Role of ascorbic acid against pathogenesis in plants
Автор: Taqi Ahmed Khan, Mohd Mazid, Firoz Mohammad
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.7, 2011 года.
Бесплатный доступ
Plants vary considerably in their physiological response to various kinds of environmental stress. To prevent damage caused by pathogenic attack and to acclimate to change in their environment, plants have evolved direct and indirect mechanism for sensing and responding to pathogenic stimuli. Ascorbic acid (AA) is found in all eukaryotes including animals and plants and lack completely in prokaryotes except cyanobactaria, have been reported to have a small amount. AA has now gained significant place in plant science, mainly due to its properties (antioxidant and cellular reductant etc.), and multifunctional roles in plant growth, development, and regulation of remarkable spectrum of plant cellular mechanisms against environmental stresses. As it is evident from the present review, recent progress on AA potentiality in tolerance of plants to pathogenic attack has been impressive to a greater extent. AA produced in plants as indirect response against pathogenic attack at different sites in plants and its intertwined network cause changes in nuclear gene expression via retrograde signaling pathways, or even into systemic responses, all of which are associated with pathogenic resistance. Indeed, AA plays an important role in resistance to pathogenesis.
Ascorbic acid, pathogenesis, plant hormones, ros signaling, pr protein
Короткий адрес: https://sciup.org/14323533
IDR: 14323533
Текст обзорной статьи Role of ascorbic acid against pathogenesis in plants
AA is a small antioxidant molecule, vitamin C (L-ascorbic acid), fulfils essential metabolic functions in the life of animals and plants. It is found in plants, animals and single cell organisms. Some fungi can synthesize erythro-ascorbic acid, a vitamin-C analogue with similar metabolic functions. Among prokaryotes, only blue green algae have been reported to have a small amount of AA (Arrigoni and De Tullio, 2002). As well as it is a small, water soluble, reductone sugar acid with antioxidant properties and acts as a primary substrate in the cyclic pathway for enzymatic detoxification of a number of reactive oxygen species (ROS) such as H2O2, and many other, harmful to normal functioning of plant metabolism. AA is an essential cofactor for α-ketoglutarate-dependent dioxygenases (e.g. prolyl hydroxylases) important for formation of covalent adducts with electrophilic secondary metabolites in plants (Traber and Stevens, 2011). In addition, it acts directly to neutralize superoxide radicals (O2∙-), singlet oxygen (O∙-) or hydroxyl radical (OH∙-) simply by acting as a secondary antioxidant during reductive recycling of the oxidized form of α-tocopherol (Noctor and Foyer, 1998). L-AA serves as a co-factor for many enzymes (Arrigioni and De-Tullio, 2000) and it contributes to the detoxification of ROS (Smirnoff and Wheeler, 2000; Conklin, 2001; Conklin and Barth, 2004). This antioxidant activity of AA is associated with resistance to oxidative stress and longevity in plants. Moreover, the endogenous level of AA has recently been suggested to be important in the regulation of developmental senescence and plant defense against pests (Pastori et al., 2003; Barth et al., 2004; Pavet et al., 2005). A recent plethora of evidences suggests that it may play a role in protection of plant against several environmental stresses such as heavy metal action, salinity, pesticides, ozone, UV-B and pathogenesis (Shalata and Neumann, 2001; Vwioko et al., 2008).
Plants under environmental stresses
Plants respond to abiotic and biotic stress factors in environment. These include osmotic stress salinity, heavy metal action, wounding, changes in amount and intensity of UV-B light, and pathogen and pest attack. Abiotic stress factors such as metal action, salinity and extremes of light have long been known as major limitations to crop productivity (Boyer, 1982). Oxidative stress, which frequently accompanies high temperature, salinity or heavy metal stress may cause denaturation of functional and structural proteins. Plants respond to the stress in part by modulating gene expression, which eventually leads to the restoration of cellular homeostasis, detoxification of toxins and recovery of growth. As a consequence, these diverse environmental stresses often activate similar cell signalling pathways and cellular responses, such as the production of stress proteins, up regulation of antioxidant and accumulation of compatible solutes. (Shinozaki and Yamaguchi-Shinozaki, 2000; Zhu 2001). Biochemical adaptation in plants involves various changes in the biochemistry of the cell. These changes include the evolution of new metabolic pathways, the accumulation of low molecular weight metabolites, the synthesis of special proteins, detoxification mechanisms and changes in plant hormone levels. Adaptation represents the ability of living organisms to fit into a changing environment, at the same time improving its chances of survival and reproducing itself (Fujita et al., 2006; Smirnoff, 1995). The complex plant responses to pathogenic stresses, which involves expression of many genes, biochemical and molecular mechanisms, is schematically represented in figure 1.
Cross talk between ascorbic acid and plant hormones during pathogenesis
Coordinated regulating of plant development in response to the environment requires a cross talk between hormones and the pathway initiated by the external cues. Plant responses to stress can be viewed as being orchestrated through a network that integrates signalling pathways characterized by the production of ET, JA, SA, ABA and GA3. Cross tolerance, the induced tolerance to additional biotic and abiotic stresses after exposure to a specific oxidative stress, is a widespread defense mechanism in higher plants. The identified regulatory step in the network involves transcription, protein interaction and targeted protein damage. In plants, the mitogen activated protein kinase (MAPK) cascade plays a key role in various abiotic and biotic stress responses and in phyto-hormone responses that include ROS signalling (Fujita et al., 2006). Molecular and genetic studies present the notion that the cross talk between AA and various plant hormones exists. It includes alternation in the expression of hormones biosynthetic genes and/or signalling intermediates. Studies of such interaction have examined changes in endogenous levels of other hormones, described synergistic effects with exogenous AA treatment.
