Role of ascorbic acid on germination indexes and enzyme activity of Vicia faba seeds grown under salinity stress
Автор: Mohsen Awatif A., Ebrahim Mohsen K. H., Ghoraba Wael F. S.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 3 т.10, 2014 года.
Бесплатный доступ
The present work aimed to investigate changes in growth and some metabolic activities in NaCl-stressed bean seedlings, and assessing the role of ascorbic acid to alleviate these changes. The germination was carried out to study the response of presoaked faba bean seeds ( Vicia faba cv. Misr 2) in freshly prepared ascorbic acid (50 ppm ≈ 0.3 mM; as recommended dose as described by El-Tayeb, 1995) or distilled water (control) for 4 hrs at natural environmental conditions, to salinity stress during germination period. The radicle and plumule lengths were inhibited at high dose of NaCl but, ascorbic acid application to salt-treated seeds seemed to increase radicle and plumule elongation. The radicle and plumule fresh and dry weights were gradually decreased with increasing NaCl concentrations but, a noticeable increase of radicle and plumule fresh and dry weights were reached in seedlings treated with ascorbic acid. The pigment biosynthesis was substantially affected by salt treatment. Addition of ascorbic acid to stressed seedlings more or less furthered the inhibitory effects of salinity. Salinity enhanced the accumulation of reducing sugars in both radicle and plumule of Vicia faba seedlings as compared with control. Ascorbic acid treatment furthered the stimulatory effects of NaCl. Salinity gradually lowered the protein content of plumules. Ascorbic acid treatments raised the accumulation of protein contents in radicle to a great extent compared to those subjected only to NaCl. Plumule alkaloid content was lowered by low and moderate levels of NaCl. Coupling ascorbic acid to salt treated seeds induced a highly significant increase in alkaloid content of plumules compared to its corresponding controls. Sodium chloride treatments to Vicia faba seeds for two days caused a drastic suppression of α- and β-amylase activities. Ascorbic acid application to non-salinized seeds seemed without effects whereas, the salt-treated seeds showed more or less furthered the same effect of salinity. From the previous results we can observed that ascorbic acid achieved a better results during germination indexes.
Vicia faba, ascorbic acid, growth, amylase, enzyme activity, nacl, pigments, salinity
Короткий адрес: https://sciup.org/14323900
IDR: 14323900
Текст научной статьи Role of ascorbic acid on germination indexes and enzyme activity of Vicia faba seeds grown under salinity stress
The most common plant response to salt stress is a general reduction in growth and yield. Neumann, (1997) and Aly et al ., (2003) stated that when the salt stressed increased the growth rate and the size of crop plants dramatically affect by salts. This growth suppression was related to osmotic potential of the soil water caused by soluble salts. Within limits, concentrations of different combinations of salts cause nearly equal reductions in growth. On the other hand, Levitt, (1980) found that excess of ions lead to yield reduction caused by ion toxicities or nutritional imbalances. The herbaceous crops are exposure to osmotic effects as a result of saline soils in the field generally consists of mixture of different salts. Soil salinity has become a serious environmental problem which affects the growth and productivity of many crops. The soil water potential is dramatically decreased by rising of salt levels in the soil. High salt content also affects the physiology of plants, both at the cellular as well as whole plant levels (Murphy et al ., 2003). Levitt, (1980) and Ebrahim, (2005) said that soybean is very sensitive to ion toxicity as it is herbaceous crop which do not exhibit leaf injury symptoms, but in reversible the woody species exhibit leaf injury when Cl- or Na+ ions accumulated.
Preeti et al. (2000) recorded the changes in the levels of amylase and ascorbic acid in horsegram, soybean and faba bean seeds during germination (until the 5th day). They found that amylase activity increased till the 3rd day post-treatment in soybean and faba bean and thereafter declined. In horsegram, it decreased on the 1st day and then increased gradually and the values on the 4th, 5th day were similar. Ascorbic acid content was maximum on the 3rd day in germinated soybean and faba bean, but decreased in soybean only. However, in horsegram seeds, its content increased continuously till the 5th day.
Ascorbic acids (C 6 H 8 O 6 ) is present in all living plant cells, the largest amounts being usually in the leaves and flowers, i.e., in actively growing parts (Smirnoff et al ., 2001 and Ebrahim, 2005).
Ascorbic acid serves as a hydrogen transport agent which involved in cellular oxidation reduction reactions. Attempts have been made to employ active vitamins to overcome the drastic effects of salinity on seed germination and seedling growth as well as on some metabolic mechanisms (Khan and Zaidi, 1985; Ansari and Khan, 1986; Samiullah and Afridi, 1988). Presowing seed treatment of responsive cultivars with vitamins could thus be exploited to enhance grain yield at harvest (Kudrev and Pandev, 1965). Moreover, Oertli (1987) reported that under certain conditions, the exogenous application of vitamins to plants stimulate their growth, Thus, a part from their main role as coenzymes, it is not improbable that vitamins may also play other independent roles in the biochemical processes of plants, repairing the injurious effects of unfavorable conditions. Ascorbic acid can be scavenged the reactive oxygen species which are very harmful on the plant growth. It is a product of D-glucose metabolism which affects some nutritional cycle activities in higher plants and plays an important role in the electron transport system (El-Kobisy et al., 2005). Several studies have shown that ascorbic acid plays an important role in improving plant tolerance to abiotic stress (Shalata and Neumann, 2001; Al-Hakimi and Hamada, 2001; Athar et al., 2008).
