Роль загрязнения воздуха взвешенными частицами в патогенезе онкологических заболеваний
Автор: Колпакова Алла Федоровна, Шарипов Руслан Нильевич, Волкова Оксана Анатольевна, Колпаков Федор Анатольевич
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Обзоры
Статья в выпуске: 2 т.20, 2021 года.
Бесплатный доступ
В обзоре освещены современные представления о роли загрязнения атмосферного воздуха взвешенными частицами (particulate matter, PM) в патогенезе онкологических заболеваний. Для этой цели были использованы материалы статей, индексированных в базах PubMed и РИНЦ. Рассмотрены результаты долговременного влияния PM в зависимости от их размера, происхождения, химического состава, концентрации в воздухе на возникновение и прогрессирование онкологических заболеваний. РМ с аэродинамическим диаметром
Загрязнение воздуха, взвешенные частицы, патогенез онкологических заболеваний
Короткий адрес: https://sciup.org/140254419
IDR: 140254419 | УДК: 616-006-092:614.7 | DOI: 10.21294/1814-4861-2021-20-2-102-109
Текст обзорной статьи Роль загрязнения воздуха взвешенными частицами в патогенезе онкологических заболеваний
Рост смертности от злокачественных новообразований (ЗНО) является серьезной медицинской и социально-экономической проблемой последних десятилетий во всем мире, в том числе и в России. Смертность от ЗНО занимает второе место после болезней сердца и сосудов в структуре летальности населения России [1]. В 2016 г. ведущими локализациями в общей структуре онкологической заболеваемости в Российский Федерации являлись: кожа (12,5 %, с меланомой – 14,2 %), молочная железа (11,5 %), трахея, бронхи, легкое (10,1 %), ободочная кишка (6,7 %), предстательная железа (6,4 %), желудок (6,2%), прямая кишка, ректосигмоидное соединение, анус (4,9 %), лимфатическая и кроветворная ткань (4,8 %), тело матки (4,2 %), почка (4,0 %), поджелудочная железа (3,1 %), шейка матки (2,9 %), мочевой пузырь (2,8 %) и яичник (2,3 %) [2].
Среди приоритетных веществ, формирующих сверхнормативное загрязнение атмосферного воздуха городских и сельских территорий РФ, первое место занимают взвешенные частицы (particulate matter, PM), т.е. все твердые и жидкие вещества малого размера, содержащиеся в воздухе в виде аэрозоля [4]. A.J. Cohen et al. проанализировали статистические данные смертности, связанной с воздействием PM, на глобальном и региональном уровнях, а также результаты спутниковых наблюдений с 1990 по 2015 г. Выявлен существенный рост заболеваемости и смертности, связанных с загрязнением воздуха, за последние 25 лет [5]. Ежегодно в мире регистрируется более 3 млн преждевременных смертей, обусловленных загрязнением воздуха, с тенденцией роста этого показателя [6].
Влияние размера и состава РМ на человека
Особенно опасны для здоровья человека мелкодисперсные частицы с аэродинамическим диаметром ≤ 2,5 мкм (РМ2,5), которые могут достигать бронхиол и альвеол, и ультрамелкодисперсные частицы с размером частиц 0,1–0,001 мкм (PM0,1), которые включают в себя наночастицы (<100 нм).
Обладая малой массой, РМ0,1 имеют относительно большую площадь поверхности, депонируются в альвеолах, могут ускользать от мукоцилиарного клиренса и макрофагов, в отличие от крупных РМ, проникают в кровоток и затем могут попасть в любую ткань организма [7, 8].
Химический состав РМ зависит от многочисленных факторов: географических, метеорологических, особенностей источников их происхождения, взаимодействия в атмосфере. Обычно РМ включают в себя неорганические компоненты, элементарный и органический углерод, биологические компоненты (бактерии, споры и пыльцу растений). Установлено, что РМ2,5 и РМ0,1 дорожно-транспортного происхождения содержат больше тяжелых металлов, чем промышленная пыль [9, 10]. Наиболее высоким потенциальным канцерогенным риском обладают РМ, содержащие металлы, в том числе Cr, Cd, Co [11]. Кроме того, в воздухе происходит формирование вторичных частиц в результате химических реакций c газообразными загрязняющими веществами, которые, в свою очередь, являются продуктом происходящей в атмосфере трансформации окислов азота и серы либо выбрасываются автотранспортом и промышленностью. Вторичные частицы в основном содержатся в мелкодисперсных РМ [12, 13].