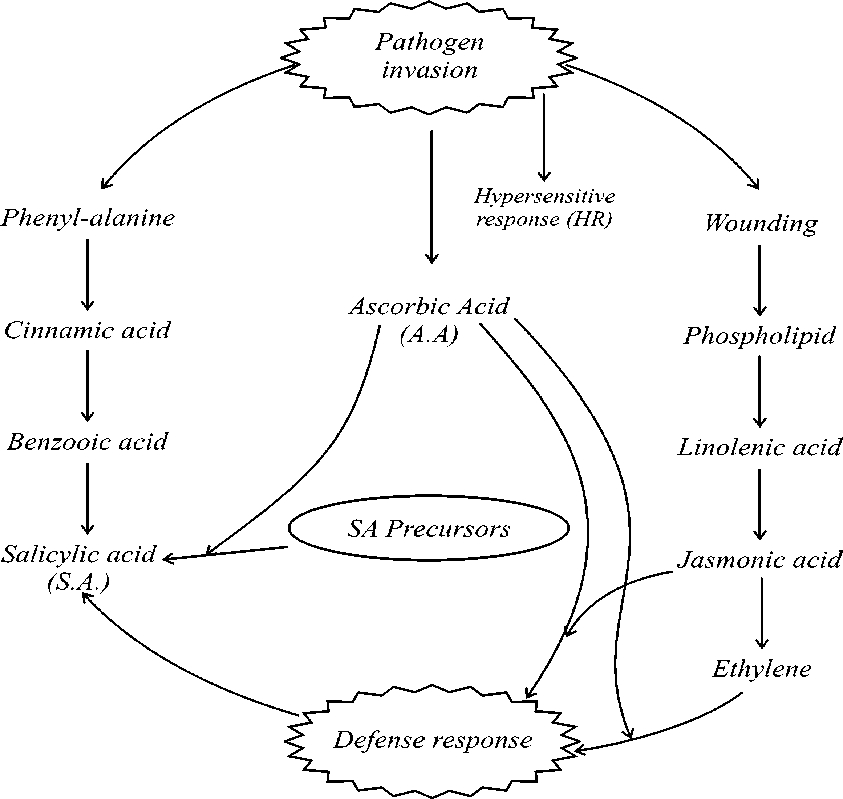
Figure 1. Activation of defence response involving SA and JA against pathogen attack.
The Combined synergistic effect of AA induced ABA and GA3 in signal transudation has been extensively studied using the cereal aleurone layer (Ritchie and Gilroy, 1998; Lovegrove and Hooley, 2000). Evidence suggests that these hormones are perceived at the plasma membrane and that phospholipases and G-proteins are involved in the early signaling events. Down streams are Ca2+ and calmodulin, which, in turn, target various ion channels, protein kinases and phosphatases. Through these systems, GA3 and ABA regulate the expression of a number of proteins in the aleorone layer, including α-amylase. However, GA can also promote cell death in this system (Bethke et al., 1999), a protectant involves AOS, and notably H2O2 which is produced in glyoxysomes by the activity of a flavin-containing acyl CoA oxidase. ABA can present cell death by promoting high activities of enzymes that destroy ROS. ABA represents a good example of a combination of genetic, molecular and biochemical approaches can lead to the elucidation of a complex biosynthetic pathway. ABA biosynthesis increases when plant cells lose turgor, raising the question of which step in the pathway is activated by various environmental stresses. The promoters of the peroxidases respond to ABA and to redox signals (Fryer et al., 2003; Baier et al., 2004). APX is synergistically induced by ABA and oxidative stress (Fryer et al., 2003) while other peroxidases (2CPA) is oxidatively induced by wounding and photo-oxidation. Stress via one MAPK2 but strongly suppressed by ABA via an antagonistically responding MAPK2 (Baier et al., 2004). Studies of ET signaling in Arabidopsis indicate that receptor gene families in plants may function similar to many of their animal counterparts, increasing their flexibility at responding to stressful environments. Also, studies in the past few years have contributed to the accumulating evidence that ET signalling has a substantial role in disease resistance (Chang and Sockeye, 1999). The bacterial spot disease symptoms were greatly reduced in one line of tomato deficient in ET synthesis (ACC deaminase/and two lines impaired in ET perception (Nr/Nr and 14893) in comparison to the wild type cultivars (Lund et al., 1998).
Response to pathogenesis
Plants are attacked by many diseases-causing organism including bacteria, fungi, viruses and nematodes. These pathogens cause large crop losses and probably since the agriculture have contributed to human hunger and malnutrition. The centre of plant disease is thus of fundamental importance and is a major objective of plant breeding and pathology programs and the agriculture chemical industry. Plant resist pathogens attacks both with performed defenses such as antimicrobial secondary compounds and by including defense responses (Hammond-Kosak and Jones, 2000; Heath, 2000). Inducible defences can be activated upon recognition of general elicitors such as bacterial flagellin and even host cell fragments released by pathogen damage (Gomez-Gamez and Boller, 2002). However plants have developed a variety of sophisticated defense mechanisms to cope with an environment in which many different microbes live by detecting proteins producing infections. This protein referred to as effectors are recognised by plant disease resistance plants in a highly specific manner first described genetically as the gene-for-gene interaction (Flor, 1971).
The identification of many R genes and in many cases their corresponding effectors proteins, has accelerated research into the molecular bases of gene-for-gene disease resistance (Cohn et al., 2001; Martin, 1999). Plant immunity is based on a complex response that is highly flexible in its capacity to recognize and counteract different invaders. To combat invasion by microbial pathogens and herbivores insects effectively, plants make use of pre-existing physical and chemical barrier as well as inducible defense mechanism that become activated upon attack. Apart from reacting locally, plants can mount a systemic response that established an enhanced defensive capacity in tissue distant from the site of primary attack. The activation of plant defense state is correlated with a stimulation of three pathogenesis related (PR) proteins, such as chitinase, β-1, 3-glucanase and peroxidase which are molecular markers of SAR (systemic acquired resistance) (Roth et al., 2000), which is triggered by necrotizing pathogens, induced systemic resistance (ISR), which is activated upon colonization of roots by selected strains of non-pathogenic rhizobacteria and wound induced defense, which is typically elicited upon tissue damage such as that caused by feeding insects. Induced defense responses are regulated by a network of inter connecting signal transduction pathways in which the hormonal signals SA, JA and ET play a major role, and other hormones such as BRs and ABA also be involved (Dong, 1998). Results of Dias et al (2011) confirmed that AA is the main precursor of oxalic acid in susceptible and resistant cocao (Theobroma cocao L.) infected by the hemibiotrophic fungus Moniliophthora perniciosa. Oxalic acid help in synthesis of H2O2 in plant-pathogen interaction play a role in inhibition of growth of biotrophic pathogens and could help in prevention of the infection/colonization process of plants by necrotrophic pathogens. Katay et al (2011) reported that the effect of ascorbigen and 1-methyl ascorbigen on the disease resistance in bean against fungal pathogen Uromyces phaseoli and also suggests that effectiveness of protection depended on the dosage of the applied 1-methylascorbigen and on the time interval between the chemical pretreatment and inoculation. Studies of Bala and Thukral (2011) established that AA along with glycerol found to be most effective in increasing the phytoremediating potential of spirodela polyrrhiza L. In addition Belide et al (2011) also suggested that hyperhydration and necrosis of Agrobacterium-infected cotyledons found to be effectively controlled by using AA along with L-cysteine and iota-carrageenan.