Beans ( Vicia faba ) are considered the first legume crop in Egypt of the arable area. Total yield and consumption as green and dry seeds are consumed in human feed because the plant has high levels of protein (18 %), carbohydrates (58 %), vitamins and other minerals. In addition to the improvement of soil texture and its fertility, the plant seeds are considered as a valuable source for energy and proteins (El-Greadly, 2002).
So far, the literary references indicated no hint for investigating the response of Vicia faba to ascorbic acid. Therefore, the present work aimed at: firstly investigating changes in crop yield, seed contents and protein pattern in NaCl-stressed bean plants, secondly assessing the role of ascorbic acid to alleviate these changes, thirdly finding an explanation for such alleviatory role and finally finding a recommended dose for treating Vicia faba with ascorbic acid.
MATERIALS AND METHODS
Germination was carried out at room temperature (23 ±2°C) in 0.02 m2 plastic dishs, each contained 780 g acid washed sterilized sandy soil. Seeds were divided into 5 groups according to the used NaCl level (0.0, 50, 100, 150 and 200 mM). Each group was classified into 2 subdivisions depending on the concentration of ascorbic acid (0.0 and 50 ppm).
Each dish contained 15 seeds that were irrigated with 50 ml NaCl solution. The photoperiod was 10:14
hours (light/darkness). At 12-day old, the germinating seeds were collected to determine the percentage of germination, growth parameters(radicle and plumule lengths, fresh and dry weights), pigment contents in cotyledonary leaves, carbohydrate contents, total soluble proteins, total alkaloid content, and the activity of alpha and beta amylases.
Plant Material and Growth Conditions
Faba bean seeds ( Vicia faba cv. Misr 2) were obtained from Gemmiza Agricultural Research Station, Gharbia, Egypt.
The seeds were selected for uniformity of size and shape and surface sterilized (2.5% clorox for 5 min.) and rinsed thoroughly in distilled water. The seeds were then soaked in freshly prepared ascorbic acid (50 ppm ≈ 0.3 mM; as recommended dose as described by El-Tayeb, (1995) or distilled water (control) for 4 hrs at natural environmental conditions. MEASURMENTS
At 12-day old the seedling radicle and plumule organs were dried in an aerated oven, at 70°C, to constant weight. Carbohydrates were extracted in borate buffer pH 8 [0.1 dry mass (10 cm3 buffer)-1]. Carbohydrates were estimated quantitatively using Nelson (1944) with some modifications was done by Naguib (1963) these modifications are ten mg of the dry plant residue after extracting in borate buffer were mixed with 0.2 ml of 0.1 % (w/v) amylase and 0.1 ml acetate buffer (6 ml 0.2 N acetic acid + 4 ml 0.2 N Na-acetate), completed to 3 ml with distilled water, left for 24 hrs at room temperature, then centrifuged for 15 min at 3000 rpm and starch can be measured quantitatively. Soluble proteins were assayed according to Bradford (1976). Alkaloids were measured quantitatively according to the method described by Harborne, (1973). Alpha and beta amylases were assayed according to Mac Gregor, (1978).
Statistical analysis - Data obtained were analyzed statistically to determine the degree of significance between treatments. The method of two ways analysis of variance (ANOVA; factorial) was applied for all data. The least significant difference (LSD) at 5 % was used to compare means (Steel and Torrie, 1980). RESULTS
(2006) found that Gossypium hirsutum seedling growth indicated by height, and fresh and dry weights was reduced by NaCl.
Soaking seeds in ascorbic acid alleviate the suppressive effects of the relatively higher salinity levels on the seed germination, radicle and plumule lengths, and fresh and dry matter yield of Vicia faba. It appears probable from the response that vitamin C may act as growth stimulants (Greulach and Adams, 1963) which can play a role in reversing the effect of NaCl on metabolic activities relevant to growth. Such promoting effects of vitamin C on growth were also obtained by Khan and Ansarie (1984); Ansari and Khan (1986); Shaddad et al . (1990); Mozafar and Oertli (1992); Azooz (1997); El-Tayeb et al. (1999); Al-Hakimi and Hamada (2001).
Azooz et al. (2013) showed that Vicia faba plants exhibited a significant reduction (p≤0.05) in their growth parameters and water status in response to 150 mM NaCl compared to control plants. Similar reduction in growth performance were found in some plants under saline conditions (Ates and Tekeli, 2007; Azooz, 2009; Ekmekçi and Karaman, 2012; Kaya et al., 2013). This might be attributed to the toxic effect of salinity or increased crucial osmotic pressure, at which the broad bean plants would not be able to absorb water due to osmotic effect and decrease in some physiological activities. The higher reduction in roots dry weight than shoots as response to salinity stress may be due to the ability of these plants to limit Na+ and Cl- transport into the shoots. This is critically important for the maintenance of high growth rates, and protection of metabolic processes in cells elongation from the toxic effects of Na+ (Razmjoo et al., 2008).