С другой стороны, M. Pedersen et al. при анализе 15 когортных исследований, выполненных в период с 1985 по 2005 г. с участием 303 431 человека, используя стандартизованные регрессионные модели из европейского проекта ESCAPE, не выявили достоверной ассоциации между уровнем РМ2,5 и риском возникновения рака мочевого пузыря [20]. Аналогичные данные получены в исследовании Nurses’ Health Study II Prospective Cohort, анализирующем связь между длительным воздействием РМ и риском заболевания раком молочной железы у медсестер [21]. Однако до сих пор остается неясной роль загрязнения воздуха РМ c аэродинамическим диаметром ≤2,5 мкм в патогенезе ЗНО, в частности на молекулярно-генетическом уровне.
Механизмы действия РМ
Доказано, что оксидативный стресс при действии PM является центральной ступенью провос-палительной реакции и канцерогенеза, а активные формы кислорода (АФК) и азота (АФА) могут быть триггерами освобождения цитокинов из тканей через транскрипционные факторы NF-κB (ядерный фактор «каппа-би»), AP-1 (белок-активатор 1), Nrf2 (ядерный фактор, подобный выделенному из эритроидных клеток-2) и AhR (рецептор ароматических углеводородов) [22–25].
Анализ многочисленных исследований привел к заключению, что митохондриальная супероксид-дисмутаза-2 (мСОД2) играет важную роль в предохранении клетки от оксидативного стресса. Однако при нарушении окислительно-восстановительного баланса, связанного с накоплением перекиси водорода, мСОД2 и глутатион пероксидаза-1 могут оказывать двоякое действие, предупреждая или увеличивая риск канцерогенеза [26].
В исследовании C. Tan et al. приняли участие 183 полицейских дорожно-патрульной службы, подвергавшихся воздействию высоких концентраций РМ2,5, содержащих металлы (Fe, Са, Сu, Zn, Cd, и Pb), и 88 офисных работников. С использованием многофакторного линейного регрессионного анализа была выявлена значимая положительная ассоциация между увеличением уровня биомаркеров оксидативного стресса, повреждений ДНК, хромосомных аберраций и высокой концентрацией РМ2,5, а также длительностью воздействия. Авторы пришли к заключению, что долгосрочное воздействие высоких концентраций РМ2,5 индуцирует кумулятивные повреждения ДНК, которые способствуют канцерогенезу и могут быть ранними биомаркерами развития рака [27].
В обзоре литературы H. Sas-Nowosielska et al. отмечено, что наиболее выраженное вредное влияние тяжелых металлов, входящих в состав РМ2,5, связано с их взаимодействием с компонентами ядра клетки: нуклеиновыми кислотами, белками и липидами. Некоторые ионы металлов, будучи микроэлементами, например Zn2+ и Cu2+, в физиологических концентрациях проникают через мембрану клеток по специализированным ионным каналам. Аналогичным образом действует большинство известных цитотоксичных металлов, которые входят в клетки, подражая ионам эссенциальных металлов, и вызывают генерацию АФК [28].
Рост уровня АФК приводит к нарушениям жизненно важных внутриклеточных процессов, таких как транскрипция, репликация и репарация ДНК, что, в свою очередь, влечет необратимые изменения в реализации программы клетки и канцерогенез [29–31].
Используя национальный раковый регистр, W.C. Pan et al. проанализировали 464 случая ГЦК у жителей Тайваня. При этом выявлена положительная ассоциация между воздействием РМ2,5, уровнем аланинтрасферазы (АЛТ) и частотой ГЦК. Установлено, что долговременное воздействие PM2,5 увеличивает риск возникновения рака печени, а хроническое воспаление печени играет ведущую роль в патогенезе ГЦК, что подтверждается высоким уровнем АЛТ [32]. В эксперименте Q. Zhang et al. обнаружили, что воздействие PM способствовало миграции и инвазии клеток ГЦК, увеличению концентрации матриксной металлопротеиназы-13 (MMP-13). Кроме того, PM2,5 индуцировали образование АФК в клетках ГЦК. Дальнейшее исследование показало, что фосфорилирование серин/треониновой протеинкиназы AKT1 увеличивалось в ответ на воздействие PM2,5 в клетках ГЦК, а антагонист AKT1 LY294002 уменьшал индуцированную PM2,5 миграцию и инвазию клеток ГЦК, а также экспрессию MMP-13. На клетках гепатоцитов линии HL7702 было показано, что высокие концентрации PM2,5 уменьшают пролиферацию клеток и способствуют апоптозу. Было также установлено, что активация AKT1 с помощью PM2,5 приводит к значительному повышению экспрессии MMP-13 и стимулирует миграцию и инвазию клеток ГЦК. Это исследование показывает, что воздействие PM2,5 способствует развитию ГЦК и объясняет потенциальный молекулярный механизм этого эффекта [33].