Indeed, AA acting simply as an antioxidant in the apoplastic space, but however it is to be involved in a complex phytohormone mediated signaling network that ties ozone and pathogen responses and influences the onset of senescence. Now it has become increasingly clear that AA function is intertwined in a complex network that meshes the plant response to pathogens and the onset of senescence. Acute exposure to ozone (150-300 p.p.b.) for a short time period (4-6h) cause necrotic lesion on leaves and induce plant reaction that resemble the hypersensitive response (HR), suggesting similarities between ozone and pathogen induce responses (Kangasjarvi et al., 1994; Sharma and Davies, 1994; Sandermann et al., 1998; Rao-Koch and Davis, 2000a). In a large number of studies associated with stress mitigation/tolerance in plants by adding the different natural and synthetic compounds such as PPGs (phenypropanoid glycosides), AA is used as a reference compound (Lopez-Munguia et al., 2011). Similarily, studies of Da Silva et al (2011) suggesting that in phosphonolibdenium assay, the Anadenanthera
Colubrina (ACHE) Libidibia ferrea (LFHE) and Pityrocarpa moniliformis (PMHE) showed increased antioxidant activity in relation to AA against ROS respectively. HR is a form of PCD in plant. HR is considered a part of a complex defense response to microbial pathogens in which death of host cells at the site of a virulent pathogen entry occurs within a few hours of pathogen attacks (incompatible interaction i.e. plant is resistant to the invading pathogen) (Crute et al., 1994; Katagiri et al., 2002). Pathogen-induced HR is a rapid oxidative burst at the site of microbial infection. Moreover, ROS production during HR (and recognition of the invading pathogen), are mediated by a NADPH oxidase localized in the plasma membrane (Levine et al., 1994; Rao and Davis, 2001) and induced signal transduction network appear to involve a MAPK signaling cascade pathways (Vranova et al., 2002).
Generally, ROS formed during HR (Leon et al., 1995; Ryals et al., 1996) activate ethylene, SA and JA signaling pathways, serve to induce defense gene expression to counteract the invading pathogen and to minimize lesion formation in plants exposed to oxidative stress like O3-ET synthesis and emission increase in plants exposed to ozone (Overmyer et al., 2000). ET triggers PCD in the accelerated cell death mutant (acd1) (Greenberg and Ausubel, 1993), and is involved in regulating PCD in plant pathogen interaction (Bent et al., 1992). SA signaling is regulated for ozone-induced cell death responses (Overmyer et al., 2003), induction of pathogens related proteins (PR) (Greenberg, 1996; Dong, 1998; Dempsey et al., 1999), and SAR (SAR increased to subsequent infection by a broad range of pathogens) (Ryals et al., 1996; Shah and Klessing, 1999). JA biosynthesis is induced in ozone treated Arabidopsis and hybrid poplar plants within several hour of treatment (Koch et al., 2000). Treatment with JA has been shown to reduce the extent of cell death in tobacco (Orvar et al., 1997). Arabidopsis mutants constitutively expressing JA, such as CEV1 are mere resistant to powdery mildew (Ellis and Turner, 2001). These plant hormones do not act independently in response to O3 and/or pathogens but rather in a complex signaling network (Dong, 1998; Rao and Davis, 2001).
Moreover, in a study of Pastori et al (2003), vtc1 (AA deficient mutant of Arabidopsis) is subjected to a large-scale microarray analysis to identify genes that are differentially expressed in the AA-deficient mutant relative to the wild type in the absence of added ROS. One hundred and seventy one transcripts that were either increased or decreased in vtc1 versus wildtype were identified. Specifically, the most dramatic changes in transcript abundance were observed in genes involved in plant defense responses against pathogenesis. PR proteins such as PR-1, PR-2, PR-5 and lytic enzymes, such as β-glucanases and chitinases are significantly increased in vtc 1 compared to wild type. Many disease resistance proteins of plants detect the presence of disease-causing bacteria, viruses or fungi by recognizing specific pathogen effectors molecule that is produced during the infection process. Effectors are often pathogen proteins that probably evolved to subvert various host processes for promotion of the pathogen life cycle. Five classes of effectors-specific R proteins are known and their sequences suggest the roles in both effector recognition and signal transduction. Although some R proteins may act as primary receptor of pathogens effectors proteins, most appear to play indirect roles in this process. The function of various R proteins requires phosphorylation, protein degradation or specific localization within the host cell. Some signaling components are shared by many R gene pathways whereas others appear to be pathway specific. New technologies arising from the genomics and proteomics revolution will greatly expand our ability to investigate the role of R proteins in plant disease resistance.
In contrast, major antioxidant enzymes such as CAT and APX are largely unaffected in vtc1. Interestingly, when the AA content is artificially elevated by feeding with 10 mM ascorbate resulting in A A levels similar to wild type treated equally, the transcript abundance is reversed in vtc1, indicating that low AA induces defense response and high ascorbate suppresses the induction of defense genes. Thus, one would predict that constitutively induced defense gene expression in vtc 1 might correlate with resistance to pathogens. Barth et al (2004) tested this hypothesis with vtc 1 and wild type using the bacterium Pseudomanas syringae Pv maculicola ES4326 and the virulent fungus Peronospore parasitica Pv Noco, the cause of downy mildew and found that induction of the PR genes in vtc1 may be due to an SA-dependent pathway as the total SA content is found to be approximately six fold higher in vtc 1 than in wild type. In addition, hyphal development and fungal conidiophores production is significantly reduced in vtc1 and also vtc 2, a second non-allelic AA-deficient mutant. PR-1 and PR-5 proteins are of higher abundance in vtc 1 than in wild type, particularly within the first 24h postinoculation with Pseudomanas syringae as revealed by western blot analysis. Virulent pathogens have been repeated to induce non-specific resistance responses via the induction of SA synthesis and PR proteins (Glazebrook et al., 1997). However, the defense responses elicited by virulent pathogens are activated more slowly and/or they are activated lower levels than the defense response induced by virulent pathogens.
