Role of ascorbic acid and tocopherol in alleviating salinity stress on flax plant ( Linum usitatissimum L.)
Автор: Sadak Mervat Sh., Dawood Mona G.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 1 т.10, 2014 года.
Бесплатный доступ
Salinity is one of the environmental challenges in the world affecting on several physiological processes and the most limiting factor of plant productivity and quality. Two pot experiments were conducted at the wire house of National Research Centre, Cairo, Egypt during two successive seasons of 2010/2011 and 2011/2012 to assess the efficiency of two antioxidant vitamins (ascorbic acid at 1.13 and 2.27 mM or α tocopherol at 0.46 and 0.93 mM) and/or salinity stress at (0.0, 3.08, 6.16, 9.23 ds/m) on photosynthetic pigments, protein, carbohydrate, minerals, oil contents and yield as well as fatty acids composition of the yielded oils of three flax cultivars (Sakha 3, Giza 8 and Ariane). The data revealed that salinity stress caused significant and gradual decreases in total photosynthetic pigments, polysaccharides, total carbohydrates, total proteins and the uptake of Mg, K, Ca and P in the leaves of three flax cultivars with increasing salinity levels (3.08, 6.16, 9.23 ds/m). Otherwise, significant and gradual increase appeared in both Na and Cl. Ascorbic acid and α tocopherol at different concentrations caused significant increases in photosynthetic pigments, total carbohydrates and protein contents in the leaves of flax plants irrigated either with tap water or saline solution as compared with their corresponding controls. Exogenous application of ascorbic and α tocopherol at different concentrations exhibited decreases in Na and Cl whereas increases appeared in Mg, K, Ca and P relative to their corresponding control. Ascorbic acid (1.13 and 2.27 mM) and α tocopherol (0.46 and 0.93 mM) caused marked increases in yield and yield attributes of three flax cultivars either in plants irrigated with tap water or saline solution as compared to corresponding control. Ascorbic acid effects were more pronounced than α tocopherol effects. In addition, the higher level of two vitamins was more pronounced than the lower level. Regarding plants irrigated with tap water, it was noted that ascorbic acid at 2.27 mM caused significant increase in oil content by 19.75 % in Giza 8 whereas α tocopherpl at 0.93 mM caused significant increase by 14.83% in Sakha 3 and 13.70% in Ariane. Regarding plants irrigated with saline solution (9.23 ds/m), it was found that α tocopherol at 0.93 mM caused significant increase in oil % by 30.84 %, 9.66 % and 35.62 % in Sakha 3, Giza 8 and Ariane cv. respectively. Responses of three flax cultivars to salt stress were more or less similar; since salinity stress caused marked increases in total saturated fatty acids accompanied by decreases in total unsaturated fatty acids as salinity levels increased. Myristic acid (C14:0) and oleic acid (C18:1) were the most affected saturated and unsaturated fatty acids in response to different salinity levels. The effect of ascorbic acid at 2.27 mM and tocopherol at 0.93 mM were found to be contrary to that of salinity as marked increases appeared in unsaturated fatty acids as compared with control plants. It could be concluded that foliar application of ascorbic acid and α tocopherol could play an enhancement role and alleviate the harmful effect of salinity stress on many metabolic and physiological processes of three flax cultivars that reflected in increasing seed yield quality and quantity.
Antioxidant vitamins, linum usitatissimum, minerals, oil quality, saline solution
Короткий адрес: https://sciup.org/14323850
IDR: 14323850
Текст научной статьи Role of ascorbic acid and tocopherol in alleviating salinity stress on flax plant ( Linum usitatissimum L.)
Flax ( Linum usitatissimum L.) is one of economically important oilseed crops over the world. In Egypt, it is grown as a dual purpose crop i.e. fibre and seed oil. The flax seed oil quality is usually valued according to the content of essential fatty acids. Flax oil is one of the richest sources of omega-3 fatty acid (α-linolenic) and omega-6 fatty acid (linoleic). The genetic constituent is the main factor controlling the fatty acids profile in flax oil and linolenic acid represents more 50% of total fatty acids. Flax seeds show a high antioxidant activity. Ascorbic acid is the most abundant antioxidant in flax seeds (Morris, 2005).
Salinity is a common environmental challenge in the world and one of the major problems that limit agricultural production. According to the report of the world’s irrigated lands, about 20-27 % may be salt affected (Ezz El-Din et al. 2005). Salinity stress can affect several physiological processes, from seed germination to plant development. Moreover, high salt content affects the physiology of plants, both at the cellular as well as whole plant levels (Murphy and Durako, 2003). In saline environment, NaCl is usually the most injurious and predominant salt but also other salts including Mg+2, Ca+2 and SO4 -2 may be presented (Yamaguchi and Blumwald, 2005). Salinity stress, similar to many abiotic stress factors, is known to induce oxidative damage to plant cells from reactive oxygen species that affect the physiology and biochemistry of plants and can lead to a reduction in plant yield (Azevedo-Neto et al. 2006). Reactive oxygen species such as superoxide radical (O2-), hydrogen peroxide (H2O2) and hydroxyl radical (OH-) are responsible for the damage of membranes and other essential macromolecules such as photosynthetic pigments, proteins, DNA and lipids (Noctor and Foyer, 1998). Egypt suffers from water shortage problems and the use of non-traditional sources such as saline water in irrigation become necessity in recent years. Overcoming deleterious effects of salinity stress and improving salt tolerance is considered one of the challenges for increasing plant growth and productivity.
Antioxidants have synergistic effects on growth, yield and yield quality of many plant species. These compounds have beneficial effects on catching the free radicals or the active oxygen that produced during photosynthesis and respiration processes (Foyer et al . 1991). Exogenous application of antioxidants in the form of vitamins has gained considerable attention as a possible approach to ameliorate the adverse effects of salinity stress on plants for improving plant growth, development and yield (quantity and quality) (El Bassiouny et al . 2005). Vitamins could be considered natural and safety bio-regulator compounds which relatively in low concentrations exerted profound influences upon many physiological processes.
Ascorbic acid (vitamin C) is one of the most important water soluble antioxidants in plants, acting as a modulator of plant development through hormone signaling and as coenzyme in reactions by which carbohydrates, fats and proteins are metabolized (Pastori et al. 2003). Ascorbic acid is involved in the regulation of many critical biological processes such as photo-inhibition and cell elongation (Noctor et al. 1998); many other important enzymatic and non enzymatic reactions (Smirnoff, 2000); as well as in regulating plant growth and development, since it plays an important role as plant growth regulator (Athar et al. 2008). Moreover, ascorbic acid is very important for the regulation of photosynthesis, flowering and senescence (Barth et al. 2006). Several investigations reported that ascorbic acid plays important roles in enhancing the salt tolerance of different plants (Athar et al. 2008; Paital and Chainy, 2010; Hussein et al. 2011; Ejaz et al. 2012).
α Tocopherol (vitamin E) is a lipophilic antioxidant synthesized by all plants; its levels vary in different tissues and fluctuate during development and in response to abiotic stresses. It interacts with the polyunsaturated acyl groups of lipids, stabilizes membranes, scavenges and quenches various ROS (Maeda and DellaPenna, 2007) thus protects polyunsaturated fatty acids from lipids peroxidation and modulates signal transduction (Noctor, 2006). In cooperation with the xanthophylls cycle, vitamin E fulfills at least two different functions in chloroplasts at the two major sites of singlet oxygen production: it preserves PSI from photoinactivation and protects membrane lipids from photooxidation (Havaux et al. 2005). α Tocopherol levels change differentially in response to environmental constraints, depending on the magnitude of the stress and the species’ sensitivity to stress. Changes in α tocopherol levels result from altered expression of pathway-related genes, degradation and, recycling, and it is generally assumed that an increase in α tocopherol contributes to plant stress tolerance (Munne-Bosch, 2005). Plants pre-treated with α-tocopherol showed induced stress tolerance and protection against oxidative damage due to various stresses (Kumar et al. 2012).
This investigation aimed to assess the efficiency of two antioxidant vitamins (ascorbic acid and α tocopherol) in alleviating salinity stress on three flax cultivars through their actions on photosynthetic pigments, minerals, protein, carbohydrate, oil contents and yield as well as fatty acids composition.