-
H. Deng et al. проанализировали истории болезней 20 221 пациента с ГЦК из регистра рака штата Калифорния (США) с использованием модели пропорционального риска (регрессии Кокса) и пришли к заключению, что воздействие высоких концентраций РМ2,5 может сократить выживаемость пациентов с ГЦК [34]. В результате анализа данных литературы R. Li et al. предложили ранжировать влияние РМ2,5 на развитие ЗНО, в
том числе РЛ, по нескольким группам: 1) активация онкогенов, опосредованная микроРНК; 2) мутации в генах; 3) инактивация супрессоров опухолей посредством ДНК-метилирования; 4) нарушение микроокружения клеток легких; 5) усиление процессов аутофагии и апоптоза [24].
C. Liu et al. изучили экспрессию микроРНК и информационной РНК (иРНК), а также их взаимодействие в клетках бронхиального эпителия после воздействия РМ2,5. Гены slc30a1 , serpinb2 и akr1c1 , обладающие повышенной экспрессией, были идентифицированы как мишени для микроРНК miR-182 и miR-185 . Кроме того, повышенная экспрессия этих генов была обнаружена и у пациентов, страдающих РЛ. Таким образом, воздействие РМ2,5, вероятно, приводит к изменению экспрессии miR-182 и miR-185 и, соответственно, их генов-мишеней, что может способствовать канцерогенезу легких [22].
Мутациям в генах, обусловленным воздействием РМ2,5, посвящено много исследований, из которых следует, что они могут взаимодействовать с ДНК, вызывая образование ДНК-аддуктов, и, как следствие, нарушать работу генов и усиливать мутагенез [35–38]. Инактивация генов онкосупрессоров путем ДНК-метилирования достаточно часто встречается в раковых опухолях. В частности, ген белка p53, хорошо известного «стража генома», был обнаружен инактивированным в опытах на клетках немелкоклеточного РЛ (НМРЛ) [29]. W. Zhou et al. исследовали изменение метилирования генома и промотора гена p53 как результат воздействия низких концентраций РМ2,5 на клетки бронхиального эпителия человека (BEAS-2B). Показано снижение уровня геномного метилирования, сопряженное со снижением экспрессии гена ДНК-метилтрансферазы DNMT1. При этом был отмечен высокий уровень метилирования промотора гена p53 на фоне повышенной экспрессии другой ДНК-метилтрансферазы DNMT3B как результат работы связки АФК-киназа Akt-DNMT3B. По мнению авторов, активность данного внутриклеточного сигнального пути не только объясняет возможное развитие ЗНО под воздействием PM2,5 из воздуха, но и может стать путеводной нитью для предотвращения их негативного влияния [30].
Установлено, что клеточное микроокружение влияет на поведение опухоли [39, 40]. B. Yang et al. исследовали влияние РМ2,5 на линии клеток аденокарциномы легких A549 (альвеолярный базальный эпителий) и НМРЛ H1299 (клетки легочного эпителия, выделенные из метастазов в лимфатическом узле). Транскриптомный анализ посредством секвенирования иРНК клеток показал, что РМ2,5 вызывают увеличение инвазивного и пролиферативного потенциала этих клеток. В результате моделирования и реконструкции сети белок-белковых взаимодействий авторы выделили два гена с наиболее повышенной экспрессией – ин- терлейкина IL1B и матриксной металлопротеиназы MMP-1 – как ключевые регуляторы, опосредующие эффекты PM2,5 [31]. Следует отметить, что повышенная активность металлопротеиназ обычно свидетельствует о худшем прогнозе.