Table 1. Plant disease resistance (R) proteins.
|
Class |
R proteins |
Plant |
Pest/pathogen |
Effector |
Reference |
|
1 |
Pto |
Tomato |
Pseudomans syringae |
Avr pto |
Kim et al. 2002 |
|
2 |
Bs2 |
Pepper |
Xanthomonas compestris |
AvrBs2 |
Tai et al. 1999 |
|
3 |
RPP1 RPS4 |
Arabidopsis Arabidopsis |
Peronospora parasitica P. Syringae |
AvrRps4 |
Doods et al. 2001 Gassmann et al. 1999 |
|
4 |
CF-2c |
Tomato |
Cladosporium fulivum |
Avr2 |
Luderer et al. 2002 |
|
5 |
Xa21 |
Rice |
Xanthomonas oryzae |
- |
Song et al. 1995 |
|
6 |
RRS1-R |
Arabidopsis |
Raestonia Solanacearum |
- |
Deslandes et al. 2002 |
Furthermore, PR gene induction in the AA deficient mutant may occur via altered SA-dependent signaling. However, these are also evidence for the possible involvement of other phytohormone signaling pathways (Pastori et al., 2003). Many recent studies suggest the specific requirement of AA as a cofactor for the activity of 2-oxo acid-dependent dioxygenases, a class of enzymes that includes those regulating the synthesis of hydroxyl proline containing proteins and hormones in plants (Arrigoni and De Tullio, 2000). As already stated before that studies of Conklin and Barth (2004) evidence that AA involved as a cofactor in the synthesis of ABA, GA, ET and AA-dependent dioxygenases are involved in ABA biosynthesis. Specifically, NCED, a dioxygenases catalyzing the formation of Xanthoxin, the precursor of ABA, can be activated before addition of both AA and Fe3+ (Schwartz et al., 1997). ABA has been demonstrated to induce PR genes in several other plant species, such as in rice (Agarwal et al., 2001) and in Lithospermum (Yu et al., 1999). AA is also strictly required by some enzymes that are involved in GA biosynthesis (Arrigoni and De Tullio, 2000). A role of GA in pathogen defense has been suggested, for example, in tomato and in arbuscular mycorrhizia plants of linum Usitatissimum when infected by fungal pathogens (Dugassa et al., 1996). ET like SA, ABA and GA also plays a role in the pathogen response and specifically in the induction of PR genes. In ET biosynthesis, AA is required for 1-aminocyclopropane-1-carboxylate (ACC) oxidase that forms ET (Dong et al., 1992) (Table 1). In last, taken together, analysis of the vtc1 mutant suggests that AA affects cell signaling during pathogenesis induced resistance. Presumably, the availability and /the redox status of AA regulate enzyme activity directly or modulate redox-sensitive proteins which trigger signaling cascades (Pignocchi and Foyer, 2003). Thereby, AA modulates the content of several signaling molecules like SA, ABA, ET and GA.
CONCLUSION
AA can act efficiently in plants as immunomodulators when applied at the appropriate concentration and the current stage of plant development. Ascorbate is implicated in plant responses to biotic stresses and to undergo profound changes in plants interacting with pathogens. AA regulated stress response as a result of a complex sequence of biochemical reactions such as activation or suppression of key enzymatic reactions, induction of stress responsive proteins synthesis, and the production of various chemical defense compounds. In addition, an attempt has been made to connect some very intriguing observations that have been reported for the AA-deficient mutant vtc1 in terms of some few developmental phenomenons. In addition, AA also open up new approaches for plant resistance against hazardous environmental conditions like pathogenesis. However, there are obviously still large gaps to fill in order to elucidate the precise role of A A in enhancing the tolerance of plant to pathogenic stress during development of plant systems.
ACKNOWLEDGEMENTS
The authors are highly thankful for the facilities obtained at AMU Aligarh. Financial support from the Department of Science and Technology, New Delhi in the form of project (SR/FT/LS-087/2007) is gratefully acknowledged.
REFRENCES
Agrawal, G.K., Rakwal, R., Jwa, N.S., Agrawal, V.P. (2001). Signaling molecules and blast pathogen attack activates rice OsPR1a and OsPR1b genes: a model illustrating components participating during defense/stress response. Plant Physiology and Biochemistry , 39 : 1095-1103.
Arrigioni, O., De Tullio, M.C. (2000). The role of ascorbic acid in cell metabolism: between gene directed functions and unpredictable chemical reactions. Journal of plant physiology, 157 : 481-488.
Arrigioni, O., De Tullio, M.C. (2002). Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta, 1569 : 1-9.
Baier, M., Stroher, E., Dietz, K.-J. (2004). The acceptor availability at photosystem I and ABA control nuclear expression of 2-Cys peroxiredoxin-A in Arabidopsis thaliana . Plant Cell Physiology , 45 : 997–1006.
Bala, R., Thukral, A.K. (2011). Phytoremediation of Cr(VI) by Spirodela polyrrhiza (L.) Schleiden employing reducing and chelating agents. Int. J. Phytoremediation , 13 (5): 465-491.
Barth, C., Moeder, W., Klessig, D.F., Conklin, P.L. (2004). The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiology , 134 : 1784–1792.
Bent, A.F., Innes, R.W., Ecker, J.R., Staskawicz, B.J. (19920. Disease development in ethyleneinsensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Molecular Plant Microbe Interactions , 5 : 372-378.
Bethke, P.C., Lonsdale, J.E., Fath, A., Jones, R.L. (1999). Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell , 11 : 1033-1045.