MATERIALS AND METHODS
Experimental procedure
Two pot experiments were conducted at the wire house of National Research Centre, Cairo, Egypt during two successive seasons of 2010/2011 and 2011/2012. Three different cultivars of flax, Sakha 3, Giza 8 (Egyptian origin) and Ariane (French origin) were obtained from Oilseed Department, Agricultural Research Centre, Giza, Egypt. The two applied vitamins, ascorbic acid and α tocopherol were supplied from Sigma Chemical Company, St. Louis, MO, USA. The salt type used in irrigation was mainly the chloride mixture suggested by Stroganov (1962). The salt components of salt mixture are shown in Table (1).
Pots containing equal amounts of homogenous clay and sand soil (2:1) were grouped into three main groups, the first main group was sown with flax seeds of Sakha 3 cv., while the second main group was sown with flax seeds of Giza 8 cv. and the third main group was sown with seeds of Ariane cv. Thinning was done after 15 days from sowing leaving 5 uniform seedlings per pot. Phosphorus, potassium and ammonium fertilizers were added to the soil at the recommended doses. Each group was divided into five sub-groups, the first sub-group of each cultivar was sprayed with tap water (control); while pots of the other four sub-groups of each cultivar were sprayed twice during vegetative growth stage (after 45 and 60 days from sowing) with either ascorbic acid (at 1.13 or 2.27 mM) or α tocopherol (at 0.46 or 0.93 mM).
Each sub-group was divided into four sub-subgroups according to irrigation with different levels of saline solutions by using Stroganov nutrient solutions at 0.0, 3.08, 6.16 and 9.23 ds/m. Every treatment consisted of 5 replicates distributed in a completely randomized design. The pots were irrigated with equal volumes of saline solution, starting at 60 days from sowing. Irrigation was run as follows 3 times with saline solutions and one with tap water. Plant samples were taken after 90 days from sowing (at beginning of flowering stage) for determination of photosynthetic pigments, proteins, total carbohydrates, and minerals content. At harvest, plant samples were collected to determine yield and yield components (capsules number/plant; seeds number/capsule; seeds weight/plant and 1000 seeds weight), oil content and fatty acids composition of the yielded oils.
Chemical analysis
Photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoids) in the fresh leaves were determined according to the method described by Moran (1982). Total carbohydrates were determined in the dry leaves using the colorimetric method described by Dubois et al . (1956). Polysaccharides were determined according to Naguib (1963). Minerals content of Na+, P, K+, Ca2+, Mg2+ and N were determined according to the method described by Chapman and Pratt (1978). N and P were determined using Spekol Spectrocolourimeter Carl Zeiss. While, Ca, K and Na contents were determined by the use of flame photometer, and Mg+2 was determined using atomic absorption spectrophotometer. Cl-1 was determined according to Johnson and Ulrich (1959). Protein content was calculated by multiplying N% x 6.25. The oil content of the seeds was determined according to the procedure reported by A.O.A.C. (1990). As the quality of the oil depends on the proportion of different fatty acids, their composition was determined quantitatively by Gas Liquid Chromatography according to the method described by Harborne (1984).
Statistical analysis
The data were subjected to the analysis of variance (ANOVA) appropriate to the randomized complete block design applied after testing the homogeneity of error variances according to the procedure outlined by Gomez and Gomez (1984). The significant differences between treatments were compared with the critical difference at 5% probability level.
RESULTS AND DISCUSSION Photosynthetic pigments
Table (2) shows that Giza 8 cv. was characterized by the highest content of total photosynthetic pigments followed by Sakha 3 cv., whereas, Ariane cv. had the least either under salt stressed conditions or unstressed conditions. Salinity stress caused significant and gradual decreases in total photosynthetic pigments with increasing salinity levels. The highest salinity level (9.23 ds/m) caused the highest significant decrease in total photosynthetic pigments by 29.08, 31.29, and 24.41 % in Sakha 3, Giza 8 and Ariane cultivars respectively relative to unstressed control. This loss of chlorophyll due to stress may be occurred due to inhibition in biosynthesis or degradation of chlorophyll (Kumar et al. 2012) and/or disorganization of chloroplasts (Camejo et al. 2006). The disruption in the fine structure of the chloroplast, instability of the pigment protein complex and enhanced chlorophylase activity are directed to decrease in chlorophyll content under saline conditions. Madan et al . (2004) reported that salinity stress marginally decreased the rate of photosynthesis and chlorophyll content in the salt tolerant cv. however in the sensitive one showed greater reduction.
Ascorbic acid and α tocopherol at different concentrations caused significant increases in total photosynthetic pigments in plants irrigated either with tap water or saline solution relative to their corresponding controls (Table, 2). Ascorbic acid treatment (2.27 mM) showed pronounced and significant effect in alleviating the harmful effect of salinity stress on photosynthetic pigments of three cultivars of flax. The increase of chlorophyll content due to ascorbic acid application depends on the scavenging of reactive oxygen species by this antioxidant molecule and removing them directly from the cytoplasm (Beyer, 1994). Moreover, it has a supplementary role in protecting or regenerating oxidized carotenoids or tocopherols (Shao et al. 2006). Gonzlez et al. (1998) mentioned that the depletion in endogenous ascorbic acid before the start of paleness and falling of plant leaves occurs under the influence of different types of stresses and exogenous application of ascorbic acid have effective role in resisting stresses.
The enhancement roles of α tocopherol on photosynthetic pigments of flax leaves (Table,2) are in agreement with those reported by Kumar et al. (2012) on stressed wheat and Al Qubaie (2012) on sunflower, since, α tocopherol may be protected the organization of the chloroplast thus minimize chlorophyll loss.
Polysaccharides, total carbohydrates and total proteins
Table (3) shows that salinity stress caused significant decreases in polysaccharides, total carbohydrate and protein contents of three flax cultivars as compared to control (irrigated with tap water). The inhibitory effect of salinity on chlorophyll synthesis as mentioned in Table (2) might be reduced the biosynthesis of carbohydrates (Table, 3). The reduction in protein content under salinity stress (Table, 3) may be due to the disturbance in nitrogen metabolism or inhibition of nitrate absorption as reported by El Zeiny et al. (2007).
Meanwhile, foliar application of ascorbic acid and α tocopherol at all concentrations showed opposite trends to salinity effects. At low salinity level (3.08 ds/m), the two applied vitamins caused significant increases in total carbohydrates and proteins not only relative to corresponding stressed plants but also to unstressed untreated plants. In addition, under moderate salinity stress at 6.16 ds/m, the higher ascorbic acid level (2.27 mM) showed the highest pronounced effect on total carbohydrates and proteins. The enhancement effect of ascorbic acid on photosynthetic pigments as shown in Table (2) reflected on total carbohydrate and protein contents (Table, 3). Khan et al. (2011) mentioned that foliar spray of ascorbic acid encouraged synthesis of chlorophyll that involved in increases of photosynthetic metabolites, which lead to the accumulation of different fractions of soluble sugars and nitrogen content in plant tissues under saline conditions or this could perhaps alleviate the inhibitory effects of salinity on glucose incorporation to cell wall polysaccharides. Moreover, Dolatabadian et al. (2010) reported that ascorbic acid scavenged reactive oxygen species and prevented protein oxidation and degradation. The positive effect of ascorbic treatments on N concentration could be explained by the finding of Talaat (2003) who showed that the accumulation of nitrate by ascorbic acid foliar application may be due to the positive effect of ascorbic on root growth which consequently increased nitrate absorption.
Regarding α tocopherol effects, Sadak et al. (2010) demonstrated that application of α-tocopherol on sunflower plants led to the accumulation of total carbohydrates, stimulation of protein synthesis and delaying senescence of sunflower plant.