H. Wei et al. обнаружили, что РМ2,5 могут индуцировать эпителиально-мезенхимальный переход и влиять на свойства стволовых раковых клеток в культуре клеток аденокарциномы легких A549. Обнаружено также, что как кратковременное, так и хроническое воздействие РМ2,5 повышает клеточную миграцию и инвазию, снижает экспрессию иРНК эпителиальных маркеров и увеличивает экспрессию иРНК мезенхимальных маркеров. Дополнительно отмечено, что хронизация процесса усиливает эпителиально-мезенхимальный переход (ЭМП) и проявление стволового потенциала раковых клеток. При ЭМП покоящиеся эпителиальные клетки теряют свои межклеточные контакты и при- нимают мезенхимальную форму. Они приобретают способность к миграции через базальную мембрану, а значит, могут по кровеносному или лимфатическому руслу попасть в любые, сколь угодно отдаленные от своего исходного местонахождения, иногда при участии фибробластов [41]. А.П. Лыков и соавт. выявили высокий пролиферативный потенциал и устойчивость к окислительному стрессу у опухоль-ассоциированных мезенхимных стволовых клеток из ткани химически индуцированной опухоли молочной железы крыс. Установлено, что мезенхимные стволовые клетки обладают тропизмом к опухолям, способствуют их росту и метастазированию [43]. В основе данного эффекта лежит паракринное влияние секретируемых мезенхимными стволовыми клетками факторов, приводящее к снижению экспрессии Е-кадгерина и повышению экспрессии протеинов, необходимых для реализации ЭМП (N-кадгерин, виментин, фи-
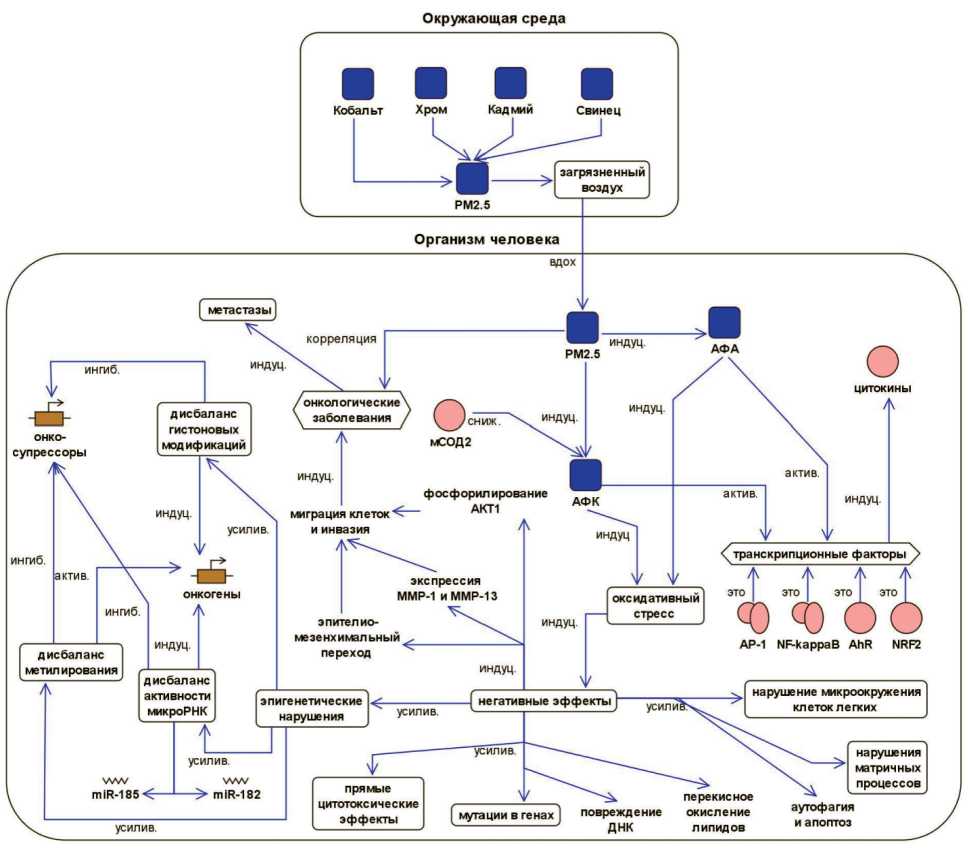
Рис. 1. Влияние взвешенных частиц PM2,5 на развитие онкологических заболеваний. Формализованное описание, платформа BioUML.
Примечание: актив. – активация, ингиб. – ингибирует, индуц. – индуцирует, сниж. – снижает, усилив. – усиливает, АФА – активные формы азота, АФК – активные формы кислорода, мСОД2 – митохондриальная супероксиддисмутаза 2 Fig. 1. A formalized description of the effect of air pollution by the particulate matter PM2.5 in pathogenesis of cancer
бронектин) [43]. Кроме того, стромальные клетки РЛ секретируют такие молекулы, как трансформирующий фактор роста бета 1 (TGF-β1), который вызывает эпителиально-мезенхимальный переход в опухолевых клетках, облегчая их инвазию, стволовость и метастазирование. Следовательно, TGF-β1 является одним из наиболее важных тканевых факторов, секретируемых при развитии эпителиальных опухолей [44]. В.Е. Шевченко и соавт. методом протеомной масс-спектрометрии высокого разрешения изучили молекулярные механизмы действия TGF-β1 на клетки A549. Повышенная экспрессия TGF-β1 в ЗНО способствовала ангиогенезу, супрессии иммунной системы, а также выживанию раковых клеток, увеличивая их рост, миграцию и инвазию [45]. Помимо перечисленных выше эффектов, РМ2,5, проникая в клетки опухоли, способны вызывать значимый рост уровня АФК и аутофагию. Впоследствии эти процессы стимулируют миграцию раковых клеток, инвазию и эпителиально-мезенхимальный переход [46, 47].