Boyer, J.S. ( 1982). Plant productivity and environment. Science , 218 : 443–448.
Chang, C., Shockey, J.A. (1999). The ethylene response pathway. Curr. Opin. Plant Biol ., 2 : 352–358.
Cohn, J., Sessa, G., Martin, G.B. (2001). Innate immunity in plants. Curr. Opin. Immunol ., 13 : 55-62.
Conklin, P.L. (2001). Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant, cell and Environment, 24 : 383-394.
Conklin, P.L., Barth, C. (2004). Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant, cell and Environment, 27 : 959-971.
Crute, I., Beynon, J., Dangl, J.L., Holub, E.B., Mauch-Mani, B., Slusarenko, A., Staskawicz, B., Ausubel, F.M. (1994). Microbial pathogenesis of Arabidopsis , in: Meyerowitz, E.M., Somerville, C.R. (eds.), Cold Spring Harbor Laboratory Press, Plainview, NY, pp. 705–747.
Da Silva, L.C., da Silva, C.A. Junior, de Souza, R.M., Jose Macedo, A., Da Silva, M.V., Dos Santos Correia, M.T. (2011). Comparative analysis of the antioxidant and DNA protection capacities of Anadenhantera colubrina,
Libidibia ferrea and Pityrocarpa moniliformis fruits. Food Chem. Toxicol ., (in press).
Dempsey, D.A., Shah, J., Klessig, D.F. (1999). Salicylic acid and disease resistance in plants. Critical Reviews in Plant Sciences , 18 : 547– 575.
Dias, C.V., Mendes, J.S., Dos Santos, A.C., Pirovani, C.P., da Silva Gesteira, A., Micheli, F., Gramacho, K.P., Hammerstone, J., Mazzafera, P., de Mattos Cascardo, J.C. (2011). Hydrogen peroxide formation in cacao tissues infected by the hemibiotrophic fungus Moniliophthora perniciosa. Plant Physiol Biochem ., (in press).
Dong, J.G., Fernandez-Maculet, J.C., Yang, S.F. (1992). Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proceedings of the National Academy of Sciences of the USA , 89 : 97899793.
Dugassa, G.D., Von Alten, H., Schonbeck, F. (1996). Effects of arbuscular mycorrhiza (AM) on health of Linum usitatissimum L. infected by fungal pathogens. Plant and Soil , 185 : 173182.
Ellis, C., Turner, J.G. (2001). The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell , 13 : 10251033.
Flor, H. (1971). Current status of the gene-for-gene concept. Annu. Rev. Phytopathol ., 9 : 275–296.
Fryer, M.J., Ball, L., Oxborough, K., Karpinski, S., Mullineaux, P.M., Baker, N.R. (2003). Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organization of Arabidopsis leaves. Plant Journal , 33 : 691–705.
Fujita, M., Fujita, Y., Noutoshi, Y., Takahashi, F., Narusaka, Y., Yamaguchi-Shinozaki, K., Shinozaki, K. (2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol ., 9 : 436–442.
Glazebrook, J., Zook, M., Mert, F., Kagan, I., Rogers, E.E., Crute, I.R., Holub, E.B., Hammerschmidt, R., Ausubel, F.M. (1997). Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics , 146 : 381–392.
Gomez-Gomez, L., Boller, T. (2002). Flagellin perception: a paradigm for innate immunity. Trends Plant Sci ., 7 : 251–256.
Greenberg, J.T. (1996). Programmed cell death: a way of life for plants. Proceedings of the National Academy of Sciences of the USA , 93 : 12094–12097.
Greenberg, J.T., Ausubel, F.M. (1993). Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant Journal , 4 : 327–341.
Grimmig, B., Gonzalez-Perez, M.N., Leubner-Metzger, G., et al. (2003). Ozone-induced gene expression occurs via ethylene dependent and independent signalling. Plant Molecular Biology , 51 : 599-607.
Hammond-Kosack, K., Jones, J.D.G. (2000). Response to plant pathogens, in: Buchanan, B., Gruissem, D., Jones, R. (ed.), Biochemistry and Molecular Biology of Plants , Rockville, MD: Am. Soc. Plant Physiol. pp. 1102–1156.
Heath, M.C. (2000). Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol ., 3 : 315–319.
Kangasjarvi, J., Talvinen, J., Utriainen, M., Karjalainen, R. (19940. Plant defence systems induced by ozone. Plant Cell and Environment , 17 : 783–794.
Katagiri, F., Thilmony, R., He, S.Y. (2002). The Arabidopsis thaliana–Pseudomonas syringae interaction, in: Somerville, C.R., Meyerowitz, E.M. (eds.), American Society of Plant Biologists, Rockville, MD, USA, pp. 1–39.
Katay, G., Tyihak, E., Katay, E. (2011). Effect of ascorbigen and 1'-methylascorbigen on disease resistance of bean plants to Uromyces phaseoli. Nat. Prod. Commun ., 6 (5): 611-615.
Knoester, M., Bol, J.F., Vanloon, L.C., Linthorst, H.J.M. (1995). Virus-induced gene-expression for enzymes of ethylene biosynthesis in hypersensitively reacting tobacco. Molecular Plant Microbe Interactions , 8 : 177–180.
Koch, J.R., Creelman, R.A., Eshita, S.M., Seskar, M., Mullet, J.E., Davis, K.R. (2000). Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiology , 123 : 487– 496.
Leon, J., Lawton, M., Raskin, I. (1995). Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiology , 108 : 1673–1678.
Levine, A., Tenhaken, R., Dixon, R., Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell , 79 : 583–593.
Lopez-Munguia, A., Hernandez-Romero, Y., Pedraza-Chaverri, J., Miranda-Molina, A., Regla, I., Martinez, A., Castillo, E. (2011). Phenylpropanoid glycoside analogues: enzymatic synthesis, antioxidant activity and theoretical study of their free radical scavenger mechanism. PLoS One , 6 (6): 201-215.
Lovegrove, A., Hooley, R. (2000). Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci ., 5 : 102–110.
Lund, S.T., Stall, R.E., Klee, H.J., 1998. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10, 371–382.
