Role of heat shock proteins and plasma membrane on thermotolerance in Saccharomyces cerevisiae-VS3 strain
Автор: Pasha Shaik Muzammil, Musfera Shaik, Venkateswar Rao L., Pasha Chand
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.19, 2023 года.
Бесплатный доступ
Aim: Study of HSPs synthesis after heat and cold shock and explanation of thermotolerance by the transport of HSPs to the plasma membrane. Methods and Results: Physical (cold and heat shock) and chemical (lignocaine) damage to plasma membrane was achieved in thermotolerant and mesophilic strains of Saccharomyces cerevisiae . In shocked yeasts K+ ion efflux, leakage of UV280 absorbing material, HSP expression profile and viability at 25 and 45°C were studied. Physical/chemical shock was given for 30 minutes and subsequently yeasts were incubated at 25°C to avoid further membrane damage by stress. In thermotolerant strain, membrane damage increased up to 70 minutes (30 min of shock and 40 min at 25°C) and reduced thereafter. De-novo HSPs in membrane were noted at 60 minutes and reached maximum at 80 minutes in thermotolerant strain. In mesophilic yeast, de-novo HSPs were not synthesized and leakage was continuous up to the studied period (100 minutes). Conclusion: These de-novo HSPs are transported to the membrane for restoring the membrane integrity and to prevent the leakage. The thermotolerant strain can grow at higher temperatures compared to mesophilic strain due to more production of HSPs and HSP associated membrane damage reversal. Significance and Impact of the Study: Several reports established the role of HSPs in thermotolerance but their mode of action is not well characterized. The current method explains the mechanism for acquiring thermotolerance in yeast.
Saccharomyces cerevisiae, vs3, heat shock proteins, hsp 104, thermotolerance, cold shock
Короткий адрес: https://sciup.org/143180109
IDR: 143180109
Текст научной статьи Role of heat shock proteins and plasma membrane on thermotolerance in Saccharomyces cerevisiae-VS3 strain
Severe heat stress causes protein denaturation in various cellular compartments. This necessitates conformational repair of vital proteins for survival because gene expression is transiently blocked after thermal insult Mühlhofer et al ., 2019; Hanninen et al., 1999).
Yeast that grows at 40°C and above is considered as thermo tolerant (Sree et al., 2000). Fermentation using thermotolerant yeasts at such temperatures also result in faster fermentation rates, cuts the over all fermentation and cooling costs, so that ethanol can be made at cheaper rates (Prado et al., 2020). There are very few reports on the selection of yeasts that are able to grow and ferment at higher temperatures (Banat et al., 2000). The Saccharomyces cerevisiae is an invaluable model for research of regulatory features of stress response (Le Breton and Mayer, 2016). In addition, budding yeast has been an outstanding model organism to elucidate the role of chaperones and correlating their functions (Verghese et al., 2012).
A substantial number of genes undergo repression after stress, including many genes associated with growth and cell division, such as actin, alpha and beta tubulin whereas stress induces more HSPs (Trinklein et al., 2004). The classical view of the HS response is that, stressing agents cause the accumulation of denatured proteins in the cell with a concomitant induction of the genes responsible for HSPs synthesis (Parsell and Lindquist, 1993; Kumar et al., 2020). Furthermore the temperature sensing mechanism is thought to be intimately associated with membrane structure and function (Chatterjee et al., 1997). The signals leading to HSF (Heat Shock Factor) activation and HSP synthesis i.e., the cellular thermometer is still a matter of debate Castells-Roca et al., 2011). Among the proposed hypotheses are the presence of abnormal, unfolded or misfolded proteins, alteration in physical state of cell membrane, second messenger induction and generation of reactive oxygen species (ROS) have been implicated in this process (Polla et al., 1997). Lignocaine (a local anaesthetic & membrane fluidizer) depresses the membrane lipid phase transition (Mizogami et al., 2002)
and fluidize the lecithin membrane by unsaturable nonspecific binding (Ueda et al., 1977).
Studies have shown that an efflux of potassium ions is a first indication of membrane damage (Fujita, 2002; Heipeiper et al. 1996). Potassium ion is a major cytoplasmic cation in growing yeast cells, and is involved in several key functions (Fujita and Kubo, 2002). Proton influx (Sikkema et al., 1995) and UV280 absorbing material leakage also follows K+ ion leakage. In addition to leakage of ions upon damage to cell membrane several other components are excreted.
In the present study, thermotolerant and mesophilic strains of Saccharomyces cerevisiae were used to investigate the effect of temperature (cold and heat shock) and chemical (lignocaine) shocks on HSP expression profile (by western blotting and autoradiography) and the damage, caused if any to the yeast plasma membrane (by efflux of potassium ions and UV280 absorbing material). HSP expression studies and cell viability assays were used to understand the mechanism of thermotolerance.
MATERIALS AND METHODS
Microorganism
Saccharomyces cerevisiae - VS3 was isolated from soil samples collected from hot regions near the Kothagudem Thermal Power Plant located in Khammam District, A.P, India. The organism was isolated, mutated by UV and identified as Saccharomyces cerevisiae - VS3 strain in our lab (Sree et al., 2000). It was maintained on Yeast extract Peptone Dextrose agar medium (YEPD) (1% Yeast Extract, 2% Peptone, 2% Glucose, 2% AgarAgar, pH 6.0). Mesophilic yeast Saccharomyces cerevisiae MTCC 3205 was obtained from Institute of Microbial Technology (IMTECH), Chandigarh, India, grown and maintained as described above at 25oC.
Radiolabelled methionine was obtained from BARC (Baba Atomic Research Center) Mumbai, India.