Minerals content
Table (4) illustrates that the uptake of Mg+2, K+, Ca+2 and P in flax leaves were decreased gradually by increasing salinity levels however increasing salinity levels caused increases in Na+ and Cl- uptake as compared to those irrigated with tap water. Excess of Na+ might cause problems with membranes, enzyme inhibition, and disturbance in metabolism which disorganize cell division, elongation and structure (Abo Kassem, 2006). In this connection, Kiarostami et al . (2010) suggested that increased accumulation of sodium (Na+) and (Cl-) ions in the tissues inhibits biochemical processes related to photosynthesis through direct toxicity and led to low water potential. The promotion of Na+ uptake by salinity was accompanied by a corresponding decline in K+ concentration, showing an apparent antagonism between K+ and Na+ (Cuin et al . 2009). The reduction in Ca+2 and Mg+2 uptake under salt stress conditions might be due to the suppressive effect of Na+ and K+ on these cations or due to reduction of transport of Ca+2 and Mg+2 ions (Asik et al . 2009). Meanwhile, Ashish et al. (2010) proposed that phosphorus does not contribute to osmotic adjustment but accumulates in cell walls of stressed plants.
On the other hand, plants irrigated either with tap water or saline solution at different levels and undergo exogenous application of ascorbic acid or α tocopherol exhibited decreases in Na+ and Cl-, whereas increases appeared in Mg+2, K+, Ca+2 and P relative to their corresponding control. Thus, the two applied vitamins partially mitigate the adverse effect of salt stress on minerals content in flax leaves. Ahmed (1996) mentioned that foliar spray of ascorbic acid might increase the organic acids excreted from the roots into the soil and consequently increase the solubility of most nutrients which release slowly into the rhizosphere zone. Moreover, the increase in Ca+2 concentration is important for preserving membrane integrity (Rengel, 1992).
Application of α tocopherol led to an increase in the contents of ions in the leaf through their role in increasing osmotolerance and/or through regulating various processes including absorption of nutrients from soil solution (Buschmann and Lichtenthaler, 1979).
Yield and yield components
Table (5) indicates that seed yield/ plant was more pronounced in Giza 8 cv. > Sakha 3 cv. > Ariane cv. at all treatments. These results were concomitant with photosynthetic pigments results that reported in Table (2). It was noted that yield and yield attributes (number of capsules/plant, number of seeds /capsule and weight of 1000 seeds) of three flax cultivars were decreased gradually by increasing salinity levels (0.0, 3.08, 6.16 and 9.23 ds/m). The highest decrease in seed yield/plant due to the highest salinity level (9.23 ds/m) were 61.70% in Giza 8 cv., 50.0 % in Sakha 3 cv. and 47.37% in Ariane cv. These results are in agreement with those reported by Sadak et al. (2010); Abdelhamid et al. (2010); Kumar et al. (2012) on different plant species. Wright et al. (1988) stated that the reduction in seed number due to increasing in salinity levels is believed to be the consequence of decreasing assimilates production associated with decreasing plant size.
On the other hand, Table (5) shows clearly that ascorbic acid (1.13 and 2.27mM) and α tocopherol (0.46 and 0.93 mM) caused marked increases in yield and yield attributes of three flax cultivars either irrigated with tap water or saline solution as compared to corresponding controls. It is worthy to mention that the effect of ascorbic acid at the higher level was the most pronounced.
Ascorbic acid was identified as a growth regulator affects many metabolic and physiological processes. Addition of ascorbic acid externally is an important factor for cell growth and division (Franceschi and Tarlyn, 2002). Emam et al . (2011) mentioned that ascorbic acid significantly increased the yield components of flax plants in terms of number of capsules/plant, number of seeds/capsule, seed yield/plant, seed yield/feddan as well as seed index compared with the control.
The enhancement effects of α tocopherol on flax yield and yield components (Table, 5) were proved earlier by El Bassiouny et al. (2005); Sadak et al. (2010); Soltani et al. (2012); Al Qubaie (2012) on different plant species. α Tocopherols plays a role in a range of different physiological phenomena including plant growth and development, senescence, preventing lipid peroxidation and to interact with the signal cascade that convey abiotic and biotic signals (Baffel and Ibrahim, 2008).
Oil contents
Table(6) shows clearly that the trend of oil contents of three flax cultivars were correlated to photosynthetic pigments (Table,2) as well as yield and yield attributes (Table,5). It was noted that, oil % in Giza 8 cv. > Sakha 3 cv. > Ariane cv. under all treatments. Three salinity levels caused significant decreases in oil % relative to control. Decreasing oil % of three flax cultivars (Table, 6) with increasing salinity could be mainly attributed to the reductions in seed yield per plant under saline conditions (Table, 5). Abdelhamid et al. (2010) mentioned that soil salinity reduced all seed yield parameters in addition to seed yield quality (protein and oil contents) of soybean.
Both applied vitamins (ascorbic acid at 2.27mM and α tocopherol at 0.93mM) caused significant increases in oil% of the three flax cultivars either irrigated with tap water or saline solution relative to corresponding controls. Regarding plants irrigated with tap water, it was noted that ascorbic acid at 2.27 mM caused significant increase in oil content by 19.75 in Giza 8 cv. whereas α tocopherpl at 0.93 mM caused significant increase by 14.83% in Sakha 3 cv. and 13.70% in Ariane cv. Regarding plants irrigated with saline solution (9.23 ds/m), it was found that α tocopherol at 0.93 mM caused significant increase in oil % by 30.84 %, 9.66 % and 35.62 % relative to corresponding control in Sakha 3, Giza 8 and Ariane cv. respectively. The promoting effect of the two applied antioxidants on seed yield and yield components surely reflected on enhancing oil yields. Gamal El Din (2005) reported that ascorbic acid significantly increased oil percentage of sunflower seeds. Contrary to the present results, Dolatabadian et al . (2010) mentioned that, the highest corn oil percentage was achieved from stressed plants while ascorbic acid treatments decreased it.
Regarding α tocopherol, Ayad et al. (2009) indicated that α-tocopherol treatments significantly increased essential oil percent and yield of Pelargonium graveolens L. and these increases might be due to a pronounced enhancement of α tocopherol on synthesis and accumulation of oil.
Fatty acids composition
Table (7-A,B,C) shows that oils extracted from three flax cultivars characterized by the presence of nine fatty acids, including six saturated fatty acids (lauric, myristic, palmitic, stearic,behenic and lignoceric) and three unsaturated fatty acids (oleic, linoleic and linolenic).
At normal conditions (untreated and unstressed plants), it was found that oil of Giza 8 cv. characterized by the least total saturated fatty acids (11.19 %) followed by Sakha 3 cv. (13.96 %) and Ariane cv.(15.96 %). Regarding unsaturated fatty acids, it was noted the two cultivars Giza 8 and Sakha 3 have approximately the same percentage (84.59 %and 84.28 %) while Ariane cv. had the least (81.64%). The predominant fatty acids were linolenic followed by oleic and linoleic. Responses of fatty acids composition of three flax cultivars to salt stress were approximately similar; since salinity stress caused marked increase in total saturated fatty acids accompanied by decreases in total unsaturated fatty acids with increasing salinity levels as compared with untreated unstressed plants.
Myristic acid (C14:0) and oleic acid (C18:1) were the most affected saturated and unsaturated fatty acids in response to different salinity levels. The highest level of salinity (9.23 ds/m) caused increases in myristic acid by 446.15% and 538.46% and decreases in oleic acid by 11.16% and 20.64% in Sakha 3 and Giza 8 cultivars respectively as compared with untreated unstressed plants. Special attention must be paid to linolenic acid content. Since, it was decreased sharply by salinity levels in Ariane cv. and slight decreases were observed in Sakha 3 and Giza 8 by salt stress. Hashem et al. (2011) indicates that mystiric acid may play an important role in salt tolerance mechanism of plants.
Under saline conditions, both osmotic and toxic stresses do occur and it is possible that water deficit occurring under salt stress might have caused a shortening of the lipid accumulation phase and some damages to all enzymatic activities, including that of oleate desaturase (Flagella et al. 2004). In addition, relative compositional changes in fatty acids induced by NaCl resulted in decreased unsaturated/saturated ratio, more so in tolerant cultivar as reported by Mansour and Salama (2004).