Martin, G.B. (1999). Functional analysis of plant disease resistance genes and their downstream effectors. Curr. Opin. Plant Biol ., 2 : 273–279.
Noctor, G., Foyer, C.H. (1998). Ascorbate and glutathione; keeping active oxygen control. Annual Review of Plant Physiology and Plant Molecular Biology, 49 : 249-279.
Orvar, B.L., McPherson, J., Ellis, B.E. (1997). Preactivating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. Plant Journal , 11 : 203–212.
Overmyer, K., Brosche, M., Kangasjarvi, J. (2003). Reactive oxygen species and hormonal control of cell death. Trends in Plant Science , 8 : 335– 342.
Overmyer, K., Tuominen, H., Kettunen, R., Betz, C., Langebartels, C., Sandermann, H., Kangasjarvi, J. (2000). Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxidedependent cell death. Plant Cell , 12 : 1849–1862.
Pastori, G.M., Kiddle, G., Antoniw, J., Bernard, S., Veljovic-Jovanovic, S., Verrier, P.J., Noctor, G., Foyer, C.H. (2003). Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell , 15 : 939-951.
Pavet,V., Olmos, E., Kiddle, G., Mowla, S., Kumar, S., Antoniw, J., Alvarez, M.E., Foyer, C.H. (2005). Ascorbic acid deficiency activates cell death and disease resistance responses in
Arabidopsis. Plant Physiology , 139 : 1291– 1303.
Pignocchi, C., Foyer, C.H. (2003). Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Current Opinion in Plant Biology , 6 : 379–389.
Rao, M.V., Davis, K.R. (2001). The physiology of ozone induced cell death. Planta , 213 : 682690.
Rao, M.V., Koch, J.R., Davis, K.R. (2000a). Ozone: a tool for probing programmed cell death in plants. Plant Molecular Biology , 44 : 345–358.
Ritchie, S., Gilroy, S. (1998). Calcium-dependent protein phosphorylation may mediate the gibberellic acid response in barley aleurone. Plant Physiol ., 116 : 765–776.
Roth, U., Friebe, A., Schnabl, H. (2000). Resistance induction in plants by a brassinosteroid-containing extract of Lychnis viscaria L. Zeitschrift Naturforschung , 55 : 552–559.
Ryals, J., Neuenschwander, U., Willits, M., Molina, A., Steiner, H., Hunt, M. (1996). Systemic acquired resistance. Plant Cell , 8 : 1809–1819.
Sandermann, H., Ernst, D., Heller, W., Langebartels, C. (1998). Ozone: an abiotic elicitor of plant defence reactions. Trends in Plant Science , 3 : 47–50.
Schwartz, S.H., Tan, B.C., Gage, D.A., Zeevaart, J.A., McCarty, D.R. (1997). Specific oxidative cleavage of carotenoids by VP14 of maize. Science , 276 : 1872–1874.
Shah, J., Klessig, D.F. (1999). Salicylic acid: signal perception and transduction. Biochemistry and Molecular Biology of Plant Hormones, in: Hooykaas, P.P.J., Hall, M.A., Libbenga, K.R. (eds.), Elsevier Science B, V., Amsterdam, The Netherlands, pp. 513–541.
Shalata, A., Peter, M., Neumann, (2001). Exogenous ascorbic acid (vitamin C) increases resistance of salt stress and reduces lipid peroxidation. Journal of Experimental Botany, 52 (364): 2207-2211.
Sharma, Y.K., Davis, K.R. (1994). Ozone-induced expression of stress-related genes in Arabidopsis thaliana . Plant Physiology , 105 : 1089–1096.
Shinozaki, K., Yamaguchi-Shinozaki, K. (2000). Molecular responses to dehydration and low temperature: difference and cross-talk between two stress signaling pathways. Current Opinion in Plant Biology , 3 : 217–223.
Smirnoff, N. (1995). Environment and Plant Metabolism: Flexibility and Acclimation, BIOS Scientific Publishers Ltd, Oxford.
Smirnoff, N., Wheeler, G.L. (2000). Ascorbic acid in plants: biosynthesis and function. Crit. Rev. Biochem. Mol. Biol ., 35 : 291–314.
Traber, M.G., Stevens, J.F. (2011). Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med ., (in press).
Van-der-Hoorn, R.A., Kruijt, M., Roth, R., Brandwagt, B.F., Joosten, M.H., De Wit, P.J. (2001). Intragenic recombination generated two distinct Cf genes that mediate AVR9 recognition in the natural population of Lycopersicon pimpinellifolium . Proc. Natl. Acad. Sci. USA , 98 : 10493-10498.
Vranova, E., Inze, D., Van Breusegem, F. (2002). Signal transduction during oxidative stress. Journal of Experimental Botany , 53 : 1227– 1236.
Vwioko, E.D., Osawaru, M.E., Eruogun, O.L. (2008). Evaluation of okro ( Abelmoschus esculentus L. Moench.) exposed to paint waste contaminated soil for growth, ascorbic acid and metal concentration. African Journal of General Agriculture , 4 (1): 39-48.
Yu, H.J., Mun, J.H., Kwon, Y.M., Lee, J.S., Kim, S.G. (1999). Two cDNAs encoding pathogenesis-related proteins of Lithospermum erythrorhizon display different expression patterns in suspension cultures. Journal of Plant Physiology, 155: 364–370.
Zhu, J.-K. (2001). Plant salt tolerance. Trends in Plant Science , 6 : 66–71.
Список литературы Role of ascorbic acid against pathogenesis in plants
- Agrawal, G.K., Rakwal, R., Jwa, N.S., Agrawal, V.P. (2001). Signaling molecules and blast pathogen attack activates rice OsPR1a and OsPR1b genes: a model illustrating components participating during defense/stress response. Plant Physiology and Biochemistry, 39: 1095-1103.
- Arrigioni, O., De Tullio, M.C. (2000). The role of ascorbic acid in cell metabolism: between gene directed functions and unpredictable chemical reactions. Journal of plant physiology, 157: 481-488.
- Arrigioni, O., De Tullio, M.C. (2002). Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta, 1569: 1-9.
- Baier, M., Stroher, E., Dietz, K.-J. (2004). The acceptor availability at photosystem I and ABA control nuclear expression of 2-Cys peroxiredoxin-A in Arabidopsis thaliana. Plant Cell Physiology, 45: 997-1006.