Membrane damage, HSP profile and viability of thermotolerant and mesophilic strains of Saccharomyces cerevisiae following temperature stress
Preliminary experiments were carried out to determine whether the two yeast strains could withstand severe cold shock (-200C) or heat shock (450C) for 30 minutes. For this purpose thermotolerant and mesophilic yeast strains were grown in YEPD broth medium of pH 6.0 for 48 hours at 150-rpm and 25oC and cultures of OD 0.3 were aliquoted, treated with lignocaine (0.1065 mg ml-1) and then subjected to the above temperatures for 30 min (since longer time periods count high mortality). After heat/cold stress, yeast was incubated at 25 oC. Cells treated in similar fashion in the absence of lignocaine served as controls and cells grown at 25oC served as control for shocked cells. The samples were aliquoted at 0 minutes to 70 minutes of incubation at 25 oC after heat/cold shock and analyzed for K+ ion efflux, UV280 absorbing material leakage, viability and membrane HSP profile. HSPs were detected both by autoradiography and western blot analysis.
Optimization of heat and cold shock temperature effect on membrane damage, HSP profile and viability
In an attempt to determine the temperatures at which cold and heat shock would be maximum, the thermotolerant yeast was subjected to temperatures ranging from -20o to 50o C for 30 minutes in the presence and absence of lignocaine (0.1065 mg ml-1). Subsequently the cultures were shifted to 25o C and after 70 minutes the cells were analyzed for K+ ion efflux, UV280 absorbing material leakage, viability and membrane HSP profile.
Cell viability assay
The effect of heat shock on yeast survival rate was studied by the plate colony count method. Yeast was given heat shock as described above and, diluted sample (0.1 ml of 10-4) was plated on YEPD agar plates in triplicates. After 2 days of incubation at 25 and 45oC, colony-forming units were recorded.
[35S] Methionine labelling, protein extraction, and electrophoresis
Thermotolerant yeast culture (10 ml) was washed and resuspended in 2 ml of YNB medium (0.67% yeast nitrogen base, 0.3% KH2PO4, 2% glucose, pH 6.0) without amino acids. After subjecting cells to thermal stress, 10 mCi of [35S] methionine (specific activity, 1,150 Ci mM) was added to control and shocked samples and the preparations were incubated at 25oC temperatures for 70 minutes. Cells were pelleted and protein was extracted by cell disruption in an ultrasonicator for detection of total cell HSPs.
In thermotolerant and mesophilic yeast, after stress induction and [35S] methionine labelling, samples were collected at 0, 10, 20, 30, 40, 50,60 and70 minute intervals from the set incubated at 25°C for detection of membrane HSPs. Protein concentrations of cell lysate and membrane were determined using Lowry method (Lowry et al., 1951). Fifty mg of extracted protein sample was resolved using 10 % SDS-PAGE. The gels were stained with Coomassie blue, dried and exposed to Hyper- film-MP (Amersham) at -70°C for 5 days before being developed.
Yeast plasma membrane isolation
Yeast plasma membrane isolation was carried out according to Santos et al. (1978). The membrane pellet equivalent to 50 mg of protein was resolved by electrophoresis and the gels were processed for autoradiography according to Sambrook et al. (1989).
Western immunoblot analysis
Following extraction and electrophoresis, proteins were transfered to Hybond-C super nitrocellulose membranes (Amersham). All the antibodies were used at a dilution of 1:10000 in phosphate buffered saline (PBS) with 0.1% Triton X 100. The appropriate secondary antibody with conjugated horse radish peroxidase was used at a dilution of 1:1000. Prior to detection, the final membranes were washed 3 times for 5 minutes each in PBS containing 0.1% Triton X100. Membranes were developed using 3,3-diaminobenzidine in 30 ml of PBS buffer containing 30 ml of H202 and developed by keeping them in darkness for a few minutes.
Membrane damage studies: Potassium efflux
Plasma membrane damage of yeast cells was monitored based on efflux of potassium ions by flame photometry. Yeast cells grown overnight in YEPD broth at 25oC as described earlier, were harvested, washed with 10 mM EDTA, and then with distilled water twice and finally re-suspended in saline to an OD450 nm equivalent to 2.0. Lignocaine treated and untreated cell suspensions of thermotolerant yeast was exposed to -20, -10, 0, 25, 40, 45 and 50oC for 30 minutes and subsequently incubated at 25 oC. From this sample the potassium ion concentration in the cell free supernatant was determined up to 70 minutes at an interval of 10 minutes. In another set, shock was given to thermotolerant and mesophilic strains at -20o, 25o, 45oC and potassium ion efflux was studied at different time intervals as explained above.
Leakage of UV 280 absorbing material
The method of Williams et al. (1991) was followed to determine the leakage of UV 280 absorbing material. The cell suspension was prepared as described above and the absorbance of the cell free supernatant was determined at 280 nm using Spectronic UV spectrophotometer.
Statistical Analysis
Statistical analysis was carried out using Two-way ANOVA (Table 1).
RESULTS
Membrane damage
Thermotolerant and mesophilic strains of yeast following exposure to –20 and 45oC showed time dependent increase in membrane damage as judged by K+ ion efflux and leakage of UV280 absorbing material. Membrane damage appeared to be more severe in the presence of lignocaine than the absence of lignocaine. The increase in membrane damage observed in 25oC cultured cells can be attributed to the presence of lignocaine. From the results of Table 1 it is clear that –
20, 45 and 50oC caused appreciable membrane damage. In an attempt to ascertain the temperatures which would cause maximum membrane damage the thermotolerant strain was subjected to temperatures ranging from –20 to 50oC. The results indicate that –20 and 50oC caused maximum damage to the membrane irrespective of the presence or absence of lignocaine. In thermotolerant strain, membrane leakage increased with time up to 70 minutes (including 30 min shock time) but decreased thereafter. Whereas in mesophilic yeast, membrane leakage was continuously increased up to the studied period of 100 minutes (Table 1).