The data of photosynthetic pigments (Fig. 2) clearly demonstrated that the biosynthesis of pigments was substantially affected by the different salt levels lowering the pigment content, particularly at the relatively high level (200 mM NaCl). This inhibitory effect is in harmony with the results obtained by Singh et al . (1994). De La Rosa and Maiti (1995) assumed that the tendency of salinity to lower the chlorophyll content might be due to the synthesis of nitrogen compounds acting as osmotic regulators e.g., proline which consumes large amount of nitrogen. Misra et al . (1997) found that chlorophyll and carotenoid contents of 15-day old rice seedlings were different in their response to salinity stress. The susceptible cultivar’s had the higher values, while the resistant one had the lower contents. Sairam et al . (2002) found that salinity decreased chlorophyll and carotenoids of wheat genotypes. Amini and Ehsanpour (2006) stated that the Chl. Content (a, b and total Chl.) was decreased with increasing salinity in Lycopersicon esculentum seedlings.
Soaking Vicia faba seeds in ascorbic acid has more or less furthered the inhibitory effects of salinity, decreasing Chl. a, Chl. b and carotenoid contents. Non-salinized seeds achieved the highest pigment content with ascorbic acid treatment. Azooz et al. (2013) stated that Salinity treatments showed a statistically significant decrease (p≤0.05) on photosynthetic pigments viz., chl. a, b, carotenoids and total chlorophyll content of Vicia faba L. leaves, compared to control. Total chlorophyll content was decreased about 42% less than control. The negative effects of salinity stress on photosynthetic pigments could be due to the inhibition of chlorophyll biosynthesis or increase its degradation by chlorophyllase, which is more active under salinity stress (Khan et al., 2006; Ziaf et al., 2009; Akça and Samsunlu, 2012).
Dolatabadian et al . (2008) reported that, salt stress leads to an increase in free radicals in chloroplasts and destruction of chlorophyll molecules by reactive oxygen species, while ascorbic acid can detoxify and neutralize the reactive oxygen species by prevention of free radicals activity, leading to increase in chlorophyll content of vitamin- treated plants. Hassanein et al . (2009) suggested that these vitamins may interfere with the protection of chloroplasts and their membrane against NaCl toxicity and; thus, maintaining their integrity. Azzedine et al . (2011) reported that, application of vitamin C was effective to mitigate the adverse effect of salt stress on plant growth due to increased leaf area, improved chlorophyll and carotenoids contents.
In addition, the results showed that monosaccharide and sucrose contents of both radicle and plumule of Vicia faba seedlings were increased by salinity treatments. On the other hand, the starch content was gradually decreased by salinity.(Fig. 3)
Soaking seeds in ascorbic acid slightly affected soluble as well as insoluble carbohydrate contents. This increase in soluble component may, in turn, play an important role in increasing the osmotic pressure of the cytoplasm, which is in accordance with the results obtained by Flowers et al. (1977), Drossopoulous et al. (1987), Shaddad et al. (1990), Ashraf and Fatima (1995), and Ebrahim (2005). On the other hand, Handa et al. (1983) found that the concentration of reducing sugars increased in the cultured tomato cells with adaptation to salinity concentrations as high as 600 mM.
Also, El-Tayeb et al . (1999) recorded that, soluble carbohydrates were increased in Trigonella foenum graecum by salinity stress. Thus, it can say that Vicia faba is fairly well adapted to salinity.
The results of total soluble protein of Vicia faba seedlings (Fig 4) showed that, the low and moderate levels of salinity raised both radicle and plumule protein contents, whereas the relatively high and very high salinity levels caused a noticeable reduction. Ascorbic acid treatment failed to make a recovery against salinity stress. It can be said that the strategy of osmotic adjustment of Vicia faba cv. Misr 2 was mediated by soluble carbohydrate rather than soluble proteins. This results are in accordance with those obtained by El-Tayeb et al . (1999); Heikal et al . (1999); Ismail and Azooz (2002). Jiang et al . (2006) stated that soluble protein content was increased in leaves and decreased in stem of Gossypium hirsutum seedlings due to salt stress, and was decreased in roots by 50 and 100 mM NaCl.
Azooz et al. (2013) said that salinity stress caused a significant increase (p≤0.05) of soluble carbohydrates, total free amino acids and proline, while soluble proteins were decreased in both roots and shoots of broad bean plants, compared to un-salinized control plants. The increase in soluble carbohydrates due to salinity stress has been reported in other plants (Ramezani et al., 2011). The reduction of protein was previously recorded by Bassuony et al. (2008) and Sadak et al. (2010). They concluded that, the reduction of protein under salinity stress was suppressed by the accumulation of total amino –N and proline. Dolatabadion et al. (2008) revealed that salt stress increased proline content, and application of vitamins scavenged reactive oxygen species and prevented the biosynthesis of extra proline in canola plant.
The data revealed that the alkaloid content of beans plumule gradually increased with increasing the level of NaCl (Fig 5). Also, the presence of ascorbic acid with stressed plants appeared the same effect on the alkaloid content of the plumule organs. It could be concluded that alkaloid might be acting as protective metabolite against salt stress (Misra and Gupta, 2006)
The results appeared that alpha and beta amylase activities of untreated seeds were higher than treated seeds with NaCl, while they were gradually decreased with increasing NaCl level. Also, application of ascorbic acid to the stressed seeds appeared to ameliorate the inhibitory effect of NaCl on the enzymatic activities (Fig 6). This protect the plants from injury and damage, where regulated the activity of amylases. In this connection, Guerrier (1991) concluded that improved axis growth of Vicia faba with KCl and CaCl2, was inversely related to NaCl accumulation coupled with an adaptive control of the hydrolytic activities. Also, Shukry and El–Bassiouny (2002) reported that amylase activity and total soluble sugars of Vicia faba seeds, during germination, decreased with different concentrations of sea water (5, 25 and 50%).In the contrast, Hassanein et al. (2009) showed that salinity caused highly significant increases in amylase activity in Zea mays plant. Also, Rather and Doering (1984) who found that salinity stimulates amylases and this accompanied with starch hydrolysis. Soaking of grains in/or spraying of plant with ascorbic acid vitamin was generally associated with marked decreases in the activities of amylases concurrently with increasing the soluble, insoluble and total carbohydrate contents indicating that vitamins could alleviate the inhibitory effects of salt stress by inhibiting amylase activity and/or enhancing photosynthetic mechanism (Kodandaramaiah, 1983).