The effects of ascorbic acid at 2.27 mM and α tocopherol at 0.93 mM were found to be contrary to that of salinity as marked increases were observed in unsaturated fatty acids as compared with control plants. The highest increase in linolenic acid was detected in Giza 8 cv. under low salinity level (3.08 ds/m) with α tocopherol treatment as well as at moderate salinity level (6.16 ds/m) with ascorbic acid. Emam et al. (2011) revealed that ascorbic acid treatment caused marked decrease in saturated fatty acids (palmitic and stearic). The increase in linolenic acid with vitamin treatments might be attributed to the acceleration of the biosynthetic pathway of linolenic acid (Joshi et al . 1998).
El Lethy et al. (2010) found that foliar application of α tocopherol, significantly affected oil yield of flax plant and linolenic acid was found to be the main fatty acid. The most prominent function of α tocopherol is protection of polyunsaturated fatty acids from lipid peroxidation (Farouk, 2011).
α Tocopherol breaks a propagation chain of lipid oxidation by reduction of radical intermediates ( Vollhardt and Schore, 2011). One molecule each of the α-, β-, γ-, δ-tocopherol is capable of protecting 220, 120, 100, 30 and 20 molecules of polyunsaturated fatty acid from oxidation, respectively (Fukuzawa et al. 1982).
The biphasic action of vitamin treatments which was generally reflected in attenuated saturated fatty acids level and augmented in unsaturated fatty acids could be a successful step in improving the quality of flax seeds. The increase of unsaturated fatty acids in response to vitamins treatments improves the nutritional value and the economic importance of the flax seed oil, as the flax seed oil health benefits are, primarily, due to it being the highest food source of omega 3 fatty acid (linolenic acid).
It could be concluded that foliar application of ascorbic acid and α tocopherol could play an enhancement role and alleviate the harmful effect of salinity stress on many metabolic and physiological processes of three flax cultivars that reflected in increasing seed yield quality and quantity.
Table 1 : The component of salt mixture used for chloride salinization expressed as % of total salt content.
|
MgSO 4 |
CaSO 4 |
NaCl |
MgCl 2 |
CaCO 3 |
|
10 |
1 |
78 |
2 |
9 |
The component of specific anions and cations in chloride mixture expressed as percentage of total milliequivalents.
|
Na+ |
Mg+2 |
Ca+2 |
SO-2 |
Cl- |
CO 3 -2 |
|
38 |
6 |
6 |
5 |
40 |
5 |
Table 2 : Effect of ascorbic acid (Asc) and α tocopherol (α Toc) on photosynthetic pigments (µg/g fresh weight) of three flax cultivars grown under salinity stress at 90 days after sowing (combined analysis of two seasons)
|
Salinity (ds/m) |
Materials (rnM) |
Cultivars |
|||||||||||||
|
Sakha 3 |
Giza 8 |
Ariane |
|||||||||||||
|
о g |
X о U |
'o о и |
С tu S 'о. *3 о |
о g |
х Я О и |
72 'о с у о и |
Е "5 1 |
а |
£ о g |
X £ о и |
"о о я и |
С о Е 2 о |
|||
|
0 |
0 |
1624 |
529 |
264 |
2417 |
1816 |
633 |
363 |
2812 |
939 |
238 |
269 |
1446 |
||
|
Asc |
1.13 |
1753 |
657 |
383 |
2793 |
2125 |
745 |
430 |
3300 |
1086 |
367 |
347 |
1790 |
||
|
2.27 |
2021 |
692 |
378 |
3091 |
2162 |
784 |
450 |
3396 |
1085 |
334 |
356 |
1775 |
|||
|
a Toe |
0.46 |
1698 |
575 |
338 |
2611 |
2115 |
664 |
433 |
3212 |
1007 |
321 |
289 |
1617 |
||
|
0.93 |
1945 |
695 |
368 |
3008 |
2147 |
703 |
466 |
3316 |
1013 |
357 |
308 |
1678 |
|||
|
3.08 |
0 |
1423 |
504 |
263 |
2190 |
1642 |
541 |
360 |
2543 |
929 |
229 |
235 |
1393 |
||
|
Asc |
1.13 |
1687 |
571 |
297 |
2555 |
2053 |
677 |
437 |
3167 |
1004 |
265 |
297 |
1566 |
||
|
2.27 |
1754 |
654 |
347 |
2755 |
2123 |
737 |
446 |
3306 |
976 |
286 |
302 |
1564 |
|||
|
a Toe |
0.46 |
1579 |
526 |
291 |
2396 |
1857 |
638 |
380 |
2875 |
988 |
252 |
263 |
1503 |
||
|
0.93 |
1628 |
594 |
310 |
2532 |
2021 |
654 |
441 |
3116 |
951 |
268 |
275 |
1494 |
|||
|
6.16 |
0 |
1322 |
501 |
242 |
2065 |
1414 |
506 |
339 |
2259 |
838 |
157 |
214 |
1209 |
||
|
Asc |
1.13 |
1436 |
547 |
272 |
2255 |
1674 |
562 |
356 |
2592 |
857 |
203 |
279 |
1339 |
||
|
2.27 |
1607 |
553 |
323 |
2483 |
1962 |
654 |
441 |
3057 |
864 |
232 |
300 |
1396 |
|||
|
a Toe |
0.46 |
1406 |
535 |
320 |
2261 |
1623 |
561 |
407 |
2691 |
838 |
215 |
224 |
1277 |
||
|
0.93 |
1483 |
575 |
282 |
2340 |
1832 |
624 |
429 |
2885 |
847 |
227 |
273 |
1347 |
|||
|
9.23 |
0 |
1081 |
425 |
208 |
1714 |
1219 |
472 |
241 |
1932 |
736 |
162 |
195 |
1093 |
||
|
Asc |
1.13 |
1237 |
474 |
246 |
1957 |
1510 |
549 |
372 |
2431 |
817 |
192 |
263 |
1272 |
||
|
2.27 |
1304 |
508 |
300 |
2112 |
1866 |
642 |
425 |
2933 |
803 |
213 |
292 |
1308 |
|||
|
a Toe |
0.46 |
1199 |
474 |
252 |
1925 |
1544 |
527 |
307 |
2378 |
797 |
186 |
239 |
1222 |
||
|
0.93 |
1360 |
496 |
258 |
2114 |
1733 |
567 |
378 |
2678 |
817 |
197 |
251 |
1265 |
|||
|
LSD 5% |
22.33 |
11.2 |
9.54 |
39.39 |
27.77 |
14.98 |
10.83 |
39.08 |
20.15 |
10.40 |
7.94 |
29.69 |
|||
Table 3 : Effect of ascorbic acid (Asc) and α tocopherol (α Toc) on polysaccharides, total carbohydrates and total proteins (%) of three flax cultivars grown under salinity stress at 90 days after sowing (combined analysis of two seasons).
