glyan’ko et al., 2013). Результаты представляют собой средние арифметические с указанием стандартной ошибки из трех независимых экспериментов. Число анализируемых срезов корней при микроскопических исследованиях не менее 10. Достоверность различий средних значений оценивали по t-критерию Стъюдента. Статистическую обработку данных проводили с помощью пакета программ Microsoft Excel.
RESULTS AND DISCUSSION
На рисунке 1 представлены кривые линии, характеризующие динамику уровня NO в течение 60 мин. В варианте «вода» четко прослеживаются ритмичность флуктуаций в содержании оксида азота: повышение через 10, 30, 50 мин и уменьшение через 20, 40 и 60 мин. Флуктуации в уровне NO наблюдаются и на фоне действия экзогенного источника кальция (100 мкМ СаСl2) (рис. 1). Однако временная зависимость флуктуаций уровня NO в этом случае иная: повышение уровня оксида азота через 20, 40, 60 мин, снижение – через 10, 30, 50 минут. Таким образом, экзогенный кальций влияет на содержание оксида азота, изменяя временную амплитуду его синтеза и сохраняя ритмичность флуктуаций. Надо полагать, что подобное влияние экзогенного кальция связано с увеличением апопластного и внутриклеточного Са2+. Об этом косвенно можно судить по содержанию общего кальция в корнях проростков спустя 60 мин. после начала экспозиции. Так, содержание общего кальция в корнях с 860±36 мкг/г сухого вещества в контроле увеличивается до 1043±28 мг/ г сухого вещества в варианте с экзогенным Са2+, что, по- видимому, может служить объяснением характера динамики флуктуаций NO в варианте с экзогенным кальцием. Опыт с экспозицией проростков на растворе СаСl2 + ЭГТА свидетельствует о нивелировании влияния экзогенного кальция на флуктуации уровня NO (рис. 1). Это, по-видимому, можно объяснить связыванием внеклеточного кальция ЭГТА.
Общий вывод, который можно сделать из полученных результатов состоит в том, что в краткосрочных опытах обнаружены ритмичные изменения уровня оксида азота и влияние на эти флуктуации ионов кальция. Можно предположить, что активация Са2+-каналов и стимуляция поступления ионов Са2+ в цитоплазму оказывает влияние на генерацию NO. Однако здесь необходимо заметить, что вопрос о путях синтеза NO в растениях остается до настоящего времени до конца нерешенным. С одной стороны бесспорно признается путь синтеза NO с участием цитозольной нитратредуктазы (НР), но функциональная связь между ионами кальция и активностью НР остается под вопросом (Courtois et al., 2008). Второй путь синтеза NO, связанный с использованием в качестве субстрата L-аргинина, подтвержден многочисленными исследованиями [Besson-Bard et al., 2008; Glyan’ko, 2013), но не доказан присутствием в растениях фермента идентичного NO-синтазе (NOS) млекопитающих, катализирующей окислительную реакцию синтеза NO из L-аргинина в животных клетках. Что же касается взаимосвязи между Ca2+ и генерацией NO по аргинин-зависимому пути, то имеются данные о необходимости ионов кальция в реакции, катализируемой предполагаемой растительной NO-синтазой (Vandelle et al., 2006). Зависимость активности конститутивной изоформы сNOS млекопитающих от кальмодулина и ионов Ca2+ (Bogdan, 2001) подтверждена также и в опытах с различными видами растений (Corpas et al., 2006). Предполагаются и другие источники генерации NO – с участием гидроксиламина, полиаминов, ксантиноксидоредуктазы, неэнзиматического пути и, возможно, другие (Freschi, 2013). Множество путей синтеза NO в растениях затрудняет определение основного из них. Можно предположить, что это будет зависеть от мест их локализации в клетках, тканях, органах. По данным Glyan’ko (2013) в этиолированных проростках гороха функционируют как НР-путь синтеза NO, так и L- аргинин зависимый путь.
NO играет специфическую роль в регуляции Cа2+-гомеостаза путем модуляции активности Са2+-каналов как на плазмалемме, так и на мембранах внутриклеточных органелл (Besson-Bard et al., 2008). Так, показана активация Са2+-кальмодулинзависимой киназы (ССаМК) изменениями концентрации кальция в цитоплазме и органеллах, что сопровождается усилением генерации NO (Courtois et al., 2008). Оксид азота в свою очередь может блокировать приток Са2+ в цитоплазму через кальциевые каналы путем S-нитрозилирования сенсора кальция кальмодулина (СаМ) (Jeandroz et al., 2013). Исходя из этого, можно говорить о перекрестном и взаимном влиянии двух сигнальных соединений на процессы метаболизма, особенно при трансдукции абиотических и биотических сигналов (Vandelle et al., 2006; Kolupaev, 2007; Besson-Bard et al., 2008a).