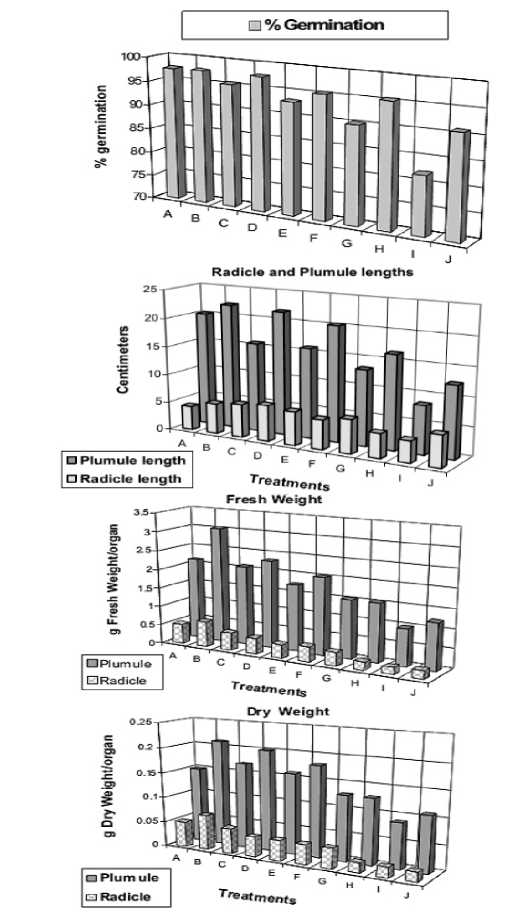
Figure 1 . Percentage of germination, radicle and plumule lengths, and fresh and dry weights of 12-day old Vicia faba seedlings as affected by NaCl level (0.0, 50, 100, 150 and 200 mM) and presoaking in 50 ppm ascorbic acid ; A: 0.0 mM NaCl, B: 50 ppm Asc., C: 50 mM NaCl, D: 50 mM NaCl + Asc., E: 100 mM NaCl, F: 100 mM NaCl + Asc., G: 150 mM NaCl, 150 mM NaCl + Asc., I: 200 mM NaCl, J: 200 mM NaCl + Asc.
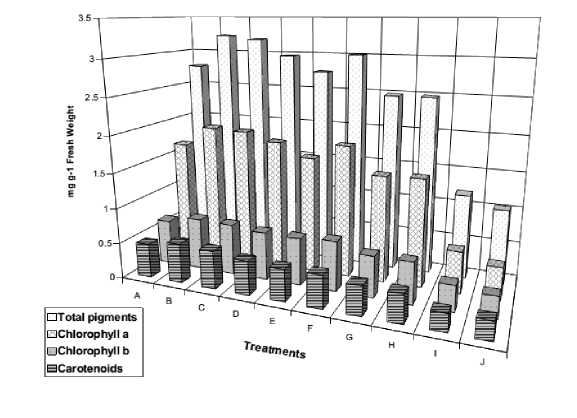
Figure 2. Pigment contents of the leaves of 12-day old Vicia faba seedlings as affected by NaCl level (0.0, 50, 100, 150 and 200 mM ) and presoaking in 50 ppm ascorbic acid; A: 0.0 mM NaCl, B: 50 ppm Asc., C: 50 mM NaCl, D: 50 mM NaCl + Asc., E: 100 mM NaCl, F: 100 mM NaCl + Asc., G: 150 mM NaCl, 150 mM NaCl + Asc., I: 200 mM NaCl, J: 200 mM NaCl + Asc.
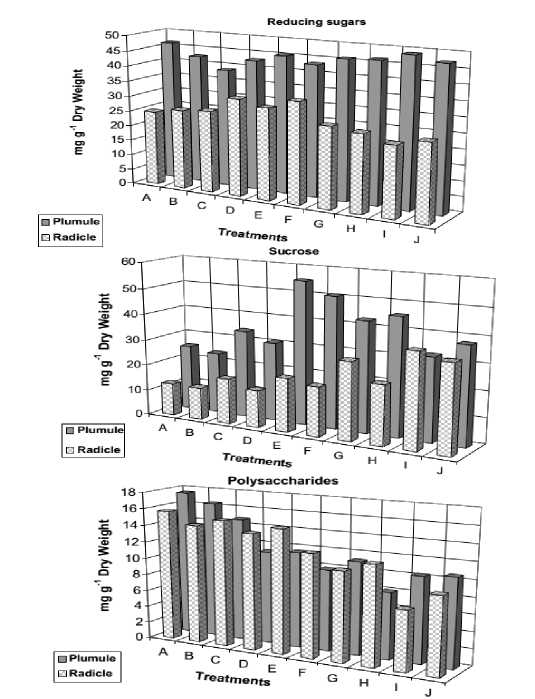
Figure 3. Reducing sugars, sucrose and polysaccharide contents of 12- day old Vicia faba seedlings as affected by NaCl level (0.0, 50, 100, 150 and 200 mM) and presoaking in 50 ppm ascorbic acid; A: 0.0 mM NaCl, B: 50 ppm Asc., C: 50 mM NaCl, D: 50 mM NaCl + Asc., E: 100 mM NaCl, F: 100 mM NaCl + Asc., G: 150 mM NaCl, 150 mM NaCl + Asc., I: 200 mM NaCl, J: 200 mM NaCl + Asc.