- Bala, R., Thukral, A.K. (2011). Phytoremediation of Cr(VI) by Spirodela polyrrhiza (L.) Schleiden employing reducing and chelating agents. Int. J. Phytoremediation, 13(5): 465-491.
- Barth, C., Moeder, W., Klessig, D.F., Conklin, P.L. (2004). The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiology, 134: 1784-1792.
- Bent, A.F., Innes, R.W., Ecker, J.R., Staskawicz, B.J. (19920. Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Molecular Plant Microbe Interactions, 5: 372-378.
- Bethke, P.C., Lonsdale, J.E., Fath, A., Jones, R.L. (1999). Hormonally regulated programmed cell death in barley aleurone cells. Plant Cell, 11: 1033-1045.
- Boyer, J.S. (1982). Plant productivity and environment. Science, 218: 443-448.
- Chang, C., Shockey, J.A. (1999). The ethylene response pathway. Curr. Opin. Plant Biol., 2: 352-358.
- Cohn, J., Sessa, G., Martin, G.B. (2001). Innate immunity in plants. Curr. Opin. Immunol., 13: 55-62.
- Conklin, P.L. (2001). Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant, cell and Environment, 24: 383-394.
- Conklin, P.L., Barth, C. (2004). Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant, cell and Environment, 27: 959-971.
- Crute, I., Beynon, J., Dangl, J.L., Holub, E.B., Mauch-Mani, B., Slusarenko, A., Staskawicz, B., Ausubel, F.M. (1994). Microbial pathogenesis of Arabidopsis, in: Meyerowitz, E.M., Somerville, C.R. (eds.), Cold Spring Harbor Laboratory Press, Plainview, NY, pp. 705-747.
- Da Silva, L.C., da Silva, C.A. Junior, de Souza, R.M., Jose Macedo, A., Da Silva, M.V., Dos Santos Correia, M.T. (2011). Comparative analysis of the antioxidant and DNA protection capacities of Anadenhantera colubrina, Libidibia ferrea and Pityrocarpa moniliformis fruits. Food Chem. Toxicol., (in press).
- Dempsey, D.A., Shah, J., Klessig, D.F. (1999). Salicylic acid and disease resistance in plants. Critical Reviews in Plant Sciences, 18: 547-575.
- Dias, C.V., Mendes, J.S., Dos Santos, A.C., Pirovani, C.P., da Silva Gesteira, A., Micheli, F., Gramacho, K.P., Hammerstone, J., Mazzafera, P., de Mattos Cascardo, J.C. (2011). Hydrogen peroxide formation in cacao tissues infected by the hemibiotrophic fungus Moniliophthora perniciosa. Plant Physiol Biochem., (in press).
- Dong, J.G., Fernandez-Maculet, J.C., Yang, S.F. (1992). Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proceedings of the National Academy of Sciences of the USA, 89: 9789-9793.
- Dugassa, G.D., Von Alten, H., Schonbeck, F. (1996). Effects of arbuscular mycorrhiza (AM) on health of Linum usitatissimum L. infected by fungal pathogens. Plant and Soil, 185: 173-182.
- Ellis, C., Turner, J.G. (2001). The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell, 13: 1025-1033.
- Flor, H. (1971). Current status of the gene-for-gene concept. Annu. Rev. Phytopathol., 9: 275-296.
- Fryer, M.J., Ball, L., Oxborough, K., Karpinski, S., Mullineaux, P.M., Baker, N.R. (2003). Control of ascorbate peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organization of Arabidopsis leaves. Plant Journal, 33: 691-705.
- Fujita, M., Fujita, Y., Noutoshi, Y., Takahashi, F., Narusaka, Y., Yamaguchi-Shinozaki, K., Shinozaki, K. (2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol., 9: 436-442.
- Glazebrook, J., Zook, M., Mert, F., Kagan, I., Rogers, E.E., Crute, I.R., Holub, E.B., Hammerschmidt, R., Ausubel, F.M. (1997). Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics, 146: 381-392.
- Gomez-Gomez, L., Boller, T. (2002). Flagellin perception: a paradigm for innate immunity. Trends Plant Sci., 7: 251-256.
- Greenberg, J.T. (1996). Programmed cell death: a way of life for plants. Proceedings of the National Academy of Sciences of the USA, 93: 12094-12097.
- Greenberg, J.T., Ausubel, F.M. (1993). Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant Journal, 4: 327-341.
- Grimmig, B., Gonzalez-Perez, M.N., Leubner-Metzger, G., et al. (2003). Ozone-induced gene expression occurs via ethylene dependent and independent signalling. Plant Molecular Biology, 51: 599-607.
- Hammond-Kosack, K., Jones, J.D.G. (2000). Response to plant pathogens, in: Buchanan, B., Gruissem, D., Jones, R. (ed.), Biochemistry and Molecular Biology of Plants, Rockville, MD: Am. Soc. Plant Physiol. pp. 1102-1156.
- Heath, M.C. (2000). Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol., 3: 315-319.
- Kangasjarvi, J., Talvinen, J., Utriainen, M., Karjalainen, R. (19940. Plant defence systems induced by ozone. Plant Cell and Environment, 17: 783-794.
- Katagiri, F., Thilmony, R., He, S.Y. (2002). The Arabidopsis thaliana-Pseudomonas syringae interaction, in: Somerville, C.R., Meyerowitz, E.M. (eds.), American Society of Plant Biologists, Rockville, MD, USA, pp. 1-39.
- Katay, G., Tyihak, E., Katay, E. (2011). Effect of ascorbigen and 1'-methylascorbigen on disease resistance of bean plants to Uromyces phaseoli. Nat. Prod. Commun., 6(5): 611-615.
- Knoester, M., Bol, J.F., Vanloon, L.C., Linthorst, H.J.M. (1995). Virus-induced gene-expression for enzymes of ethylene biosynthesis in hypersensitively reacting tobacco. Molecular Plant Microbe Interactions, 8: 177-180.
- Koch, J.R., Creelman, R.A., Eshita, S.M., Seskar, M., Mullet, J.E., Davis, K.R. (2000). Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid. The role of programmed cell death in lesion formation. Plant Physiology, 123: 487-496.