|
Salinity (ds/m) |
Materials (mM) |
Cultivars |
||||||||||
|
Sakha 3 |
Giza 8 |
Ariane |
||||||||||
|
JU У - ” 8 о CL, |
Q |
-а .s н 2 Cl |
a JU у -” 8 о CL, |
s £ о u |
-a .s н 2 Cl |
a У « ” 8 о CL, |
2 £ о ^ U |
« -B н 2 Cl |
||||
|
0 |
0 |
14.51 |
15.54 |
9.87 |
13.90 |
17.33 |
13.37 |
12.89 |
13.93 |
10.37 |
||
|
Asc. |
1.13 |
21.17 |
22.36 |
1 1.62 |
23.59 |
25.48 |
14.75 |
19.87 |
20.58 |
10.81 |
||
|
2.27 |
23.16 |
24.59 |
12.19 |
25.94 |
29.76 |
18.00 |
22.75 |
24.00 |
12.44 |
|||
|
a Toe |
0.46 |
18.55 |
19.69 |
1 |
1.19 |
19.69 |
23.59 |
13.44 |
15.79 |
17.87 |
10.69 |
|
|
0.93 |
20.98 |
22.42 |
1 1.69 |
21.98 |
26.15 |
16.06 |
18.48 |
19.61 |
1 1.75 |
|||
|
3.08 |
0 |
12.77 |
14.46 |
9.19 |
9.24 |
15.51 |
12.56 |
1 1.45 |
12.81 |
9.94 |
||
|
Asc. |
1.13 |
15.70 |
16.26 |
10.50 |
11.49 |
22.16 |
14.06 |
15.79 |
18.64 |
10.75 |
||
|
2.27 |
17.20 |
19.14 |
11.12 |
16.78 |
25.03 |
15.94 |
18.48 |
20.14 |
11.19 |
|||
|
a Toe |
0.46 |
14.69 |
15.99 |
10.56 |
11.47 |
18.58 |
13.94 |
13.69 |
15.69 |
10.62 |
||
|
0.93 |
16.54 |
18.35 |
10.50 |
14.67 |
20.90 |
15.44 |
15.80 |
17.56 |
1 1.00 |
|||
|
6.15 |
0 |
12.14 |
13.87 |
7.94 |
6.75 |
13.34 |
1 1.87 |
10.36 |
11.93 |
9.12 |
||
|
Asc. |
1.13 |
13.70 |
15.48 |
8.62 |
9.88 |
15.48 |
13.44 |
12.46 |
13.69 |
9.44 |
||
|
2.27 |
13.69 |
18.52 |
9.06 |
1 1.72 |
18.80 |
14.75 |
14.70 |
16.64 |
9.94 |
|||
|
a Toe |
0.46 |
12.69 |
14.59 |
8.50 |
8.80 |
15.13 |
12.56 |
11.88 |
12.36 |
9.50 |
||
|
0.93 |
14.70 |
15.35 |
9.00 |
10.61 |
17.90 |
14.12 |
13.26 |
13.71 |
9.81 |
|||
|
9.23 |
0 |
9.74 |
11.66 |
6.81 |
5.38 |
12.36 |
1 1.00 |
9.33 |
11.19 |
8.50 |
||
|
Asc. |
1.13 |
10.24 |
12.55 |
7.81 |
7.89 |
13.62 |
12.44 |
10.24 |
12.59 |
9.25 |
||
|
2.27 |
12.57 |
14.58 |
8.50 |
8.23 |
15.48 |
12.56 |
11.17 |
13.26 |
9.11 |
|||
|
a Toe |
0.46 |
10.26 |
12.33 |
7.50 |
7.90 |
12.60 |
1 1.75 |
10.37 |
12.36 |
8.75 |
||
|
0.93 |
12.37 |
13.33 |
8.31 |
8.52 |
14.99 |
12.50 |
11.63 |
13.53 |
9.44 |
|||
|
LSD 5% |
0.23 |
0.30 |
0.25 |
0.31 |
0.38 |
0.41 |
0.26 |
0.32 |
0.24 |
|||
Table 4 : Effect of ascorbic acid (Asc) and α tocopherol (α Toc) on minerals content (mg/g) of three flax cultivars grown under salinity stress at 90 days after sowing (combined analysis of two seasons).
|
и |
ОС |
9 |
у ci |
s |
ил |
4- |
un ri |
ri |
S |
о |
|||||
|
РЭ и |
а о |
d |
о A 00 |
О ri |
n d |
n A d |
a A 00 |
4G 00 Tt |
m |
G A d |
о G |
||||
|
и |
Ок |
d |
un С п |
d |
rj A |
d |
un |
oc |
ri |
ri |
A |
rn О |
Gt- |
о |
|
|
'С |
во S |
q тГ |
А d |
n A d |
A A |
о Tt |
00 Tt |
A ГЛ |
rl A Tt |
=. Tt |
d |
rl ri |
s Tt |
ОС А G |
|
|
00 ri N |
А Th ТГ |
00 Th Tt |
Th ri n |
Tt 00 N |
00 d N |
n d |
ri d N |
A d n |
ri d |
q |
A |
00 G |
|||
|
Я Z |
ОС » А |
А |
-t ri A |
3 ЦП |
so ГП |
00 Th |
d |
MD un |
-■ A d |
A d |
00 00 d |
q sO |
ос G О |
||
|
□ |
5 |
ОС G |
1 |
ГП ГЛ |
«л |
ri |
A |
MD |
rl A |
rl 00 |
m A |
Vl A |
О G |
||
|
и |
ОС А |
ОС А |
A |
ОС |
МЛ ri |
3 |
e 9 |
00 |
© oc 00* |
un A Th |
S @4 |
un oc |
о ri |
||
|
1 |
ОС |
@к |
А d |
А |
Г1 ri n |
МП ГЛ d |
d |
A UH |
ri |
un |
=: d |
sC d |
A |
A tn |
ГП N d |
|
N и |
|||||||||||||||
|
u |
во 3 |
5 ос |
ил А о |
о 00 |
Th |
A |
un |
tn rt * |
3 |
-+ |
o A ГП |
d |
ос в О |
||
|
К |
Г1 ri |
Г1 А ГП |
о s |
Г1 ГП п |
ri n |
d n |
n d |
rl d rl |
q un n |
a 2 |
00 ri ri |
о о м |
я ОО О |
||
|
я 2 |
ос Tt |
сч А |
8 Tt |
ОС ОС А |
о ОС Tt |
g u*| |
4G ГП d |
4C d |
3 d |
4C A |
3 |
00 Г| |
q G |
||
|
и |
ос d |
с О |
c Ci |
— |
ил 00 d |
00 00 d |
QS rl |
^ |
ri |
A |
^ |
00 А |
Q О |
||
|
РЭ и |
g А d |
00 А |
00 о |
о 00 Th |
n d |
Q d |
Q4 О ГЛ |
о A od |
1Л A d |
a |
a A d |
с А d |
O'. A d |
||
|
ГЛ я |
ь |
А ГЛ |
40 ОС |
A |
О |
A |
40 d |
О n d |
G A 00* |
un un d |
O' d |
■л А ос* |
Tt tn d |
||
|
И 3 |
г] d |
00 |
00 |
rl А |
00 d |
n d |
тг ri ЦП |
A d |
un A d |
A Vl |
wn 00 d |
А d |
oc G О |
||
|
А МЛ п |
00 ri гл |
A A rn |
А Th п |
00 00 N |
A МЛ N |
A |
00 d n |
G d n |
G ri |
ri Os |
А d |
d |
|||
|
Z |
ОС А ил |
< |
oc oc ■* |
ос ОС А |
A МП |
00 A 1Л |
4 UD |
d |
Ф d |
© oc d |
4 sO |
А sO |
ri d |
||
|
'В 'J 5 |
f |
о |
R ri < |
A d =1 |
о |
R ri I |
A d H =1 |
e |
R Ci < |
гл A d =1 |
e |
R ri < |
А А d V н =1 |
1Л Q |
|
|
3 |
|||||||||||||||
|
"E и |
75 |