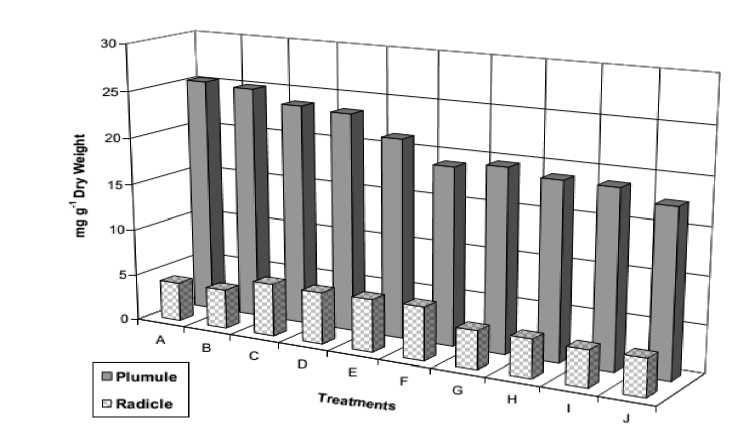
Figure 4. Soluble protein contents of 12-day old Vicia faba seedlings as affected by NaCl level (0.0, 50, 100, 150 and 200 mM) and presoaking in 50 ppm ascorbic acid; A: 0.0 mM NaCl, B: 50 ppm Asc., C: 50 mM NaCl, D: 50 mM NaCl + Asc., E: 100 mM NaCl, F: 100 mM NaCl + Asc., G: 150 mM NaCl, 150 mM NaCl + Asc., I: 200 mM NaCl, J: 200 mM NaCl + Asc.
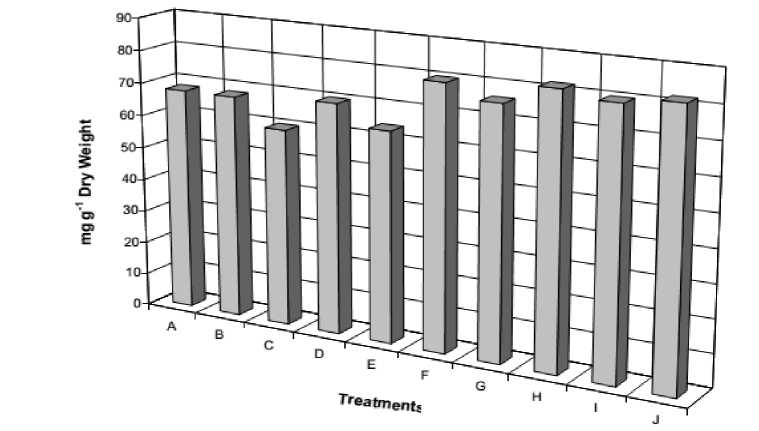
Figure 5. Total plumule alkaloids content of 12-day old Vicia faba seedlings as affected by NaCl level (0.0, 50, 100, 150 and 200 mM) and presoaking in 50 ppm ascorbic acid; A: 0.0 mM NaCl, B: 50 ppm Asc., C: 50 mM NaCl, D: 50 mM NaCl + Asc., E: 100 mM NaCl, F: 100 mM NaCl + Asc., G: 150 mM NaCl, 150 mM NaCl + Asc., I: 200 mM NaCl, J: 200 mM NaCl + Asc.
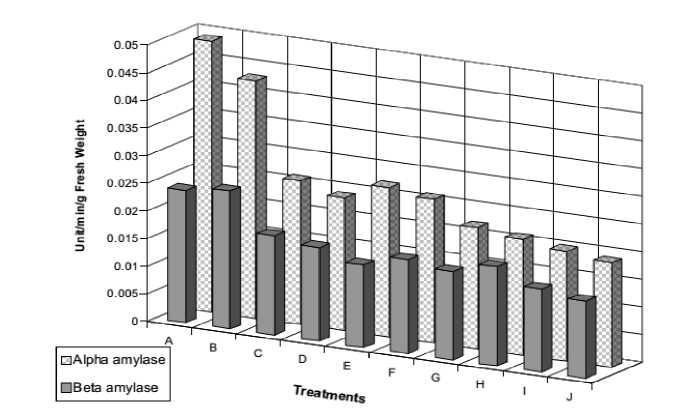
Figure 6. Alpha (α) and β amylase activities of 2-day old Vicia faba seedling as affected by NaCl level (0.0, 50, 100, 150 and 200 mM ) and presoaking in 50 ppm ascorbic acid; A: 0.0 mM NaCl, B: 50 ppm Asc., C: 50 mM NaCl, D: 50 mM NaCl + Asc., E: 100 mM NaCl, F: 100 mM NaCl + Asc., G: 150 mM NaCl, 150 mM NaCl + Asc., I: 200 mM NaCl, J: 200 mM NaCl + Asc.