Induction of thermotolerance after heat and cold shocks
Yeast cells were grown at 25oC and then given shock at different temperatures ranging from -20 to 50oC for 30 minutes. Subsequently the cultures were shifted to 25oC for 70 minutes and their viability was recorded following plating and incubation at 25 and 45oC respectively. The results indicate that culture subjected to cold shock is comparatively more thermotolerant than culture subjected to heat shock. Further when cells treated with lignocaine were compared with cells in the absence of lignocaine it appeared that heat shock at 30, 40oC made the cells more thermotolerant in the presence of lignocaine. In contrast to thermotolerant strain, increase in thermotolerance was not observed following cold and heat shock in mesophilic yeast strain. The experiments were replicated thrice in triplicates and mean values varied by no more than + 5% except values otherwise indicated (Table 2 & Fig 1).
Induction of heat shock proteins after cold and heat shock in thermotolerant Saccharomyces cerevisiae-VS3 strain
Autoradiographic and SDS-PAGE analysis after total proteins of thermotolerant yeast following cold (0 to – 20oC) and heat (25 to 50oC) shock for 30 min and subsequent incubation at 25oC for 70 min indicated the synthesis of a number of proteins. Following cold shock, increase in the synthesis of proteins of molecular weight 30, 60, 70, 90, 104 KD was observed whereas following heat shock only proteins of molecular weight 60, 70 KD increased in levels up to 45oC.
Table 1a: Potassium efflux (ppm) and leakage of UV280 absorbing material (OD) following heat and cold shock of thermotolerant Saccharomyces cerevisiae- VS3 yeast and mesophilic Saccharomyces cerevisiae MTCC 3205 strains in the absence or presence of lignocaine*.
|
Time (min) |
Saccharomyces cerevisiae ( VS3) ** |
Mesophilic S . cerevisiae (MTCC 3205)** |
||||||||||
|
Potassium efflux (ppm) |
Leakage of UV280 absorbing material (OD) |
Potassium efflux (ppm) |
Leakage of UV280 absorbing material(OD) |
|||||||||
|
-20oC |
25oC |
45oC |
-20oC |
25 o C |
45 o C |
-20oC |
25oC |
45oC |
-20oC |
25 o C |
45 o C |
|
|
30 |
1.2± |
1.3± |
1.7± |
0.11± |
0.09± |
0.19± |
1.4± |
1.3± |
1.6± |
0.13± |
0.10± |
0.20± |
|
0.01 |
0.03 |
0.01 |
0.03 |
0.01 |
0.01 |
0.01 |
0.02 |
0.02 |
0.01 |
0.02 |
0.02 |
|
|
40 |
1.8± |
1.4± |
2.0± |
0.26± |
0.15± |
0.28± |
1.8± |
1.6± |
2.2± |
0.27± |
0.15± |
0.30± |
|
0.01 |
0.02 |
0.01 |
0.04 |
0.02 |
0.04 |
0.02 |
0.02 |
0.01 |
0.04 |
0.02 |
0.04 |
|
|
50 |
2.5± |
1.6± |
2.3± |
0.39± |
0.18± |
0.37± |
2.1± |
1.9± |
2.4± |
0.39± |
0.18± |
0.41± |
|
0.01 |
0.02 |
0.02 |
0.05 |
0.02 |
0.05 |
0.02 |
0.03 |
0.02 |
0.05 |
0.02 |
0.04 |
|
|
60 |
2.9± |
1.7± |
2.6± |
0.49± |
0.21± |
0.41± |
2.5± |
2.3± |
2.7± |
0.51± |
0.26± |
0.47± |
|
0.02 |
0.01 |
0.02 |
0.04 |
0.04 |
0.04 |
0.03 |
0.03 |
0.01 |
0.03 |
0.02 |
0.04 |
|
|
70 |
3.3± |
1.9± |
2.9± |
0.50± |
0.22± |
0.43± |
2.8± |
2.4± |
3.2± |
0.54± |
0.29± |
0.49± |
|
0.02 |
0.01 |
0.02 |
0.03 |
0.02 |
0.04 |
0.03 |
0.02 |
0.03 |
0.03 |
0.04 |
0.04 |
|
|
80 |
3.0± |
1.6± |
2.7± |
0.46± |
0.14± |
0.40± |
3.2± |
3.0± |
3.4± |
0.59± |
0.32± |
0.54± |
|
0.01 |
0.01 |
0.03 |
0.04 |
0.03 |
0.04 |
0.03 |
0.04 |
0.04 |
0.03 |
0.05 |
0.03 |
|
|
90 |
2.5± |
1.3± |
2.0± |
0.30± |
0.11± |
0.31± |
3.5± |
3.1± |
3.5± |
0.62± |
0.39± |
0.56± |
|
0.02 |
0.01 |
0.03 |
0.01 |
0.04 |
0.02 |
0.03 |
0.01 |
0.04 |
0.03 |
0.03 |
0.01 |
|
|
100 |
1.8± |
1.1± |
1.4± |
0.15± |
0.09± |
0.20± |
4.1± |
3.2± |
3.9± |
0.70± |
0.46± |
0.62± |
|
0.03 |
0.02 |
0.03 |
0.02 |
0.02 |
0.01 |
0.02 |
0.01 |
0.04 |
0.03 |
0.02 |
0.02 |
|
Both strains in the absence of lignocaine showed time dependent increase in K+ ion efflux and UV280 absorbing material leakage. But, the values are significantly lower compared to cells treated with lignocaine (data not shown).
** All the experiments were repeated thrice using three replicates each time (n=9) and values are represented as ppm + SD for K+ ion efflux and OD280 for UV absorbing material. For two way ANOVA analysis three average results of replicates was taken (n=3).