о |
oc О A |
40 d |
m ri d |
||||||||||
Table 5 : Effect of ascorbic acid (Asc) and α tocopherol (α Toc) on yield (g/plant) and yield components of three flax cultivars grown under salinity stress (combined analysis of two seasons)
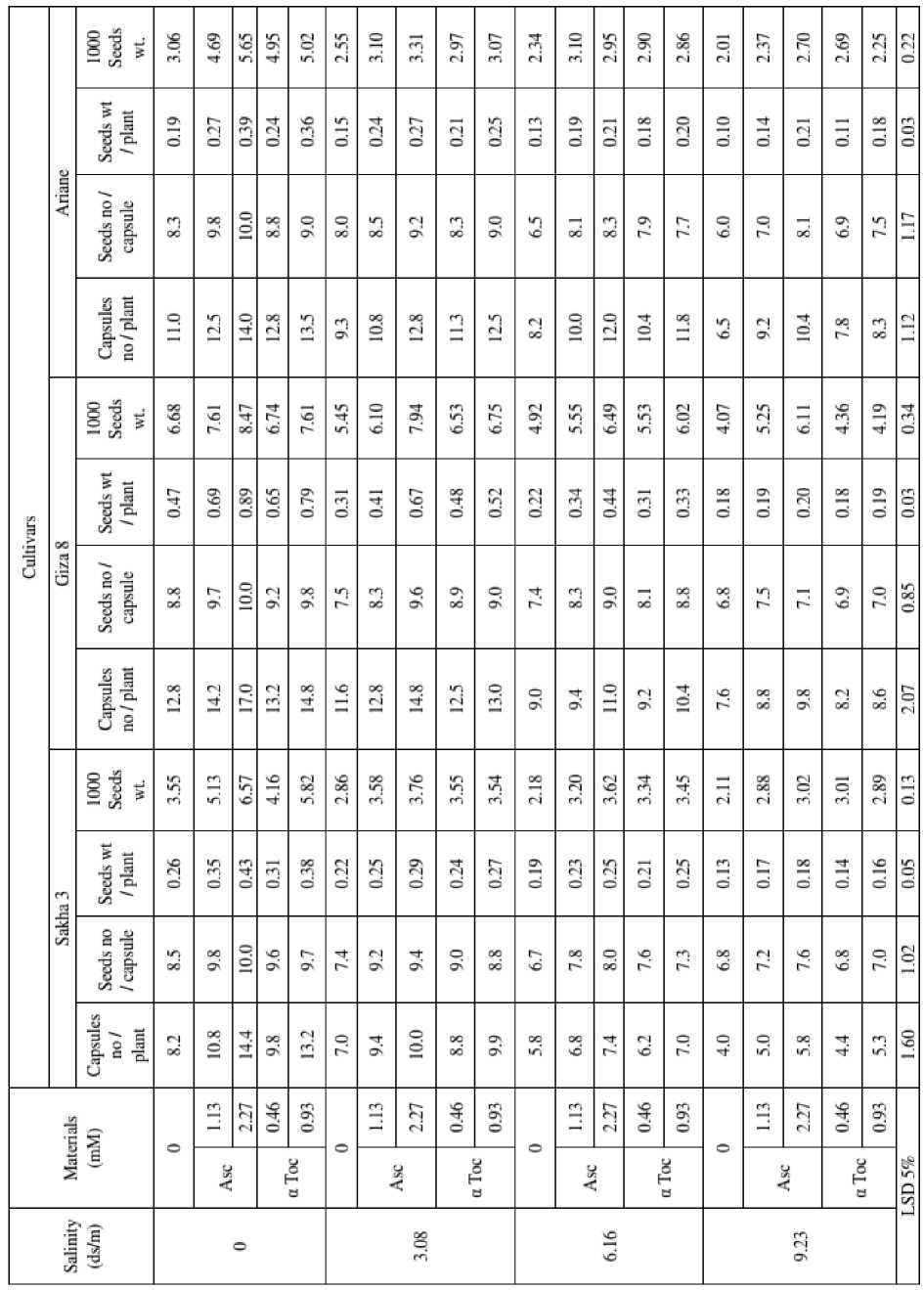
Table 6 : Effect of ascorbic acid and α tocopherol on oil contents (%) of three flax cultivars grown under salinity stress (combined analysis of two seasons).
|
Salinity (ds/m) |
Materials (mM) |
Cultivars |
||
|
Sakha 3 |
Giza 8 |
Ariane |
||
|
0 |
0 |
35.54 |
38.62 |
32.99 |
|
ascorbic acid (2.27) |
39.39 |
46.25 |
35.89 |
|
|
α tocopherol (0.93) |
40.81 |
42.85 |
37.51 |
|
|
3.08 |
0 |
30.97 |
33.27 |
28.04 |
|
ascorbic acid (2.27) |
33.58 |
36.61 |
29.89 |
|
|
α tocopherol (0.93) |
35.19 |
37.81 |
30.61 |
|
|
6.16 |
0 |
26.21 |
30.56 |
22.25 |
|
ascorbic acid (2.27) |
30.64 |
36.33 |
25.89 |
|
|
α tocopherol (0.93) |
34.39 |
34.59 |
31.82 |
|
|
9.23 |
0 |
20.46 |
29.50 |
17.94 |
|
ascorbic acid (2.27) |
23.44 |
31.50 |
23.59 |
|
|
α tocopherol (0.93) |
26.77 |
32.35 |
24.33 |
|
|
LSD 5% |
0.78 |
1.28 |
0.86 |
|
Table 7 : (A) Effect of ascorbic acid (Asc) and αtocopherol (αToc) on fatty acids composition of oil content of Sakha 3 cultivar grown under salinity stress.
|
Fatty acid % |
Salinity level (ds/m) / Materials (mM) |
|||||||||||
|
ri |
3.08 ds/m |
6.16 ds/m |
9.23 ds/m |
|||||||||
|
0.0 |
Asc (2.27) |
a Toe (0.93) |
0.0 |
Asc (2.27) |
a Toe (0.93) |
0.0 |
Asc (2.27) |
aToc (0.93) |
0.0 |
Asc (2.27) |
aToc (0.93) |
|
|
Lauric (Cl2:0) |
0.05 |
0.13 |
0.13 |
0.08 |
0.14 |
0.15 |
0.16 |
0.35 |
0 |
0 |
0.12 |
0.18 |
|
Myristic (Cl4:0) |
0.1 ? |
0.28 |
0.26 |
0.36 |
0.37 |
0.39 |
038 |
0.62 |
039 |
0.71 |
0.78 |
0.74 |
|
Palmitic (C16:0) |
7.10 |
7.90 |
7.90 |
8.36 |
7.76 |
7.76 |
9.42 |
7.42 |
825 |
10.65 |
1037 |
7.93 |
|
Stearic (C18:O) |
4.36 |
2.35 |
3.96 |
4.05 |
3.98 |
4.78 |
3.98 |
4.24 |
4.12 |
3.68 |
4.07 |
4.35 |
|
Oleic (C18:l) |
22.22 |
23.37 |
25.37 |
20.26 |
30.52 |
32.12 |
20.26 |
24.23 |
23.98 |
19 74 |
23.03 |
22.28 |
|
Linoleic (Cl8:2) |
18.51 |
19.35 |
16.05 |
16.59 |
10.47 |
7.05 |
15.16 |
14.09 |
14.32 |
16.02 |
12.06 |
15.29 |
|
Linolenic (C18:3) |
43.55 |
43.91 |
44.46 |
43.61 |
42.71 |
44.35 |
42.35 |
44.62 |
44.25 |
42.35 |
45.35 |
44.35 |
|
Behenic (C22:0) |
1.07 |
0.45 |
0.64 |
0.64 |
0.64 |
0.65 |
132 |
0.87 |
039 |
1.17 |
0.95 |
1.06 |
|
Lignoceric (C24:0) |
1.25 |
0.45 |
1.05 |
1.03 |
1.05 |
1.06 |
1.05 |
1.91 |
0.95 |
1.10 |
1.06 |
1.69 |
|
Total saturated |
13.96 |
11.56 |
13.94 |
1432 |
13.94 |
14.79 |
16.71 |
15.41 |
14.50 |
17.31 |
1735 |
15.95 |
|
Total unsaturated |
84.28 |
86.63 |
85.88 |
80.46 |
83.70 |
83.52 |
77.77 |
82.94 |
82.55 |
78.11 |
80.44 |
81.92 |
|
TUS/TS |
(.<14 |
7.49 |
6.16 |
5.41 |
6 00 |
5.65 |
4.65 |
5.38 |
5.69 |
431 |
4.58 |
5.13 |
-
(B) Effect of ascorbic acid (Asc) and αtocopherol (αToc) on fatty acids composition of oil content of Giza 8 cultivar grown under salinity stress.
Fatty acid %
Salinity level (ds/m) / Materials (mM)
0
3.08 ds/m
6.16 ds/m
9.23 ds/m
0.0
Asc (2.27)
a Toe (0.93)
0.0
Asc (2.27)
a Toe (0.93)
0.0
Asc (2.27)
aToc (0.93)
0.0
Asc (2.27)
aToc (0.93)
Lauric (Cl 2:0)
0.00
0.02
0.15
0.26
035
0.18
021
0
0.25
033
0.25
0.18
Myristic (C14:0)
0.13
0.07
0
0.36
0.06
0.06
053
0
0.16
0.83
0.18
0.21
Palmitic (Cl6:0)
6.96
6.65
5.69
8.83
624
6.69
935
7.61
6.66
10.63
6.42
8.37
Steanc (Cl8:0)
2.23
2.25
3.25
3.01
3.69
2.98
3.14
3.02
3.25
3.01
3.96
3.25
Oleic (Cl 8:1)
18.36
26.98
25.94
1732
23.52
22.77
15.14
20.97
27.26
14.57
24.67
25.62
Linoleic (Cl8:2)
18.87
10.98
7.51
18.22
5.62
7.10
17.06
5.88
5.25
15.71
6.08
8.55
Linolenic (Cl8:3)
47.36
50.98
54.58
4536
53.36
57.36
48.03
5735
54.58
47.35
5535
51.35
Behenic (C22:O)
0.89
0.62
0.89
1.25
0.98
0.59
1.8
0.36
0.87
0.68
0.98
0.36
Lignoceric (C24:0)
0.98
0.25
0.14
1.58
035
0.58
057
0.68
035
0.87
0.68
0.68
Total saturated
11.19
9.86
10.12
1526
11.67
11.08
15.60
11.67
11.54
16.35
12.47
10.05
Total unsaturated
84.59
88.94
88.03
80.90
82.50
87.23
80.23
84.20
87.09
77.63
86.10
85.52
TUS/TS
7.56
9.02
8.70
5.30
7.07
7.87
5.14
7.21
7.55
4.75
6.90
8.51
-
(C) Effect of ascorbic acid (Asc) and αtocopherol (αToc) on fatty acids composition of oil content of Ariane cultivar grown under salinity stress.