CONCLUSION
The present work demonstrated that presoaking Vicia faba seeds with 50 ppm ≈ 0.3 mM ascorbic acid for 4 hrs before germination ameliorated the negative effect of NaCl- stress on growth criteria and some metabolic activity.
ACKNOWLEDGMENT
My deep appreciation goes to Dr. Bahia Abd El-Salam Abd El-Ghaffar, Professor of Plant Physiology (Tanta University, Egypt), for suggesting the point of research. I ask ALLAH to forgive her and the deceased Muslims as a whole and mercy on her and rest in peace and enter paradise.
Список литературы Role of ascorbic acid on germination indexes and enzyme activity of Vicia faba seeds grown under salinity stress
- Abd-El-Samad, H.M. (1993) Counteraction of NaCl with NaH2PO4 and NaNO3 on pigment, saccharide and protein contents in broad bean. Biol. Plant. 35 (4): 561-566
- Ahmed, A.M. Heikal, M.M. and Zedan, M.A. (1980) Effect of salinization treatments on growth and some related physiological activities of some leguminous plants. Can. Plant Sci. 60: 713-718
- Akça, Y., Samsunlu, E. (2012) The effect of salt stress on growth, chlorophyll content, proline and nutrient accumulation, and k/Na ratio in walnut. Pak. J. Bot. 44: 1513-1520
- Al-Hakimi, A.M. (2000) Interactive effect of Ca2+ and NaCl salinity on gas exchange and growth of broad bean (Vicia faba). Union Arab Biol. Cairo. Physiol. And algae. 8B: 33-43
- Al-Hakimi, A.M. and Hamada, A.M. (2001) Counteraction of salinity stress on wheat plants by grain soaking in ascorbic acid, thiamin or sodium salicylate. Biol. Plant. 44(2): 253-261
- Aly, M.M., El-sabbagh, S.M., El-shouny, W.A. and Ebrahim, M.K. (2003) Physiological response of Zea mays to NaCl stress with respect to Azotobacter chroococcum and Streptomyces niveus. Biol. Sci. 6(24): 2073-2080
- Amini, F. and Ehsanpour, A.A. (2006) Response of tomato (Lycopersicon esculentum Mill.) cultivars to MS, water agar and salt stress in vitro culture. Biol. Sci. 9(1): 170-175
- Ansari, S.A. and Khan, F.A. (1986) Effect of pre-sowing seed treatment with pyridoxine on growth and yield performance of summer moong. Indian Bot. Soc. 65: 316-322
- Ashraf, M. and Fatima, H. (1995) Responses of salt-tolerant and salt-sensitive lines of safflower Carthannus tinctorius L. to salt stress. Acta Physiol. Plant. 17: 61-70
- Ates, E., Tekeli, A.S. (2007) Salinity tolerance of Persian clover (T. resupinatum var. majus boiss.) lines at germination and seedling stage. World J. Agric. Sci. 3: 71-79
- Athar, H., Khan, A., Ashraf, M. (2008) Exogenously applied ascorbic acid alleviates salt induced oxidative stress in wheat. Environ. Exp. Bot. 63: 224-231
- Azooz, M.M. (1997) Interactive Effects of Some Vitamins and Salinity on Some Broad Bean Lines. Ph.D. Thesis, Fac. Sci., South Valley Univ., Qena, Egypt
- Azooz, M.M. (2009) Foliar application with riboflavin (Vitamin B2) enhancing the resistance of Hibiscus sabdariffa L. (Deep red sepals variety) to salinity stress. J. Biol. Sci. 9: 109-118
- Azooz, M.M., Alzahrani, A.M. and Youssef, M.M. (2013) The potential role of seed priming with ascorbic acid and nicotinamide and their interactions to enhance salt tolerance in broad bean (Vicia faba L.). Aus. J. of Crop Sci. 7(13): 2091-2100
- Azzedine, F., Gherroucha, H., Baka, M. (2011) Improvement of salt tolerance in durum wheat by ascorbic acid application. J. Stress Physiol. Biochem. 7: 27-37
- Bassuony, F.M., Hassanein, R.A., Baraka, D.M., Khalil, R.R. (2008) Physiological effects of nicotinamide and ascorbic acid on Zea mays plant grown under salinity stress II-Changes in nitrogen constituent, protein profiles, protease enzyme and certain inorganic cations. Aust. J. Appl. Sci. 2: 350-359
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254
- De La Rosa, I.M. and Maiti, R.K. (1995) Biochemical mechanism in glossy sorghum lines for resistance to salinity stress. Plant Physiol. 146: 515-519
- Dolatabadian, A., Sanavy, S.A.M.M., Chashmi, N.A. (2008) The effects of foliar application of ascorbic acid (vitamin C) on antioxidant enzymes activities, lipid peroxidation and proline accumulation of canola (Brassica napus L.) under conditions of salt stress. J. Agron. Crop. Sci. 194: 206-213
- Drossopoulus, J.B.; Karamannos, A.J. and Niavis, C.A. (1987) Changes in ethanol soluble carbohydrates during development of two cultivars subjected to different degrees of water stress. Ann. Bot. 59: 173-188