Table 1b: Two-way ANOVA of Potassium efflux (ppm) following heat and cold shock of thermotolerant Saccharomyces cerevisiae- VS3 yeast and mesophilic Saccharomyces cerevisiae MTCC 3205 strains in the presence of lignocaine.
|
Source of Variation |
Sum of Squares |
Total (n) |
d.f. |
Mean Square |
F Ratio |
F0. 95 |
|
Total |
51.9 |
144 |
143 |
|||
|
Between-means of Products |
617.49 |
8 |
7 |
88.21 |
19.38 |
2.09 |
|
Between-means of judges |
165 |
18 |
17 |
9.70 |
2.13 |
1.75 |
|
Error |
542.19 |
119 |
4.55 |
For Error d.f. = 143-17-7= 119
Note : Potassium efflux (ppm) following heat and cold shock of thermotolerant and mesophilic yeast strains in the presence of lignocaine is significant at 5% level.
Table 1c: Two-way ANOVA of leakage of UV280 absorbing material following heat and cold shock of thermotolerant Saccharomyces cerevisiae- VS3 yeast and mesophilic Saccharomyces cerevisiae MTCC 3205 strains in the presence of lignocaine.
|
Source of Variation |
Sum of Squares |
Total (n) |
d.f. |
Mean Square |
F Ratio |
F0. 95 |
|
Total |
5.25 |
144 |
143 |
|||
|
Between-means of Products |
11.98 |
8 |
7 |
1.71 |
57 |
2.09 |
|
Between-means of judges |
12.94 |
18 |
17 |
0.76 |
25.33 |
1.75 |
|
Error |
4.29 |
119 |
0.036 |
For Error d.f. = 143-17-7= 119
Note: Leakage of UV280 absorbing material following heat and cold shock of thermotolerant and mesophilic yeast strains in the presence of lignocaine is significant at 5% level.
Table 2: Potassium efflux, UV 280 absorbing material leakage and viability at 25 and 45 oC of shocked VS3 strain.
|
Yeast |
Lignocaine untreated † Saccharomyces cerevisiae-VS3 Strain |
Lignocaine treated † Saccharomyces cerevisiae-VS3 Strain |
||||||
|
Parameter |
K+ ion efflux |
UV280 |
Viability CFU |
K+ efflux |
UV280 |
Viability CFU |
||
|
Temp.(oC) |
25 oC |
45 oC |
25 o C |
45 o C |
||||
|
-20 |
1.75± |
0.15± |
96 |
98 |
2.0± |
0.16± |
92 |
72 |
|
0.018 |
0.003 |
0.012 |
0.001 |
|||||
|
-10 |
2.1± |
0.217± |
102 |
104 |
2.4± |
0.228± |
103 |
105 |
|
0.022 |
0.029 |
0.019 |
0.013 |
|||||
|
0 |
1.5± |
0.145± |
97 |
98 |
1.7± |
0.142± |
94 |
96 |
|
0.029 |
0.019 |
0.014 |
0.019 |
|||||
|
25 |
1.1± |
0.057± |
105 |
80 |
1.1± |
0.081± |
92 |
102 |
|
0.036 |
0.015 |
0.017 |
0.022 |
|||||
|
30 |
1.3± |
0.181± |
104 |
90 |
1.4± |
0.117± |
92 |
98 |
|
0.041 |
0.023 |
0.015 |
0.025 |
|||||
|
35 |
1.2± |
0.145± |
101 |
92 |
1.5± |
0.179± |
91 |
97 |
|
0.044 |
0.041 |
0.030 |
0.036 |
|||||
|
40 |
1.3± |
0.225± |
90 |
98 |
1.4± |
0.281± |
94 |
96 |
|
0.042 |
0.012 |
0.032 |
0.033 |
|||||
|
45 |
1.2± |
0.213± |
98 |
93 |
1.5± |
0.225± |
92 |
97 |
|
0.011 |
0.026 |
0.039 |
0.046 |
|||||
|
50 |
3.1± |
0.360± |
22 |
17 |
3.5± |
0.414± |
11 |
08 |
|
0.015 |
0.024 |
0.041 |
0.029 |
|||||
†: All the experiments were repeated thrice using three replicates each time (n=9) and values are represented as ppm + SD for K+ ion efflux , OD280 for UV absorbing material and colony forming units (CFU) for viability.
Time in minutes
-20oC shocked growth at 25oC
Unshocked growth at 25oC '
~“— 45oC shocked growth at 25oC '
' -20o shocked growth at 25oC 11
Unshocked growth at 25oC "
45oC shocked growth at 25oC '1
Unshocked growth at 45oC 1
~* 45oC shocked growth at 45oC 1
--- -20oC shocked growth at 45oC 1 1
Unshocked growth at 45oC 1 1
--45oC shocked growth at 45oC ' '
* Thermotorant Yeast
“ Mesophilic Yeast
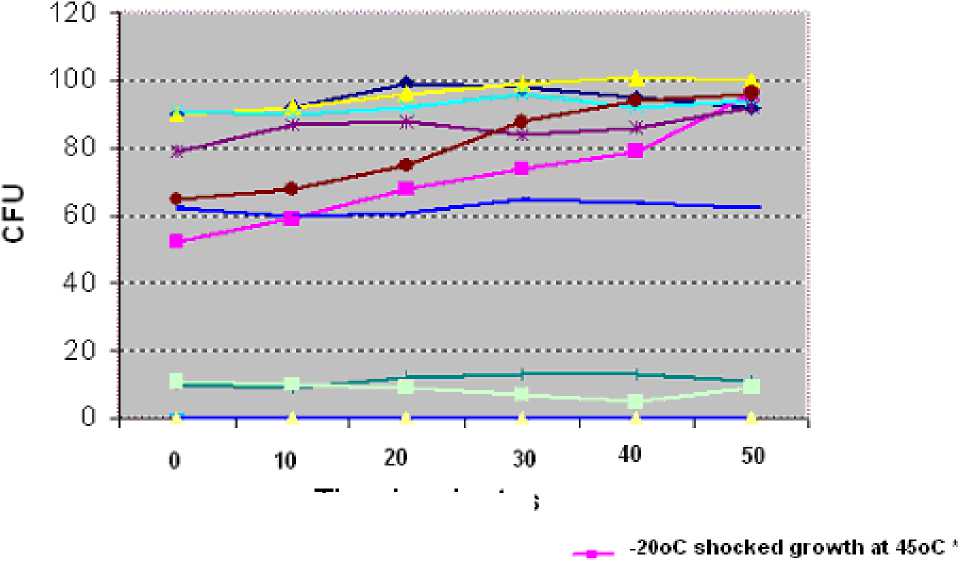
Figure 1: Colony forming units per ml at 10-4 dilution of heat and cold shocked yeast with lignocaine treatment at various time intervals of post shock incubation at 25oC.