Накопление цитозольного NO будет вести к стимуляции процессов, оказывающих влияние на гомеостаз Ca2+ во внутриклеточных органеллах путем создания в цитоплазме кальциевых волн (осцилляций), составляющих звенья в трансдукции информации в геном (Medvedev, 2010; Jeandroz et al., 2013). Кальциевые волны осуществляются за счет мембранного транспорта, Са2+-АТФаз, киназ, белков-сенсоров (Tarchevsky, 2002). Действие NO на генерацию АФК, которые влияют на Са2+-каналы, может проявляться в ингибировании активности НАДФН-оксидазы за счет S-нитрозилирования цистеина (Cys890) фермента (Yun et al., 2011).
Необходимо отметить роль АФК (О2•-, Н2О2), основными продуцентами которых являются мембранная НАДФН-оксидаза и апопластная супероксиддисмутаза (Marino et al., 2012). АФК оказывают влияние, как и NO, на проницаемость Са2+-каналов, связывают оксид азота с образованием пероксинитрита (ООNO-), образуют положительную обратную связь по регуляции НАДФН-оксидазы путем фосфорилирования фермента (Kimura et al., 2012). О волновой природе функционирования АФК (Н2О2) в растительных клетках сообщается в ряде работ, в которых доказывается наличие в клетках арабидопсиса АФК-волн, выполняющих функцию системного сигнала на длинные дистанции, связанного с формированием системной устойчивости к действию стрессоров (Miller et al., 2009; Mittler et al., 2011). И главную роль в этих процессах играет НАДФН-оксидаза (RbohD oxidase) – генератор АФК, активность которой связана с Са2+-сигналингом и Са2+-регулируемыми киназами (Steinhorst, Kudla, 2013). Показаны колебания в активности микросомальной НАДФН-оксидазы этиолированных проростков гороха, что может влиять на уровень генерации АФК (Glyan’ko, Ischenko, 2013).
Флуктуации NO могут поддерживаться за счет связывания этой молекулы «NO-мишенями», к которым, в частности, относятся несимбиотический гемоглобин и нитрозоглутатион (GSNO) (Besson-Bard et al ., 2008; Sanchez et al ., 2011). GSNO является внутриклеточным резервуаром NO и может спонтанно освобождать оксид азота и выполнять роль проводника NO между клетками (Neill et al., 2008).
Все эти процессы ведут к формированию NO-волн, которые оказывают влияние на выход Са2+ из внутриклеточных органелл (вакуолей, ядра и др.) в цитоплазму и на обратный процесс. Наличие в клетке многочисленных «Са2+-мишеней» создает условия для уменьшения концентрации этого элемента в цитоплазме и формированию кальциевых осцилляций, которые наряду с NO-волнами, инициируют экспрессию ядерных генов. Эти процессы осуществляются за счет различных процессов (Medvedev, 2010). Особую роль в этом выполняют кальмодулин (CaM) и протеинкиназы, зависимые от Са2+ и осуществляющие фосфорилирование белков – факторов регуляции транскрипции (Steinhorst, Kudla, 2013; Tarchevsky, 2002).
Необходимо отметить, что существенным компонентом сигнальных систем являются Са2+-спайки (calcium spiking), образующие в результате флуктуаций в концентрации кальция. Са2+-спайки локализуются в околоядерном пространстве и формируются с участием белков ионных каналов (CASTOR, POLLUX), нуклепоринов (NUP85, NUP133) и связаны с осцилляциями кальция в нуклеоплазме (Oldroyd, Downie, 2008). Они, в частности, активируют кальций-зависимую кальмодулинкиназу (CCaMK) и далее рецептор цитокинина - гистидинкиназу (LjHK1, MtCRE1), что инициирует процесс деления клеток. В то же время действие цитокинина тесно связано с NO (Freschi, 2013).
Эндогенный ритм генерации NO в корнях, по всей вероятности, свидетельствует о наличии в клетках механизма, регулирующего как образование NO, так и АФК. Это подтверждается данными литературы о том, что в ответ на различные факторы генерация NO и АФК в клетках происходит одновременно и изменения в концентрации одного из компонентов оказывает влияние на уровень другого (Neill et al., 2008). Подобный механизм, очевидно, связан с гомеостазом ионов Са2+, выражающийся в пульсирующем изменении концентрации этого иона в цитоплазме (Са2+-волны). В последнее время это явление в литературе обозначается термином “Са2+-сигнатура” (саlcium signatures), которое характеризует флуктуации в концентрации внутриклеточного кальция по таким параметрам как: амплитуда, частота, продолжительность пульсов (Whalley, Knight, 2013). Показано влияние АФК (Н2О2) на синтез NO с участием НР у проростков арабидопсиса (Wang et al., 2010).