Таким образом, на основе литературных данных можно заключить, что канцерогенез является результатом взаимодействия генетических, эпигенетических и факторов окружающей среды. Основными моментами патогенеза онкологических заболеваний являются повреждение генома эпителиальной клетки, изменения в метилилирова-нии ДНК, экспрессии микроРНК, моделировании хроматина и посттранскрипционных регуляторов,
Список литературы Роль загрязнения воздуха взвешенными частицами в патогенезе онкологических заболеваний
- Zaridze D.G., Kaprin A.D., Stilidi I.S. Dinamika zabolevaemosti zlokachestvennymi novoobrazovaniyami i smertnosti ot nikh v Rossii. Voprosy onkologii. 2018; 64(5): 578-591.
- Kaprin A.D., Starinskii V.V., Petrova G.V. Zlokachestvennye novoobrazovaniya v Rossii v 2016 godu (zabolevaemost' i smertnost'). M., 2018; 250 s.
- International Agency for Research on Cancer; World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France, 2016. 2019.
- Meshkov N.A. Prioritetnye faktory riska okruzhayushchei sredy v razvitii onkopatologii. Nauchnyi al'manakh. 2016; 5-3(19): 309-318.
- Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., Balakrishnan K., Brunekreef B., Dandona L., Dandona R., Feigin V., Freedman G., Hubbell B., Jobling A., Kan H., Knibbs L., Liu Y., Martin R., Morawska L., Pope C.A. 3rd, Shin H., Straif K., Shaddick G., Thomas M., van Dingenen R., van Donkelaar A., Vos T., Murray C.J.L., Forouzanfar M.H. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017 May; 389(10082): 1907-18. https://doi.org/10.1016/S0140-6736(17)30505-6.
- Lelieveld J., Evans J.S., Fnais M., Giannadaki D., Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015 Sep 17; 525(7569): 367-71. https://doi.org/10.1038/nature15371.
- Jantzen K., Moller P., Karottki D.G., Olsen Y., Beko G., Clausen G., Hersoug L.G., Loft S. Exposure to ultrafine particles, intracellular production of reactive oxygen species in leukocytes and altered levels of endothelial progenitor cells. Toxicology. 2016; 359-360: 11-18. https://doi.org/10.1016/j.tox.2016.06.007.
- Traboulsi H., Guerrina N., Iu M., Maysinger D., Ariya P., Baglole C.J. Inhaled Pollutants: The Molecular Scene behind Respiratory and Systemic Diseases Associated with Ultrafine Particulate Matter. Int J Mol Sci. 2017 Jan 24; 18(2): 243. https://doi.org/10.3390/ijms18020243.
- Rakhmanin Yu.A., Levanchuk A.V. Gigienicheskaya otsenka atmosfernogo vozdukha v raionakh s razlichnoi stepen'yu razvitiya dorozhnoavtomobil'nogo kompleksa. Gigiena i sanitariya. 2016; 95(12): 1117-1121.
- Jia Y.Y., Wang Q., Liu T. Toxicity Research of PM2.5 Compositions In Vitro. Int J Environ Res Public Health. 2017 Feb 26; 14(3): 232. https://doi.org/10.3390/ijerph14030232.
- Li K., Liang T., Wang L. Risk assessment of atmospheric heavy metals exposure in Baotou, a typical industrial city in northern China. Environ Geochem Health. 2016 Jun; 38(3): 843-53. https://doi.org/10.1007/s10653-015-9765-1.
- Treskova Yu.V. Problemy normirovaniya melkodispersnykh chastits v Rossii i za rubezhom. Molodoi uchenyi. 2017; 23: 17-19.
- Yun Y., Gao R., Yue H., Guo L., Li G., Sang N. Sulfate Aerosols Promote Lung Cancer Metastasis by Epigenetically Regulating the Epithelial-to-Mesenchymal Transition (EMT). Environ Sci Technol. 2017 Oct 3; 51(19): 11401-11. https://doi.org/10.1021/acs.est.7b02857.