Lignocaine treated yeasts showed less colony forming units (CFU) than untreated yeasts at 25oC. In thermotolerant strain after cold shock at -20o C, few cells were killed initially (low count), but the CFU were gradually increased with the post shock incubation at 25o C and attained the maximum at 80th minute. In mesophilic yeast, heat and cold shock has decreased the initial viable cells and there are no CFU at 45o C, indicating that there is no increase in thermotolerance.
HSP 90
Lane 123 456789 10 11
9 10 11
HSP 104
HSP 90
HSP 70
HSP 60
HSP 30
Lane
HSP104
HSP 70
HSP 60
2 3 4 5 6 7 8
ВЕТД-АСТМ - hsp 30 -•
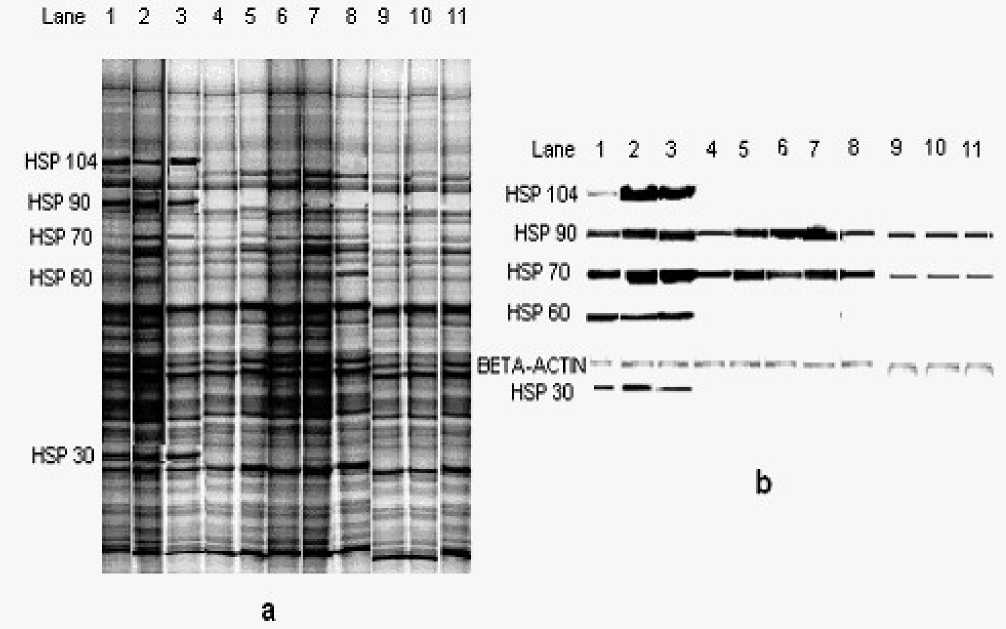
Figure 2:Total cell HSP profile of Thermotolerant yeast
-
(a) Autoradiograph (b) Western blot
Lane 1-8: Heat and cold shocked samples of thermo tolerant yeast at -20, -10, 0, 25, 35, 40, 45 and 50o C temperatures Lane 9-11 mesophilic yeast shocked at-20, 25 and 45 o C
-
a) In thermotolerant yeast de -novo proteins were produced after heat and cold shock. De-novo proteins of molecular weight 104, 90, 70, 60, and 30 were detected in cold shocked yeast. Their levels are more in -10o C shocked yeast. Proteins of molecular weight 70 and 60 were produced by heat shock. Apart from these proteins, many other proteins were also produced but their levels were not increased with the intensity of shock temperature. In mesophilic yeast presence of de-novo proteins in the molecular weight range of HSPs was not noted.
-
b) Five types of HSPs (104,90,70,60 &30) were produced by cold shock and maximum production was observed at -10o C shock. HSPs 70 & 60 were synthesized by heat shock and their levels increased up to 45o C shock and there after decreased. In 50o C heat shocked sample, many of the yeasts were killed, which may be leading to the decreased HSP levels (Table 2). Lignocaine treated and shocked (heat and cold) yeast produced HSPs similar to particular shocked yeast, but HSP levels were increased. In lignocaine treated and unshocked yeast (25o C), HSP 70 and 60 were noted and the levels were more than the base line (unshocked yeast). Induction of HSP 70, 60 by lignocaine resembles the results of heat shock. In stressed mesophilic yeast only base-line Hsps were noted as seen in un shocked yeast.