Таким образом, из вышеизложенного можно сделать вывод об определенном ритме в изменении уровня NO в клетках проростков гороха и о тесном взаимовлиянии Са2+, NO и АФК – как компонентов сигнальных систем растения, функционирование которых регулируется, вероятно, сходным(и) механизмом(ами). В этой связи предстоит выяснить механизм действия кальция и его влияние на перекрестное взаимодействие других сигнальных молекул, в частности, NO и Н2О2. Все эти механизмы, по-видимому, инициируются действием внешних факторов.
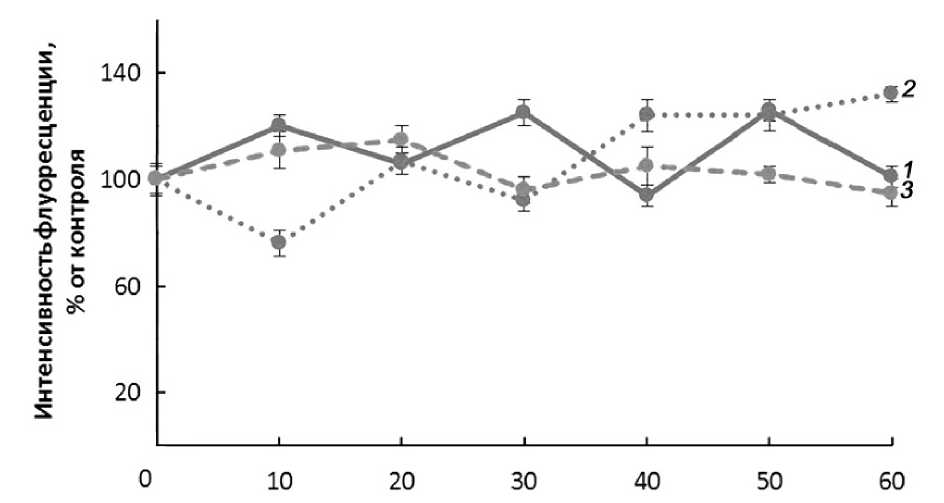
мин
Рисунок 1. Рис. Динамика интенсивности флуоресценции в корнях этиолированных проростков гороха на фоне воды, экзогенного кальция и ЭГТА.
1 - вода; 2 - 100 мкМ СаСl 2 ; 3 - 100 мкМ СаСl 2 + 100 мкМ ЭГТА
ACKNOWLEDGMENT
Степанову, Г.Г. Васильевой за помощь в проведении исследований.
Список литературы Rhythmical changes of a level nitric oxide (NO) in roots etiolated seedlings of pea ( Pisum sativum L.) and influence of exogenous calcium
- Besson-Bard A., Pugin A. and Wendehenne D. (2008) New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol., 59, 21-39
- Besson-Bard A., Courtois C., Gauthier A., Dahan J., Dobrowolska G., Jeandroz S., Pugin A. and Wendehenne D. (2008a) Nitric oxide in plants production and cross-talk with Ca2+ signaling. Mol. Plant, 1, 218-228
- Bogdan C. (2001) Nitric oxide and the regulation of gene expression. Trends Cell Biol., 11, 66-75
- Сlementi E. (1998) Role of nitric oxide and its intracellular signaling pathways in the control of Ca2+ homeostasis. Biochem. Pharmacol., 55, 713-718
- Corpas F.J., Barroso J.B., Carreras A., Valderrama R., Palma J.M., Leon A.V., Sandalio L.M. and del Rio L.A. (2006) Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta, 224, 246-254
- Courtois C., Besson A., Dahan J., Bourque S., Dobrowolska G., Pugin A. and Wendehenne D. J. (2008) Nitric oxide signaling in plants: interplays with Ca2+ and protein kinases. Exp. Bot., 59, 155-163
- Denisenko V.Yu. and Kuz’mina T.I. (2013) On the problem of identification of intracellular signaling pathways. Biochemistry (Moskow), 78, 431-433
- Detari L. and Karcagi V. (1984) Biorhithms (Ed. V.B. Chernyshov). M.: Mir Press, 160 p
- Dmitriev A.P. (2004) The signaling role of nitric oxide in plants. Cytol. Genet. (Ukraine), 38, 67-75
- Freschi L. (2013) Nitric oxide and phytohormone interactions: current status and perspectives. Front. Plant Sci., 4, Article 398
- Garcia-Mata C., Gay R., Sokolovski S., Hills A., Lamattina L. and Blatt M.R. (2003) Nitric oxide regulates K+ and Cl-channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc. Natl. Acad. Sci. USA, 100, 11116-11121
- Glyan’ko A.K., Mitanova N.B. and Stepanov A.V. (2010) The physiological role of nitric oxide (NO) in plants. The Bulletin of Kharkiv National Agrarian University. Series Biology, 1 (19), 6-20
- Glyan‘ko A.K. and Ischenko A.A. (2013) Influence of rhizobial inoculation and calcium ions on the NADPH oxidase activity in roots of etiolated pea seedlings. Appl. Biochem. Microbiol., 49, 215-219
- Glyan’ko A.K. (2013) Initiation of nitric oxide (NO) synthesis in roots of etiolated seedlings of pea (Pisum sativum L.) under the influence of nitrogen-containing compounds. Biochemistry (Moskow), 78, 471-476
- Glyan’ko A.K., Ischenko A.A., Stepanov A.V., Vasil’eva G.G. and Projdakova O.A. (2013) Dynamics of synthesis nitric oxide (NO) in roots etiolated seedlings of pea (Pisum sativum L.). The Bulletin of Kharkiv National Agrarian University. Series Biology, 3 (30), 32-38