Fatty acid %
Salinity level (ds/m) / Materials (mM)
0
3.08 ds/m
6.16 ds/m
9.23 ds/m
0.0
Asc (2.27)
a Toe .(0.93)
0.0
Asc (2.27)
aToc (0.93)
0.0
Asc (2.27)
aToc (0.93)
0.0
Asc (2.27)
aToc (0.93)
Lauric (С12Ю)
0.35
0
0
0.25
0.12
0.35
0.54
0.72
0.33
0.28
0.46
0.49
Myristic (C14:0)
0.53
0.65
0.68
0.66
0.47
0.76
0.87
0.6
0.25
1.68
0.94
050
Palmitic (Cl6:0)
7.96
8.69
9.98
10.13
8.31
9.51
11.66
11.46
9.30
10.16
8.41
11.60
Stearic (08:0)
4.21
4.21
4.68
435
5.13
5.01
5.06
4.98
5.36
5.35
5.32
5.47
Oleic (08:1)
30.14
33.68
32.46
30.63
32.83
32.41
31.18
3033
3338
32.12
31.16
32.72
Linoleic (Cl 8:2)
9.14
8.30
8.65
9.02
851
931
1035
10.87
10.97
10.45
10.36
11.83
Linolenic (Cl 8:3)
42 3 6
42.57
42.20
40.97
42 59
40.53
36.81
39.61
3631
34.45
38.97
36.24
Behenic (C22:0)
1.56
1 07
0.64
0.78
0.68
0 11
1.52
0.39
1.61
1.61
0.90
0.70
Lignoceric (C24:0)
1.35
0.54
0.03
0.45
038
0.411
0.58
0.58
0
1.35
0.98
0.12
Total saturated
15.96
15.16
16.01
16.62
15.09
16.14
20.23
18.73
16.85
20.43
17.01
18.88
Total unsaturated
81.64
84.55
83.31
80.62
83.93
82.25
7834
80.81
80.66
77.02
80.49
80.79
TUS/TS
5.11
5.58
5.20
4.85
5.56
5.10
3.87
431
4.78
3.77
4.73
428
Список литературы Role of ascorbic acid and tocopherol in alleviating salinity stress on flax plant ( Linum usitatissimum L.)
- Abdelhamid, M., Gaballah, M.S., Rady, M. and Gomaa, A. (2010). Biofertilizer and ascorbic acid alleviated the detrimental effects of soil salinity on growth and yield of soybean. Proceedings of the Second Science Africa Conference -2010 pp.73-81
- Abo Kassem, E.E.M. (2006). Effect of salinity: calcium interaction on growth and nucleic acid metabolism in five species of chenopodiaceae. Turk. J. Bot., 31: 125-134
- Ahmed, A.H. (1996). Physiological studies on tiploun and nitrate accumulation in lettuce plants. J. Agric. Sci., Mansoura Univ., 21: 3971-3994
- Al Qubaie, A.I. (2012). Response of sunflowers cultivar Giza 102 (Helianthus annuus L) plants to spraying some antioxidants. Nature and Sci., 10: 1-6
- A.O.A.C. (1990). Official Methods of Analysis. 20th_edition. Association of Official Analytical Chemists, Arlington, Virginia, U.S.A
- Ashish, D.P., Nilesh, S.P., InduBhushan, P. and Amarnath, P. (2010). Growth, water status and nutrient accumulation of seedlings of jatropha curcas L. (euphorbiaceae) in response to soil salinity. Anals de Biologia, 32: 59-71
- Asik, B.B., Turan, M.A., Celik, H. and Katkat, A.V. (2009). Effects of humic substances on plant growth and mineral nutrients uptake of wheat (Triticum durum cv. Salihli) under conditions of salinity. Asian J. Crop Sci., 1: 87-95
- Athar, H.R., Khan, A. and Ashraf, M. (2008). Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ. Exp. Bot., 63: 224-231
- Ayad, H.S., Gamal El-Din, K. M. and Reda, F. (2009). Efficiency of stigmastrol and α-tocopherol application on vegetative growth, essential oil pattern, protein and lipid peroxidation of geranium. J. Appl. Sci. Res., 5: 887-892
- Azevedo-Neto, D., Prisco, J., Eneas, J., De Abreu, C. and Gomes. E. (2006). Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt sensitive maize varieties. Environ. Exp. Bot., 56: 87-94
- Baffel, S.O. and Ibrahim, M.M. (2008). Antioxidants and accumulation of α-tocopherol induce chilling tolerance in Medicago sativa. Int. J. Agric. Biol., 10: 593-598
- Barth, C., De Tullio, M. and Conklin, P.L. (2006). The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot., 57: 1657-1665
- Beyer, R.E. (1994). The role of ascorbate in antioxidant protection of biomembranes interaction with vitamin-E and Coenzyme-Q. J. Bioenerg Biomember., 26: 349-358
- Buschmann, C. and Lichtenthaler, H.K. (1979). The influence of phytohormones on prenyllipid composition and photosynthetic activities of thylakoids. In Appelgvist L.A. and Lilj Enberg, C. (eds.) Advances in Biochemistry and Physiology of Plant Lipids, Elsevier, Amsterdam. pp. 145-150
- Camejo, D., Jime´nez, A., Alarco´n, J.J., Torres, W., Go´mez, J.M. and Sevilla, F. (2006). Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Biol., 33: 177-187
- Chapman, H.D. and Pratt, P. F. (1978). “Methods of Analysis for Soils, Plant and Water”. Univ. California, Div. Agric. Sci. Publ. no. 4034. p. 162-165
- Cuin, T.A., Tian, Y., Betts, S.A., Chalmandrier, R. and Shabala, S. (2009). Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Funct. Plant Biol., 36: 1110-1119
- Dolatabadian,A., Mohammad,S.A., Sanavy,M. and Asilan.K.S. (2010). Effect of ascorbic acid foliar application on yield, yield component and several morphological traits of grain corn under water deficit stress conditions. Notulae Scientia Biologicae, 2: 45-50
- Dubois, M., Guilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith. F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem., 28: 350-356
- Ejaz, B., Sajid, Z.A. and Aftab,F. (2012). Effect of exogenous application of ascorbic acid on antioxidant enzyme activities, proline contents, and growth parameters of Saccharum spp. hybrid cv. HSF-240 under salt stress. Turk. J. Biol., 36: 1-11
- EL Bassiouny, H.M.S., Gobarah, M.E. and Ramadan, A.A. (2005). Effect of antioxidants on growth, yield and favism causative agents in seeds of Vicia faba L. plants grown under reclaimed sandy soil. J. Agron., 4: 281-287
- El Lethy, S.R., Ayad, H.S. and Talaat, I.M. (2010). Physiological effect of some antioxidants on flax plant (Linum usitatissimum L.). World J. Agric. Sci., 6: 622-629
- El Zeiny, H.A., Abou, L.B., Gaballah, M.S. and Khalil, S. (2007). Antitranspirant application to sesame plant for salinity stress Augmentation. Res. J. Agric. Biologic. Sci. 3: 950 -959
- Emam, M. M., El-Sweify, A. H. and Helal, N. M. (2011). Efficiencies of some vitamins in improving yield and quality of flax plant. African J. Agric. Research, 6: 4362-4369
- Ezz El-Din, A. A., Aziz, E.E., Hendawy, S.F. and Omar, E.A. (2005). Response of Thymus vulgaris L. to salt stress in newly reclaimed soil. J. Applied Sci. Res., 5: 2165-2170
- Farouk, S. (2011). Ascorbic Acid and α-Tocopherol minimize salt-induced wheat leaf senescence. J. Stress Physiol. Biochem., 7: 58-79
- Flagella, Z., Giuliani, M.M., Rotunno, T., Di Caterina, R., De Caro, A. (2004). Effect of saline water on oil yield and quality of a high oleic canola (Brassica napus L.) hybrid. Eur. J. Agron., 21: 267-272