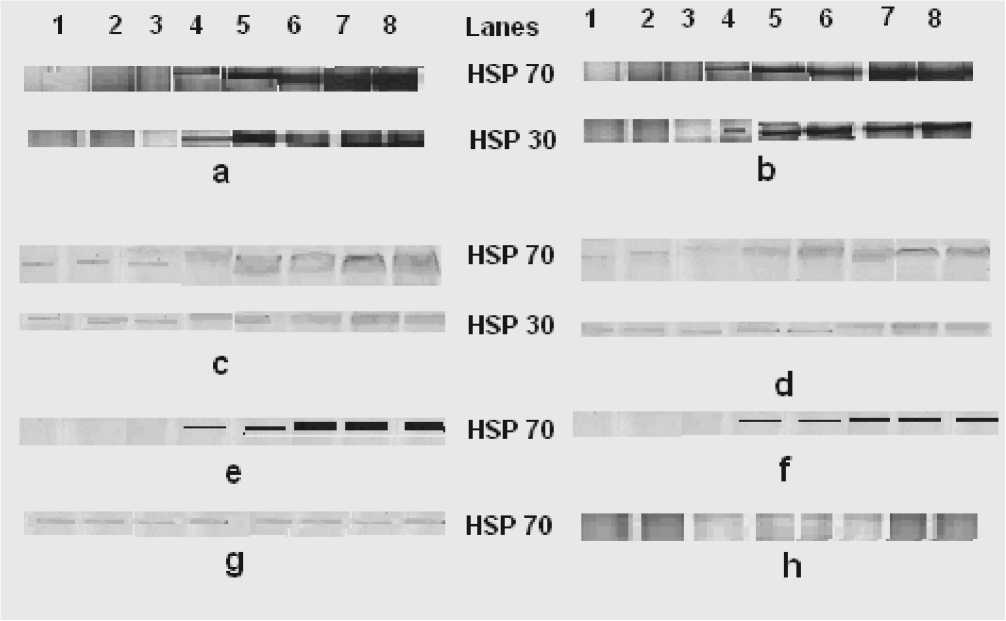
Fig: 3 Cell membrane HSP profile of Thermotolerant yeast
-
(a) Autoradiograph of lignocaine treated,
-
(b) Autoradiograph of lignocaine untreated,
-
(c) Western blot of lignocaine treated,
-
(d) Western blot of lignocaine untreated and cold shocked(–20oC)
-
(e) Autoradiograph of lignocaine treated ,
-
(f) Autoradiograph of lignocaine untreated and heat shocked (45oC)
-
(g) Western blot of lignocaine treated and heat shocked (45oC) mesophilic yeast
-
(h) Autoradiograph of lignocaine treated and heat shocked (45oC) mesophilic yeast
Lanes 1-8: Protein sample from membrane extracted at 0, 10, 20, 30, 40 ,50,60 and 70 minutes of 30 minutes post shock incubation at 25oC after shock.
De-novo HSPs (HSP 70, 30) in plasma membrane were noted only after 30 minutes of incubation at 25o C after the shock at -20o C (a). In lignocaine treated and -20o C shocked yeast HSP levels are more than lignocaine untreated yeast (b). In western blot analysis increase of HSPs from base line was noted similar to autoradigraph (c, d). In 45oC heat shocked yeast, only de-novo HSP 70 was detected in the membrane after 30 minutes of incubation at 25oC. The level of this HSP was more in lignocaine treated (e) compared to lignocaine untreated yeast (f). In stressed mesophilic yeast only base-line Hsps were noted in membrane and de-nova Hsps were not found as seen in un-shocked yeast.
However when the cells were subjected to 50oC heat shock both the above proteins decreased in their intensity. Western blot analysis confirmed the presence of above molecular weight proteins as respective HSPs (Fig 2). Lignocaine treated and shocked yeast cells showed HSP profile similar to that of heat shocked yeast. In mesophilic yeast de-novo HSPs were not synthesized after heat and cold shock and their levels were maintained constant like the un shocked yeast. Beta–Actin levels were maintained stable in heat/cold shocked yeasts at all studied periods (Fig 2).
Membrane stress proteins
In thermotolerant yeast, after giving cold and heat shock only baseline levels of HSPs were found in the membrane and no de-novo HSPs were detected up to 50 minutes (shock time and time at 25oC). Thereafter gradual increase in HSPs was found up to 80 minutes, after this it was stable (Fig 2). In mesophilic yeast, baseline HSP levels were maintained up to 100 minutes indicating that no more HSPs were produced. In lignocaine treated and unshocked thermotolerant strain, HSP expression was similar to that in heat shocked yeast. De-novo HSP expression observed in thermotolerant strain was not found in mesophilic yeast. Membrane western blots and autoradiographs (Fig 3) indicated that de-novo HSP 70 and 30 are transported to the membrane from 60 minutes.
DISCUSSION
Exposure to a mild non-lethal heat shock renders cells resistant to subsequent challenge at higher temperatures in all organisms (Sanchez et al., 1993). Experiments were carried out to determine the effect of different heat shock temperatures on inducing thermotolerance. The thermotolerant strain of Saccharomyces cerevisiae (VS3 ) survives, at 45oC but grows very poorly. However prior heat or cold shock confers thermotolerance as judged by increased number of colony forming units compared to the control (Table 2 & Fig 1). Though earlier studies have indicated improvement in thermotolerance following heat shock (Piper et al., 1997; Silva et al., 1994; Trinklein et al., 2004) this study demonstrates first time that the cold shock also is capable of improving thermotolerance in yeast.
Increased thermotolerance may occur due to synthesis of HSPs, which are known to confer protection to high temperature (Hanninen et al., 1999; Lindquist and Kim, 1996; Sanchez et al., 1993) but their exact involvement and mode of action was not studied. Saccharomyces cerevisiae cells exposed to 43oC (normal being 30oC) exhibit synthesis of heat shock proteins (HSPs). Time course studies indicated that the major HSPs (97, 85 and 70 kDa family) are induced within 10 minutes of heat shock attaining maximum levels within two hrs of treatment (Kaul et al., 1992). In the present study yeasts were shocked at various temperatures for 30 minutes and subsequently incubated at 25 oC for 70 minutes (Total HSP expression time was 100 minutes) in order to observe the membrane damage reversal at different time intervals. Yeasts were incubated at 25oC to block further damage of membrane by heat or cold shock. In our study, an increase in the synthesis of Heat shock proteins HSP 60 & 70 was observed, confirming earlier published results (Kaul et al., 1992). However it is also observed that following cold shock a number of heat shock proteins such as HSP 30,60,70,90 & 104 were produced. Heat/cold shock is known for down regulation of several genes including beta actin (Trinklein et al., 2004). As beta–Actin is heat/cold stable, its expression is reduced/blocked after heat shock therefore, it was used for normalization of western blots. Thus implying that HSPs may also have a role to play in protecting the cells during cold shock and subsequently during high temperature tolerance. Earlier reports have indicated a role of HSP30 & 104 in yeast thermotolerance (Hanninen et al., 1999;
HSP 30 has been identified earlier as the only plasma membrane integrated HSP in Saccharomyces cerevisiae. This protein is induced by several physical and chemical stress (Piper et al., 1997) and has been implicated in preventing denaturation of membrane bound enzymes (Patriarca and Maresca, 1990). In thermotolerant yeast strain in addition to HSP 30 another Heat shock protein HSP 70 has also been identified in the membrane.