- Jeandroz S., Lamotte O., Astier J., Rasul S., Trapet P., Besson-Bard A., Bourque S., Nicolas-Frances V., Berkowitz G.A. and Wendehenne D. (2013) There’s more to the picture than meets the eye: nitric oxide cross talk with Ca2+ signaling. Plant Physiol., 163, 459-470
- Kimura S., Kaya H., Kawarazaki T., Hiraoka G., Senzaki E., Michikawa M., Kuchitsu K. (2012) Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim. Biophys. Acta, 1823, 398-405
- Kolupaev Yu.Ye. and Karpets Yu.V. (2009) Participation of nitrogen oxide (NO) in transduction of abiotic stressors signals in plants. The Bulletin of Kharkiv National Agrarian University. Series Biology, 3 (18), 6-19
- Kolupaev Yu. Ye. (2007) Calcium and stress reactions of plants. The Bulletin of Kharkiv National Agrarian University. Series Biology, 3 (10), 24-41
- Lamotte O., Gould K., Lecourieux D., Sequeira-Legrand A., Lebrun-Garcia A., Durner J., Pugin A., Mori I.C. and Schroeder J.I. (2004) Analysis of nitric oxide signaling functions in tobacco by the elicitor cryptogein. Plant Physiol., 135, 516-529
- Marino D., Dunand C., Puppo A. and Pauly N. (2012) A burst of plant NADPH oxidase. Trends Plant Sci., 17, 9-15
- Meilhoc E., Boscan A., Bruand C., Puppo A. and Brouquisse R. (2011) Nitric oxide in legume-rhizobium symbiosis. Plant Sci., 181, 573-581
- Medvedev C.C. (2010) Calcium signaling systems. In: Signaling in cells (Ed. A.N. Grechkin). Kazan: FEN Press, pp. 26-36
- Miller G., Schlauch K., Tam R., Cortes D., Torres M.A., Shulaev V., Dangi J.L. and Mittler R. (2009) The plant NADPH oxidase RBohD mediates rapid systemic in response to diverse stimuli. Sci. Signal., 2(84), ra 45
- Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V. and Van Breusegem F. (2011) ROS signaling: the new wave? Trends Plant Sci., 16, 300-309
- Nakatsuboa A., Kojimaa H., Kikuchia K., Nagoshib H., Maedaa D., Imaia Y., Irimuraa T., Naganoa T. (1998) Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Letters, 427, 263-266
- Neill S., Bright J., Desikan R., Hancock J., Harrison J. and Wilson I. (2008) Nitric oxide evolution and perception. J. Exp. Bot., 59, 25-35
- Oldroyd G.E.D. and Downie J.A. (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol., 59, 519-546
- Sanchez C., Cabrera J.J., Gates A., Bedmar E.J., Richardson D.J. and Delgado M.J. (2011) Nitric oxide detoxification in the rhizobium-legume symbiosis. Biochem. Soc. Transactions, 39, 184-188
- Steinhorst L. and Kudla J. (2013) Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol., 163, 471-485
- Tarchevsky I.A. (2002) Plant cell signaling systems (Ed. A.N. Grechkin). M: Nauka, 294 p
- Vandelle E., Poinsson B., Wendehenne D., Bentejac M. and Pugin A. (2006) Integrated signaling network involving calcium, nitric oxide, and active oxygen species but not mitogen-activated protein kinases in BcPG1-elicited grapevine defenses//Mol. Plant-Microbe Interact.,19, 429-440
- Wang P., Du Y., Li Y., Ren D. and Song C.-P. (2010) Hydrogen peroxide -mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell, 22, 2981-2998
- Whalley H.J. and Knight M.R. (2013) Calcium signatures are decoded by plants to give specific gene responses. New Phytol. 197, 690-693
- Yun B.W., Feechan A., Yin M., Saidi N.B., Le Bihan T., Yu M., Moore J.W., Kang J.G., Kwon E., Spoel S.H., Pallas J.A. and Loake G.J. (2011) S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature, 478, 264-268