- Wong C.M., Tsang H., Lai H.K., Thomas G.N., Lam K.B., Chan K.P., Zheng Q., Ayres J.G., Lee S.Y., Lam T.H., Thach T.Q. Cancer Mortality Risks from Long-term Exposure to Ambient Fine Particle. Cancer Epidemiol Biomarkers Prev. 2016 May; 25(5): 839-45. https://doi.org/10.1158/1055-9965.EPI-15-0626.
- Lamichhane D.K., Kim H.C., Choi C.M., Shin M.H., Shim Y.M., Leem J.H., Ryu J.S., Nam H.S., Park S.M. Lung Cancer Risk and Residential Exposure to Air Pollution: A Korean Population-Based CaseControl Study. Yonsei Med. J. 2017; 58(6): 1111-1118. https://doi.org/10.3349/ymj.2017.58.6.1111.
- Wang Y., Li M., Wan X., Sun Y., Cheng K., Zhao X., Zheng Y., Yang G., Wang L. Spatiotemporal analysis of PM2.5 and pancreatic cancer mortality in China. Environ Res. 2018 Jul; 164: 132-139. https://doi.org/10.1016/j.envres.2018.02.026.
- Gharibvand L., Shavlik D., Ghamsary M., Beeson W.L., Soret S., Knutsen R., Knutsen S.F. The Association between Ambient Fine Particulate Air Pollution and Lung Cancer Incidence: Results from the AHSMOG-2 Study. Environ Health Perspect. 2017 Mar; 125(3): 378-384. https://doi.org/10.1289/EHP124.
- Nagel G., Stafoggia M., Pedersen M., Andersen Z.J., Galassi C., Munkenast J., Jaensch A., Sommar J., Forsberg B., Olsson D., Oftedal B., Krog N.H., Aamodt G., Pyko A., Pershagen G., Korek M., De Faire U., Pedersen N.L., Östenson C.G., Fratiglioni L., Sørensen M., Tjønneland A., Peeters P.H., Bueno-de-Mesquita B., Vermeulen R., Eeftens M., Plusquin M., Key T.J., Concin H., Lang A., Wang M., Tsai M.Y., Grioni S., Marcon A., Krogh V., Ricceri F., Sacerdote C., Ranzi A., Cesaroni G., Forastiere F., Tamayo-Uria I., Amiano P., Dorronsoro M., de Hoogh K., Beelen R., Vineis P., Brunekreef B., Hoek G., Raaschou-Nielsen O., Weinmayr G. Air pollution and incidence of cancers of the stomach and the upper aerodigestive tract in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Int J Cancer. 2018; 143(7): 1632-43. https://doi.org/10.1002/ijc.31564.
- VoPham T., Weaver M.D., Vetter C., Hart J.E., Tamimi R.M., Laden F., Bertrand K.A. Circadian Misalignment and Hepatocellular Carcinoma Incidence in the United States. Cancer Epidemiol Biomarkers Prev. 2018 Jul; 27(7): 719-727. https://doi.org/10.1158/1055-9965.EPI-17-1052.
- Pedersen M., Stafoggia M., Weinmayr G., Andersen Z.J., Galassi C., Sommar J., Forsberg B., Olsson D., Oftedal B., Krog N.H., Aamodt G., Pyko A., Pershagen G., Korek M., De Faire U., Pedersen N.L., Östenson C.G., Fratiglioni L., Sørensen M., Eriksen K.T., Tjønneland A., Peeters P.H., Bueno-de-Mesquita B., Vermeulen R., Eeftens M., Plusquin M., Key T.J., Jaensch A, Nagel G., Concin H., Wang M., Tsai M.Y., Grioni S., Marcon A., Krogh V., Ricceri F., Sacerdote C., Ranzi A., Cesaroni G., Forastiere F., Tamayo I., Amiano P., Dorronsoro M., Stayner L.T., Kogevinas M., Nieuwenhuijsen MJ, Sokhi R., de Hoogh K., Beelen R., Vineis P., Brunekreef B., Hoek G., Raaschou-Nielsen O. Is There an Association Between Ambient Air Pollution and Bladder Cancer Incidence? Analysis of 15 European Cohorts. Eur Urol Focus. 2018 Jan; 4(1): 113-120. https://doi.org/10.1016/j.euf.2016.11.008.
- Hart J.E., Bertrand K.A., DuPre N., James P., Vieira V.M., Tamimi R.M., Laden F. Long-term Particulate Matter Exposures during Adulthood and Risk of Breast Cancer Incidence in the Nurses’ Health Study II Prospective Cohort. Cancer Epidemiol Biomarkers Prev. 2016; 25(8): 1274-6. https://doi.org/10.1158/1055-9965.EPI-16-0246.