Increase in heat shock proteins following heat shock is probably a consequence of a change in membrane physical state, which in turn would induce the expression of heat shock genes (Polla et al., 1997; Vigh et al., 1998; Lloyd et al., 1993; Sanchez. et al., 1993). Therefore it would be logical to assume that a membrane perturbing agent like lignocaine, which is known to fluidizing the membrane should also induce changes similar to heat shock (Mizogami et al., 2002; Steels et al., 1994). In fact, in the present study it is observed that in the thermotolerant strain, lignocaine does increase the levels of HSPs. Lignocaine treated and shocked yeast showed HSP profile corresponding to the type of shock, but the HSP levels are more than the lignocaine untreated and shocked yeast (Fig 2 & 3). The study also confirms similar changes in HSP levels following cold shock probably due to membrane damage. It may be worth while to mention that in addition to HSP 60 & 70 which are induced both following cold and heat shock, a few other proteins such as HSP 30, 90, 104 are induced only during cold shock. Therefore it is tempting to postulate that the later 3 proteins may be associated with the cold shock.
Damage to the cell membrane following cold and heat shock was confirmed by the efflux of K+ ions, leakage of UV280 absorbing material from the two strains. This confirms earlier observation of Fujitha and Kubo (2002) in yeast and Williams et al. (1991) in Staphylococcus aureus. It was also observed that in thermotolerant strain the above two (efflux of K+ ions, leakage of UV280 absorbing material) processes slowed down after 60 minutes coinciding with the synthesis of HSPs in the membrane. Therefore it appears that membrane integrity is recovering with time and probably the heat shock proteins are facilitating the recovery.
Mechanism of HSPs and plasma membrane in thermotolerance
Physical (cold and heat shock) or chemical (lignocaine) damage which alters the physical state of the membrane stimulates the cellular thermometer (Polla et al., 1997). Changed electro potential after stress due to intracellular acidification by influx of protons and efflux of K+ and other ions or cell protein content leakage may be stimulating the cellular thermometer (Coss et al., 2003). Cellular thermometer activates HSF and HSPs are synthesized (Polla et al. 1997). Cellular thermometer of a particular organism may be genetically determined. In thermotolerant strain, little stimulation may be enough to synthesize HSPs where as more stimulation may be needed in mesophilic yeast. HSP production is effected, if the stimuli are less or more than the optimum. In thermotolerant strain, HSPs 70 & 30 produced are transported to the membrane to recover the damage created by physical or chemical stress. This causes the decrease of leakage and increased intake of ions for achieving the balance. In mesophilic yeast, the sensitivity levels may be more and HSPs may be produced at a later period. Meanwhile heat shock causes irreversible damage of proteins and DNA, which leads to the death of the organism.
CONCLUSION
In present study using a mesophilic and a thermotolerant yeast it is clearly demonstrated that both cold and heat shock confer thermotolerance. The mechanism underlying this survival process is closely associated with the induction in the synthesis of heat shock proteins. These HSPs in turn stabilize the damage caused in the membrane and thus facilitate the thermotolerance. It is interesting that the subset of HSPs that are involved in conferring thermotolerance following cold and heat shock are different. This indicates that the involvement of Heat shock proteins along with plasma membrane are associated with the increased thermotolerance in thermotolerant Saccharomyces cerevisiae strain. In mesophilic yeast as de-novo HSPs were not produced after shock, it was unable to grow at high temperature.
ACKNOWLEDGEMENTS
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Role of heat shock proteins and plasma membrane on thermotolerance in Saccharomyces cerevisiae-VS3 strain
- Banat I.M., Singh D., Nigam P. and Marchant R. (2000) Potential use of thermotolerant fermentative yeasts for industrial ethanol production. Rec. Adv. in Food Sci. 1, 41-55.
- Castells-Roca L, García-Martínez J, Moreno J, Herrero E, Belli G, Pérez-Ortín JE. Heat shock response in yeast involves changes in both transcription rates and mRNA stabilities. PLoS One. 2011 25;6,2
- Chatterjee M.T. , Khalawan S.A. and Curran B.P.G. (1997) Alterations in cellular lipids may be responsible for the transient nature of the yeast heat shock response. Microbiol., 143, 3063-3068.
- Coss A. R., Storck W. C., Daskalakis C., Berd D. and Miriam Wahl L. (2003) Intracellular Acidification Abrogates the Heat Shock Response and Compromises Survival of Human Melanoma Cells. Mol Can Ther,2, 383- 388.
- Fujita K. and Kubo I. (2002) Plasma membrane injury induced by nonyl gallate in Saccharomyces cerevisiae J Appl Microbiol., 99,1035-1042.
- Hanninen A.-L., Simola M., Saris N. and Makarow M. (1999) The Cytoplasmic Chaperone Hsp104 is Required for Conformational Repair of Heat-denatured Proteins in the Yeast Endoplasmic Reticulum. Mol. Biol. Cell., 11, 3623- 3632.
- Heipeiper H.J., Meulenbeld G., Oirschot Q. and deBont J.A.M. (1996) Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in
- Pseudomonas putida S12. Appl. Environ. Microbiol., 62, 6665-6670.