- Ebrahim, M.K. (2005) Amelioration of sucrose-metabolism and yield changes, in storage roots of NaCl-stressed sugarbeet, by ascorbic acid. Agrochimica, XLIX (3 -4): 93-103
- Ekmekçi, B. A., Karaman, M. (2012) Exogenous ascorbic acid increases resistance to salt of Silybum marianum (L.) Afr. J. Biotech. 11: 9932-9940
- El-Greadly, N. H. M. 2002. Effect of foliar application of ascorbic acid, ethrel and their combinations on growth, yield and endogenous hormones in cucumber plants. J. Agric. Sci. Mansoura Univ. 27(8): 5269-5281
- El-Kobisy, D.S, Kady, K.A. Medani, R.A. and Agamy, R.A. 2005. Response of pea plant pisum sativum L. to treatment with ascorbic acid. Egypt. J. Appl. Sci. 20: 36-50
- El-Tayeb, M.A. (1995) Effect of thiamin seed presoaking on the physiology of Sorghum bicolor plants grown under salinity stress. Egypt. Bot. 35(2): 201-214
- El-Tayeb, M.A., Ahmed, A.M., Ismail, A.M. and Hamed, S.T. (1999) Response of seeds of Cicer arietinum, Lens culinaris and Trigonella foenum-graecum to the interactive effect of salinity and thiamine or ascorbic acid. Acta Agron. Hungarica, 47(3): 265-275
- Flowers, T.J., Troke, P.F. and Yeo, A.R. (1977) The mechanism of salt tolerance in halophytes. Ann. Rev. Plant Physiol. 28: 89-121
- Greulach, V.A. and Adams, J.E. (1963) An Introduction to Modern Botany, pp. 327-328. John Wiley and Sons, New York
- Guerrier, G. (1991) Improvement of seed vigor by KCl and CaCl2 pretreatments: relation with the regulation of hydrolytic enzyme activities. Agrochimica. 35(5-6): 396-407
- Hamada, A.M. and El-Enany, A.E. (1994) Effect of NaCl salinity on growth, pigment and mineral element contents, and gas exchange of broad bean and pea plants. Biol. Plant. 36(1): 75-81
- Handa, S., Bressan, R.A., Handa, A.K., Carpita, N.C. and Husegawa, P.M. (1983) Solutes contribution to osmotic adjustment in cultured plant cells adapted to water stress. Plant physiol. 28: 834-849
- Harbone, J.B. (1973) Phytochemical methods, a guide to modern techniques of plant analysis. Chapman and Hall. London, pp 185-186
- Hassanein, R.A., Bassuony, f.M., Baraka, D.M. and Khalil, R.R. (2009) Physiological Effect of Nicotinamide and Ascorbic Acid on Zea mays Plant Grown Under Salinity Stress. I-Changes in Growth, Some Relevant Metabolic Activities and Oxidative Defense System. Res. J. of Agric. and Biolo. Sci. 5(1):72-81
- Heikal, M.M., El-Tayeb, M.A., Ismail, A.M. and Hamed, S.T. (1999) Effect of thiamine and ascorbic acid on growth, carbon and nitrogen metabolism of some salinity stressed crop plants. Bull. Fac. Sci., Assiut Univ., 28(2-D): 279-294
- Ismail, A.M. and Azooz, M.M. (2002) Response of Vicia faba to salinity and vitamins. Indian Plant Physiol. 7(3): 298-301
- Jiang, L.; Duan, L.; Tian, X.; Wang, B.; Zhang, H.; Zhang, M. and Li, Z. (2006) NaCl salinity stress decreased Bacillus thuringiensis (Bt) protein content of transgenic Bt cotton (Gossypium hirsutum L.) seedlings. Enviro. and Exp. Bot. 55(3): 315-320
- Kaya, C., Ashraf M., Dikilitas M., Tuna A.L. (2013) Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients. Aust. J. Crop Sci. 7: 249-254
- Khan, F.A. and Ansari, S.A. (1984) Enhancement of lateral roots differentiation in urd (black gram) by pyridoxine application. Indian. Bot. Soc. 63: 102-108
- Khan, F.A. and Zaidi, A.K. (1985) On optimizing growth and yield of mustard by pre-sowing seed treatment with pyridoxine. Indian Bot. Soc. 64: 91-94
- Khan, M.A., Ahmed, M.Z., Hameed, A. (2006) Effect of sea salt and L-ascorbic acid on the seed germination of halophytes. J Aird Environ. 67: 535-540
- Kodandaramaiah, J. (1983) Physiological studies on the influence of B-vitamins on leaf and fruit metabolism in cluster beans Cyamopsis tetragonoloba L. Tanb. Ph.D. Thesis submitted to Srivenkateswera Univ. Tirupati, India
- Kudrev, T. and Pandev, S. (1965) Raising yield of swamp damaged wheat by vitamin B1 spraying. C.R. Acad. Bull. Sci. 18: 555-557
- La Haye, P.A. and Epstein, E. (1971) Calcium and salt tolerance by bean plants. Physiol. Plant. 25: 213-215
- Levitt, J. (1980) Salt stresses. In: “Responses of Plants to Environmental Stresses” Vol. П. “Water, Radiation, Salt and Other stresses” Academic Press, New York p. 365-454
- Mac Gregor, A.W. (1978) Changes in α-amylase enzymes during germination. Am. Soc. Brewing Chem. 36: 1-5
- Meiri, A. and Poljakoff-Mayber, A. (1970) Effect of various salinity regions on growth, leaf expansion and transpiration rate of bean plants. Soil Sci. 109: 26-29