- Foyer, Ch., Lelandais, M., Edwards, E. A. and Mulineawx, P. M. (1991). The role of ascorbate in plants, interactions with photosynthesis and regulatory significance. In: Active oxygen oxidative stress and plant metabolism. Pell, E.J., and Steffen. K.L., eds. Current Topics in plant physiology. Vol. 6. American Society of Plant Physiologists, Rockville, M. D. pp. 131 -144
- Franceschi, V.R. and Tarlyn, N. M. (2002). L-ascorbic is accumulated in source leaf phloem and transported to sink tissue in plants. Plant Physiol., 130: 649-656
- Fukuzawa, K., Tokumura, A., Ouchi, S. and Tsukatani, H. (1982). Antioxidant activities of tocopherols on Fe2+ascorbate-induced lipid peroxidation in lecithin liposomes. Lipids 17: 511-513
- Gamal El-Din, K.M. (2005). Physiological studies on the effect of some vitamins on growth and oil content in sunflower plant. Egypt. J. Appl. Sci., 20: 560-571
- Gomez, K.A. and Gomez, A.A. (1984). “Statistical Procedures for Agricultural Research”. p.680. John Wiley & Sons Inc., Singapore
- Gonzlez, A., Steffen, K. L. and Lynch, J. P. (1998). Light and excess manganese. Implications for oxidative stress in common bean. Plant Physiol., 118: 493-504
- Harborne, J.B. (1984).Phytochemical methods: A guide to modern techniques of plant analysis. 2nd Edition, London, N.Y., P.15
- Hashem, H.A., Bassuony, F.M., Hassanein, R.A., Baraka, D.M. and Khalil, R. R. (2011). Stigmasterol seed treatment alleviates the drastic effect of NaCl and improves quality and yield in flax plants. Austalian J. Crop Sci., 5: 1858-1867
- Havaux, M., Eymery, F., Porfirova, S., Rey, P. and Do¨rmann, P. (2005). Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell, 17: 3451-3469
- Hussein, M.M., Abd El-Rheem, Kh. M. Khaled, S. M. and Youssef, R. A. (2011). Growth and nutrients status of wheat as affected by ascorbic acid and water salinity. Nature and Sci., 9: 64-69
- Johnson, C.M. and Ulrich, A. (1959). "Analytical Methods for use in Plant Analysis". Bull., 766. Berkeley: University of California, Agric., Experiment Station., pp: 26-78
- Joshi, N.L., Mali, P.C. and Anurag, S. (1998). Effect of nitrogen and sulphur application on yield and fatty acid composition of mustard (Brassica juncea L.) oil. J. Agron. Crop Sci., 180: 59-63
- Khan, T.A., Mazid, M. and Mohammad, F. (2011). A review of ascorbic acid potentialities against oxidative stress induced in plants. J. Agrobiol., 28: 97-111
- Kiarostami, Kh., Mohseni, R. and and Saboora, A. (2010). Biochemical changes of Rosmarinus officinalis under salt stress. J. Stress Physiol. Biochem., 6: 114-122
- Kumar, S.; Singh, R. and Nayyar, H. (2012). α-Tocopherol application modulates the response of wheat (Triticum aestivum L.) seedlings to elevated temperatures by mitigation of stress injury and enhancement of antioxidants. J. Plant Growth Regul., 32(2), 307-314
- Madan-Pal, Singh, D.K., Rao, L.S. and Singh, K.P. (2004). Photosynthetic characteristics and activity of antioxidant enzymes in salinity tolerant and sensitive rice cultivars. Indian J. of Plant Sci., 9(4): 407-412
- Maeda, H. and DellaPenna, D. (2007). Tocopherol functions in photosynthetic organisms. Curr. Opin. Plant Biol., 10: 260-265
- Mansour, M.M.F. and Salama, K.H.A. (2004). Cellular basis of salt tolerance in plants. Environ. Exp. Bot., 52: 113-122
- Moran, R. (1982). Formula for determination of chlorophyllous pigments extracted with N.N. dimethylformamide. Plant Physiol., 69: 1371-1381
- Morris, D.H. (2005). Flax-A smart choice. New Flax Facts. Flax Council of Canada, 465-167 Lombard Ave., Winnipeg, MB, Canada R3B 0T6, Website: www.flaxcouncil.ca
- Munne´-Bosch, S. (2005). The role of α tocopherol in plant stress tolerance. J. Plant Physiol., 162: 743-748
- Murphy, K.S.T. and Durako, M.J. (2003). Physiological effect of short term salinity changes on Ruppia maritime. Aquat. Bot., 75: 293-309
- Naguib, M.I. (1963). Colourimetric estimation of plant polysaccharides. Zeit. Zucher., 16: 15-22
- Noctor, G. (2006). Metabolic signaling in defense and stress: the central roles of soluble redox couples. Plant Cell Environ., 29: 409-425
- Noctor, G., Arisi, A., Jouanin, L., Kunert, K. J., Rennenberg, H. and Foyer, C. H. (1998). Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot., 49: 623-647
- Noctor, G. and Foyer, C. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol., 49: 249-279
- Paital, B. and Chainy, G.B.N. (2010). Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to change salinity. Comparative Biochemistry and Physiology, Part C: Toxicology and Pharmacology, 151: 142-147
- Pastori, G.M., Kiddle, G., Antoniw, J., Bernard, S., Veljovic-Jovanovic, S., Verrier, P.J., Noctor, G. and Foyer, C.H. (2003). Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell, 15: 939-951
- Rengl, Z. (1992). The role of calcium in salt toxicity. Plant Cell Environ., 15: 625-632
- Sadak, M,Sh., Rady, M.M., Badr, N.M. and Gaballah, M.S. (2010). Increasing sunflower salt toleramce using nicotinamide and α-tocopherol. Int. J. Acad. Res., 2: 263-270
- Shao, H. B., Chu, L. Y., Zhao, C. X., Guo, Q. J., Liu, X.A. and Ribaut, J.M. (2006). Plant gene regulatory network system under abiotic stress. Acta Biol. Sezeged, 50: 1-9
- Smirnoff, N. (2000). Ascorbic acid: metabolism and functions of a multi-facetted molecule. Current Opinion in Plant Biol., 3: 229-235
- Soltani, Y., Saffari, V.R., Moud, A. A. M. and Mehrabani, M. (2012). Effect of foliar application of α-tocopherol and pyridoxine on vegetative growth, flowering, and some biochemical constituents of Calendula officinalis L. plants. African J. Biotechnol., 11: 11931-11935
- Stroganov, B. P. (1962). Physiological basis of the salt tolerance of plants (under different types of soil salinization) Izd. Akad. Nauk. USSR. Moscow
- Talaat, N.B. (2003). Physiological studies on the effect of salinity, ascorbic acid and putrescine of sweet pepper plant. Ph.D. Thesis, Fac. Agric. Cairo Univ., Egypt
- Vollhardt, K.P.C. and Schore, N. E. (2011). Organic chemistry: structure and function, 6th Ed., New York, W.H. Freeman
- Wright, G.C., Smith, C.J. and Woodroofe, M.R. (1988). The effect of irrigation and nitrogen fertilizer on rapeseed (Brassica napus) production in southeastern Australia: l. Growth and seed yield. Irrig. Sci., 9: 1 -13
- Yamaguchi, T. and Blumwald, E. (2005). Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci., 10: 615-620