- Kaul SC, Obuchi K, Iwahashi H, Komatsu Y. (1992) Cryoprotection provided by heat shock treatment in Saccharomyces cerevisiae. Cell Mol Biol. 38(2):135-43.
- Kaul S.C., Obuchi K., Iwahashi H. and Komatsu Y. (1992) Cryoprotection provided by heat shock treatment in Saccharomyces cerevisiae. Cell Mol. Biol., 38, 135-43.
- Kumar A., Sharma S., Chunduri V. Kaur A, Kaur S, Malhotra N, Kumar A, Kapoor P, Kumari A, Kaur J, Sonah H & Garg M. (2020) Genome-wide Identification and Characterization of Heat Shock Protein Family Reveals Role in Development and Stress Conditions in Triticum aestivum L.. Sci Rep, 10, 7858.
- Le Breton L. and Mayer M.P. (2016) Heat Shock Response: A model for handling cell stress eLife 5:e22850.
- Lindquist S. and Kim G .(1996) Heat-Shock Protein 104 Expression is Sufficient for Thermotolerance in Yeast. Proc Natl Acad Sci., 93, 5301-5306.
- Lloyd D. Morrell S., Carlsen H.N., Degn,H., James P.E. and Rowlands C.C. (1993) Effects of growth with ethanol on fermentation and membrane fluidity of Saccharomyces cerevisiae. Yeast, 9, 825-833.
- Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.L. (1951) Protein measurement with the Folin Phenol Reagent. J. Biol. Chem., 193, 265.
- Mizogami M., Tsuchiya H., and Harada J. (2002) Membrane effects of Ropivacaine compared with those of Bupivacaine and Mepivacaine. Fundam. Clin. Pharmacol., 16, 325-330.
- Mühlhofer M., Berchtold E., Stratil C. G., Csaba G., Kunold E., Bach N.C., Sieber S.A., Haslbeck M., Zimmer R., & Buchner J. (2019). The heat shock response in yeast maintains protein homeostasis by chaperoning and replenishing proteins. Cell reports, 29(13), 4593-4607.
- Parsell D.A. and Lindquist S. (1993) The function of heat shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet., 27, 437-496.
- Patriarca E.J. and Maresca B. (1990) Acquired thermotolerance following heat shock protein synthesis prevents impairment of mitochondrial ATPase activity at elevated temperatures in Saccharomyces cerevisiae. Exp. Cell Res., 190, 57 - 64.
- Piper PW, Ortiz-Calderon C, Holyoak C, Coote P. and Cole M. (1997) Hsp30,the integral plasma membrane heat shock protein of Saccharomyces cerevisiae, is a stress- inducible regulator of plasma membrane H(+)-ATPase. Cell Stress Chaperones., 2, 12-24.
- Polla B.S. , Dwight R.M.J., Robinson R. and Maresca B. (1997) Effetcs of membrane fatty acids on thermal and oxidative injury in the human premonocytic line U937. Biochem. Pharmacol., 54, 773-780.
- Prado C.D., Mandrujano G.P.L., Souza J.P. et al. (2020) Physiological characterization of a new thermotolerant yeast strain isolated during Brazilian ethanol production, and its application in high-temperature fermentation. Biotechnol Biofuels, 13, 178 .
- Sambrook J., Fritsch E.F. and Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring HarborLaboratory, Cold Spring Harbor, NY.
- Sanchez Y., Parsell D. A., Taulien J., Vogel J. L., Craig E. A., S Lindquist S. (1993). Genetic evidence for a functional relationship between Hsp104 and Hsp70. Journal of Bacteriology, 175(20), 6484-6491.
- Santos E., Villanueva J.R., and Sentandrem R. (1978) The plasma membrane of Saccharomyces cerevisiae. Isolation and some properties. Biochem et Biophys Acta (BBA)-Biomem., 508, 39-54.
- Sikkema J., De Bont J.A. and Poolman B. (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev., 59, 201-222.
- Silva, J.T., Vericimo, M.A., Floriano, W.B., Dutra, M.B. and Panek, A.D. (1994) On the Hsp 26 of Saccharomyces cerevisiae. Biochem. Mol. Biol. Int. , 33, 211-220.
- Sree N.K., Sridhar M., Suresh K., Banat I.M., & Rao L.V. (2000). Isolation of thermotolerant, osmotolerant, flocculating Saccharomyces cerevisiae for ethanol production. Bioresource Technology, 72(1), 43-46.
- Steels E.L., Learmonth R.P. and Watson K. (1994) Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiol., 40, 569-576.
- Suutari M., Liukkonen K. and Laakso S. Temperature adaptation in yeast : The role of fatty acids. J. Gen. Microbiol. 1990, 136, 1469-1474.
- Trinklein N.D., Murray J.I, Hartman S.J., Botstein. D. and Myers R.M. (2004) The Role of Heat Shock Transcription Factor 1 in the Genome-wide Regulation of the Mammalian Heat Shock Response. Mol Biol Cell., 15, 1254-1261.
- Ueda I. Tashiro C. and Arakawa K. (1977) Depression of phase transition temperature in a model cell membrane by local anesthetics. Anesthesiology., 46, 327-332.
- Verghese J, Abrams J, Wang Y, Morano KA. (2012) Biology of the heat shock response and protein chaperones: budding yeast (Saccharomyces cerevisiae) as a model system. Microbiol Mol Biol Rev. 76(2):115-58.
- Vigh L., Maresca B., and Harwood J.L. (1998) Does the Membrane's Physical State Control the Expression of Heat Shock and Other Genes? TIBS, 23, 369374.
- Williams D.E., Swango L. J., Wilt G. R. and Worley S. D. (1991) Effect of organic N-halamines on selected membrane functions in intact Staphylococcus aureus cells. Appl. Environ. Microbiol., 57, 11211127.