- Leon, J., Lawton, M., Raskin, I. (1995). Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiology, 108: 1673-1678.
- Levine, A., Tenhaken, R., Dixon, R., Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell, 79: 583-593.
- Lopez-Munguia, A., Hernandez-Romero, Y., Pedraza-Chaverri, J., Miranda-Molina, A., Regla, I., Martinez, A., Castillo, E. (2011). Phenylpropanoid glycoside analogues: enzymatic synthesis, antioxidant activity and theoretical study of their free radical scavenger mechanism. PLoS One, 6(6): 201-215.
- Lovegrove, A., Hooley, R. (2000). Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci., 5: 102-110.
- Lund, S.T., Stall, R.E., Klee, H.J., 1998. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10, 371-382.
- Martin, G.B. (1999). Functional analysis of plant disease resistance genes and their downstream effectors. Curr. Opin. Plant Biol., 2: 273-279.
- Noctor, G., Foyer, C.H. (1998). Ascorbate and glutathione; keeping active oxygen control. Annual Review of Plant Physiology and Plant Molecular Biology, 49: 249-279.
- Orvar, B.L., McPherson, J., Ellis, B.E. (1997). Pre-activating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. Plant Journal, 11: 203-212.
- Overmyer, K., Brosche, M., Kangasjarvi, J. (2003). Reactive oxygen species and hormonal control of cell death. Trends in Plant Science, 8: 335-342.
- Overmyer, K., Tuominen, H., Kettunen, R., Betz, C., Langebartels, C., Sandermann, H., Kangasjarvi, J. (2000). Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxidedependent cell death. Plant Cell, 12: 1849-1862.
- Pastori, G.M., Kiddle, G., Antoniw, J., Bernard, S., Veljovic-Jovanovic, S., Verrier, P.J., Noctor, G., Foyer, C.H. (2003). Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell, 15: 939-951.
- Pavet,V., Olmos, E., Kiddle, G., Mowla, S., Kumar, S., Antoniw, J., Alvarez, M.E., Foyer, C.H. (2005). Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiology, 139: 1291-1303.
- Pignocchi, C., Foyer, C.H. (2003). Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Current Opinion in Plant Biology, 6: 379-389.
- Rao, M.V., Davis, K.R. (2001). The physiology of ozone induced cell death. Planta, 213: 682-690.
- Rao, M.V., Koch, J.R., Davis, K.R. (2000a). Ozone: a tool for probing programmed cell death in plants. Plant Molecular Biology, 44: 345-358.
- Ritchie, S., Gilroy, S. (1998). Calcium-dependent protein phosphorylation may mediate the gibberellic acid response in barley aleurone. Plant Physiol., 116: 765-776.
- Roth, U., Friebe, A., Schnabl, H. (2000). Resistance induction in plants by a brassinosteroid-containing extract of Lychnis viscaria L. Zeitschrift Naturforschung, 55: 552-559.
- Ryals, J., Neuenschwander, U., Willits, M., Molina, A., Steiner, H., Hunt, M. (1996). Systemic acquired resistance. Plant Cell, 8: 1809-1819.
- Sandermann, H., Ernst, D., Heller, W., Langebartels, C. (1998). Ozone: an abiotic elicitor of plant defence reactions. Trends in Plant Science, 3: 47-50.
- Schwartz, S.H., Tan, B.C., Gage, D.A., Zeevaart, J.A., McCarty, D.R. (1997). Specific oxidative cleavage of carotenoids by VP14 of maize. Science, 276: 1872-1874.
- Shah, J., Klessig, D.F. (1999). Salicylic acid: signal perception and transduction. Biochemistry and Molecular Biology of Plant Hormones, in: Hooykaas, P.P.J., Hall, M.A., Libbenga, K.R. (eds.), Elsevier Science B, V., Amsterdam, The Netherlands, pp. 513-541.
- Shalata, A., Peter, M., Neumann, (2001). Exogenous ascorbic acid (vitamin C) increases resistance of salt stress and reduces lipid peroxidation. Journal of Experimental Botany, 52 (364): 2207-2211.
- Sharma, Y.K., Davis, K.R. (1994). Ozone-induced expression of stress-related genes in Arabidopsis thaliana. Plant Physiology, 105: 1089-1096.
- Shinozaki, K., Yamaguchi-Shinozaki, K. (2000). Molecular responses to dehydration and low temperature: difference and cross-talk between two stress signaling pathways. Current Opinion in Plant Biology, 3: 217-223.
- Smirnoff, N. (1995). Environment and Plant Metabolism: Flexibility and Acclimation, BIOS Scientific Publishers Ltd, Oxford.
- Smirnoff, N., Wheeler, G.L. (2000). Ascorbic acid in plants: biosynthesis and function. Crit. Rev. Biochem. Mol. Biol., 35: 291-314.
- Traber, M.G., Stevens, J.F. (2011). Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med., (in press).
- Van-der-Hoorn, R.A., Kruijt, M., Roth, R., Brandwagt, B.F., Joosten, M.H., De Wit, P.J. (2001). Intragenic recombination generated two distinct Cf genes that mediate AVR9 recognition in the natural population of Lycopersicon pimpinellifolium. Proc. Natl. Acad. Sci. USA, 98: 10493-10498.
- Vranova, E., Inze, D., Van Breusegem, F. (2002). Signal transduction during oxidative stress. Journal of Experimental Botany, 53: 1227-1236.
- Vwioko, E.D., Osawaru, M.E., Eruogun, O.L. (2008). Evaluation of okro (Abelmoschus esculentus L. Moench.) exposed to paint waste contaminated soil for growth, ascorbic acid and metal concentration. African Journal of General Agriculture, 4(1): 39-48.
- Yu, H.J., Mun, J.H., Kwon, Y.M., Lee, J.S., Kim, S.G. (1999). Two cDNAs encoding pathogenesis-related proteins of Lithospermum erythrorhizon display different expression patterns in suspension cultures. Journal of Plant Physiology, 155: 364-370.
- Zhu, J.-K. (2001). Plant salt tolerance. Trends in Plant Science, 6: 66-71.