- Liu C., Guo H., Cheng X., Shao M., Wu C., Wang S., Li H., Wei L., Gao Y., Tan W., Cheng S., Wu T., Yu D., Lin D. Exposure to airborne PM2.5 suppresses microRNA expression and deregulates target oncogenes that cause neoplastic transformation in NIH3T3 cells. Oncotarget. 2015 Oct 6; 6(30): 29428-39. https://doi.org/10.18632/oncotarget.5005.
- Liu X., Chen Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J Transl Med. 2017; 15(1): 207. https://doi.org/10.1186/s12967-017-1306-5.
- Li R., Zhou R., Zhang J. Function of PM2.5 in the pathogenesis of lung cancer and chronic airway inflammatory diseases. Oncol Lett. 2018 May; 15(5): 7506-14. https://doi.org/10.3892/ol.2018.8355.
- Cho C.C., Hsieh W.Y., Tsai C.H., Chen C.Y., Chang H.F., Lin C.S. In Vitro and In Vivo Experimental Studies of PM2.5 on Disease Progression. Int J Environ Res Public Health. 2018 Jul 1; 15(7): 1380. https://doi.org/10.3390/ijerph15071380.
- Ekoue D.N., He C., Diamond A.M., Bonini M.G. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim Biophys Acta Bioenerg. 2017 Aug; 1858(8): 628-32. https://doi.org/10.1016/j.bbabio.2017.01.006.
- Tan C., Lu S., Wang Y., Zhu Y., Shi T., Lin M., Deng Z., Wang Z., Song N., Li S., Yang P., Yang L., Liu Y., Chen Z., Xu K. Long-term exposure to high air pollution induces cumulative DNA damages in traffic policemen. Sci Total Environ. 2017 Sep; 593-594: 330-336. https://doi.org/10.1016/j.scitotenv.2017.03.179.
- Sas-Nowosielska H., Pawlas N. Heavy metals in the cell nucleus - role in pathogenesis. Acta Biochim Pol. 2015; 62(1): 7-13. https://doi.org/10.18388/abp.2014_834.
- Toyooka S., Mitsudomi T., Soh J., Aokage K., Yamane M., Oto T., Kiura K., Miyoshi S. Molecular oncology of lung cancer. Gen Thorac Cardiovasc Surg. 2011 Aug; 59(8): 527-37. https://doi.org/10.1007/s11748-010-0743-3.
- Zhou W., Tian D., He J., Wang Y., Zhang L., Cui L., Jia L., Zhang L., Li L., Shu Y., Yu S., Zhao J., Yuan X., Peng S. Repeated PM2.5 exposure inhibits BEAS-2B cell P53 expression through ROS-Akt-DNMT3B pathway-mediated promoter hypermethylation. Oncotarget. 2016 Apr 12; 7(15): 20691-703. https://doi.org/10.18632/oncotarget.7842.
- Yang B., Chen D., Zhao H., Xiao C. The effects for PM2.5 exposure on non-small-cell lung cancer induced motility and proliferation. Springerplus. 2016 Dec 1; 5(1): 2059. https://doi.org/10.1186/s40064-016-3734-8.
- Zhou Y.H. RE: Fine Particle Pollution, Alanine Transaminase, and Liver Cancer: A Taiwanese Prospective Cohort Study (REVEAL-HBV). J Natl Cancer Inst. 2016 Aug 31; 109(1). https://doi.org/10.1093/jnci/djw184.
- Zhang Q., Luo Q., Yuan X., Chai L., Li D., Liu J., Lv Z. Atmospheric particulate matter2.5 promotes the migration and invasion of hepatocellular carcinoma cells. Oncol Lett. 2017 May; 13(5): 3445-50. https://doi.org/10.3892/ol.2017.5947.
- Deng H., Eckel S.P., Liu L., Lurmann F.W., Cockburn M.G., Gilliland F.D. Particulate matter air pollution and liver cancer survival. Int J Cancer. 2017 Aug 15; 141(4): 744-749. https://doi.org/10.1002/ijc.30779.
- Sancini G., Farina F., Battaglia C., Cifola I., Mangano E., Mantecca P., Camatini M., Palestini P. Health risk assessment for air pollutants: alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). PLoS One. 2014 Oct 8; 9(10): e109685. https://doi.org/10.1371/journal.pone.0109685.