- Misra, A.N., Sahu, S.M. and Misra, M. (1997) Sodium chloride induced changes in leaf growth, pigment and protein contents in two rice cultivars. Biol. Plant. 39(2): 257-262
- Misra, N. and Gupta, A.K. (2006) Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. Plant physiol. 163(1): 11-18
- Mola-Diola, Y.A., Kumar, B. and Daysal, J. (1998) Effect of salinity and boron on germination and early seedling growth of broad bean (Vicia faba L.). Research. 28(2-3): 63-72
- Mozafar, A. and Oertli, J.J. (1992) Uptake and transport of thiamin (vitamin B1) by barley and soybean. Plant Physiol. 139: 436-442
- Munns, R., Greenway, H., Delane, R. and Gibbs, J. (1982) Ion concentration and carbohydrate status of the elongating leaf tissue of Hordium vulgare growing at high external NaCl. Π Cause of the growth reduction. Exp. Bot. 33: 574-580
- Murphy, K.S.T. and Durako, M.J. (2003) Physiological effect of short term salinity changes on Ruppia maritime. Aquat. Bot. 75: 293-309
- Naguib, M.I. (1963) Colorimetric estimation of plant polysaccharides. Zucker, 16: 15-18
- Nelson, N. (1944) A Photometric adaptation of Somogi method for the determination of glucose. Biol. Chem. 153: 275-280
- Neumann, P. (1997) Salinity resistance and plant growth revisited. Plant Cell Environ. 20: 1193-1198
- Oertli, J.J. (1987) Exogenous application of vitamins as regulators for growth and development of plants. A review. Z. Pflanzenernähr. Bodenk. 150: 375-391
- Pascale, S., Barbieri, G. and De-Pascale, S. (1997) Effect of soil salinity and top removal on growth and yield of broad bean as a green vegetable. Sci. Horticul. 71(3-4): 147-165
- Preeti, D., Ameeta, B., and Kushwah, H. (2000) Amylase activity and ascorbic acid content in legume seeds during germination. Indian. Animal-Nutrition. 17(3): 249-251
- Ramezani, E., Sepanlou, M. G., Badi, H. A. N. (2011) The effect of salinity on the growth, morphology and physiology of Echium amoenum Fisch. & Mey. Afric. J Biotech. 10: 8765-8773
- Rather, G. and Doering, H.W. (1984) Influence of extreme potassium to sodium ratio and high substrate salinity on plant metabolism of crops differing in salt tolerance J. Plant Nutr. 6(7): 583-595
- Razmjoo, K., Heydarizadeh, P., Sabzalian, M.R. (2008) Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int. J. Agric. Biol. 10: 451-454
- Sadak, M.Sh., Rady M.M., Badr N.M., Gaballah M.S. (2010) Increasing sunflower salt tolerance using nicotinamide and α-tocopherol. Int. J. Acad. Res. 2(4): 263-270
- Sairam, R.K. and Srivastava, G.C. (2002) Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci. 162(6): 897-904
- Sairam, R.K., Veeravhadra, R.K. and Srivastava, G.C. (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 163(5): 1037-1046
- Sallam, H.A. (1999) Effect of some seed soaking treatments on growth and chemical components on faba bean plants under saline conditions. Annal. Agricul. Sci. Cairo. 44(1): 159-171
- Samiullah, S.A. and Afridi, M.M. (1988) B-vitamins in relation to crop productivity. Indian Rev. Life Sci. 8: 51-74
- Shaddad, M.A, Radi, A. F., Abdel-Rahman, A.M. and Azooz, M.M. (1990) Response of seeds of Lupinus termis and Vicia faba to the interactive effect of salinity and ascorbic acid or pyridoxine. Plant and soil. 122(2): 177-183
- Shaddad, M.A. (1990) The effect of proline application on the physiology of Raphanus sativus plants grown under salinity stress. Biol. Plant. 32: 104-107
- Shalata, A. and Neumann, M. (2001) Exogenous ascorbic acid (Vitamin C) increases resistance to salt stress and reduces lipid peroxidation. Exp. Bot. 52(364): 2207-2211
- Shukry, W.M. and El-Bassiouny, H.M. (2002) Gibberellic acid effects on protein pattern, hydrolytic enzyme activities and ionic uptake during germination of Vicia faba in sea water. Acta-Botanica-Hungarica. 44(1-2) 145-162
- Singh, S.P., Singh, B.B. and Singh, M. (1994) Effect of Kinetin on chlorophyll, nitrogen and proline in mungbean Vigna radiata under saline conditions. Indian Plant Physiol. 37: 37-39
- Smirnoff, N., Conklin, P. and Loewus, F.A. (2001) Biosynthesis of ascorbic acid in plants: a renaissance. Ann. Rev. Plant Physiol. Plant Mol. Biol. 52: 437-440
- Steel, R.G. and Torrie, J.H. (1980) Principles and Procedures of Statistics, 2nd ed, McGraw-Hill Book Company Inc., New York, London
- Ziaf, K., Amjad, M., Pervez, M.A., Iqbal, Q., Rajwana, I.A., Ayub, M. (2009) Evaluation of different growth and physiological traits as indices of salt tolerance in hot pepper (Capsicum annuum L.). Pakistan J. Bot. 41: 1797-1809