- Zhou Z., Liu Y., Duan F., Qin M., Wu F., Sheng W., Yang L., Liu J., He K. Transcriptomic Analyses of the Biological Effects of Airborne PM2.5 Exposure on Human Bronchial Epithelial Cells. PLoS One. 2015 Sep 18; 10(9): e0138267. https://doi.org/10.1371/journal.pone.0138267.
- Weichenthal S., Crouse D.L., Pinault L., Godri-Pollitt K., Lavigne E., Evans G., van Donkelaar A., Martin R.V., Burnett R.T. Oxidative burden of fine particulate air pollution and risk of cause-specific mortality in the Canadian Census Health and Environment Cohort (CanCHEC). Environ Res. 2016 Apr; 146: 92-9. https://doi.org/10.1016/j.envres.2015.12.013.
- Wan R., Mo Y., Zhang Z., Jiang M., Tang S., Zhang Q. Cobalt nanoparticles induce lung injury, DNA damage and mutations in mice. Part Fibre Toxicol. 2017 Sep 18; 14(1): 38. https://doi.org/10.1186/s12989-017-0219-z.
- Drifka C.R., Tod J., Loeffler A.G., Liu Y., Thomas G.J., Eliceiri K.W., Kao W.J. Periductal stromal collagen topology of pancreatic ductal adenocarcinoma differs from that of normal and chronic pancreatitis. Mod Pathol. 2015 Nov; 28(11): 1470-80. https://doi.org/10.1038/modpathol.2015.97.
- Okladnikova E.V., Ruksha T.G. Rol' mikrookruzheniya v razvitii i progressii raka podzheludochnoi zhelezy. Sibirskii onkologicheskii zhurnal. 2016; 15(3): 82-90. https://doi.org/10.21294/1814-4861-2016-15-3-85-92.
- Wei H., Liang F., Cheng W., Zhou R., Wu X., Feng Y., Wang Y. The mechanisms for lung cancer risk of PM2.5: Induction of epithelialmesenchymal transition and cancer stem cell properties in human non-small cell lung cancer cells. Environ Toxicol. 2017 Nov; 32(11): 2341-51. https://doi.org/10.1002/tox.22437.
- Lykov A.P., Kabakov A.V., Bondarenko N.A., Poveshchenko O.V., Raiter T.V., Kazakov O.V., Strunkin D.N., Surovtseva M.A., Poveshchenko A.F., Konenkov V.I. Opukhol'-assotsiirovannye mezenkhimnye stvolovye kletki pri khimicheski indutsirovannom rake molochnoi zhelezy u krys Wistar. Sibirskii onkologicheskii zhurnal. 2019; 18(1): 56-64. https://doi.org/10.21294/1814-4861-2019-18-1-56-64.
- Lacerda L., Debeb B.G., Smith D., Larson R., Solley T., Xu W., Krishnamurthy S., Gong Y., Levy L.B., Buchholz T., Ueno N.T., Klopp A., Woodward W.A. Mesenchymal stem cells mediate the clinical phenotype of inflammatory breast cancer in a preclinical model. Breast Cancer Res. 2015 Mar 20; 17(1): 42. https://doi.org/10.1186/s13058-015-0549-4.
- Miyazono K., Ehata S., Koinuma D. Tumor-promoting functions of transforming growth factor-β in progression of cancer. Ups J Med Sci. 2012 May; 117(2): 143-52. https://doi.org/10.3109/03009734.2011.638729.
- Shevchenko V.E., Bryukhovetskii I.S., Nikiforova Z.N., Kovalev S.V., Kudryavtsev I.A., Arnotskaya N.E. Transformiruyushchii faktor rosta beta-1 v onkogeneze adenokartsinomy legkogo cheloveka. Uspekhi molekulyarnoi onkologii. 2017; 4(3): 67-74.
- Deng X., Feng N., Zheng M., Ye X., Lin H., Yu X., Gan Z., Fang Z., Zhang H., Gao M., Zheng Z.J., Yu H., Ding W., Qian B. PM2.5 exposureinduced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim Biophys Acta Gen Subj. 2017 Feb; 1861(2): 112-125. https://doi.org/10.1016/j.bbagen.2016.11.009.
- Rachkovskii K.V., Vtorushin S.V., Stepanov I.V., Naumov S.S., Zav'yalova M.V., Afanas'ev S.G. Rol' protsessov autofagii i angiogeneza pri kolorektal'nom rake. Sibirskii onkologicheskii zhurnal. 2017; 16(6): 86-92. https://doi.org/10.21294/1814-4861-2017-16-6-86-92.


