Rooting technique of double haploids obtained in culture of microspore in vitro for European radish
Автор: Kozar Elena V., Kozar Elena G., Soldatenko Alexey V., Domblides Elena A.
Журнал: Овощи России @vegetables
Рубрика: Селекция и семеноводство сельскохозяйственных растений
Статья в выпуске: 5 (55), 2020 года.
Бесплатный доступ
Relevance. Doubled haploids (DH-plants) are excellent material for genetic research and breeding due to their complete homozygosity. The genus Raphanus from the Brassicaceae family is the toughest to produce doubled haploid plants through isolated microspore culture in vitro (IMC). The study of the causes of disturbed root formation and the development of elements of this stage of technology will significantly increase the effectiveness of the IMC technology for European radish. Methods. The study included three varieties from the collection of the Federal State Budgetary Scientific Institution Federal Scientific Vegetable Center (FSBSI FSVC): Teplichny Gribovsky, Rozovo-krasniy s belim konchikom and Rhodes. The experiments used a standard protocol for obtaining DH plants using IMC technology in a standard form and with a modification of the rooting stage. The solid MS medium (with agar 7g/L): MS without hormones, MS medium supplemented with IAA at concentrations of 0.5; 1 and 2 mg / L and liquid MSm medium supplemented with 0.1 mg / L kinetin were used for rooting of regenerated plants. All media were supplemented with 20 g/L sucrose. We used three types of techniques for transplanting plant explants onto a solid hormone-free MS medium: planting micro-shoots with their basal part immersed by 2-3 mm into the medium; planting in a well made in a nutrient medium using tweezers under sterile conditions; and landing on the surface of the medium without embedment. Results. In this work, we studied the features of the stage of rooting of regenerated European radish plants in vitro conditions. The transplant technique has been proven to be important for the successful establishment of radish micro-shoots. Plant explants must be planted strictly on the surface of a solid hormone-free nutrient medium MS, without embedment. The use of tubes with bridges made of filter paper and MSm liquid medium with the addition of 0.1 mg/L kinetin for the induction of root formation also showed high efficiency. For plants prone to the formation of root-like structures (RLS) with secondary tumors (ST), multiple dissection of abnormal formations with successive transplants s necessary. Modification at the rooting stage of micro-shoots growing has increased the percentage of successfully adapted DH plants in vivo conditions from 0-14% to 95-98%.
Dh-растения, raphanus satvus, культура изолированных микроспор in vitro, регенерация в культуре in vitro, укоренение in vitro, dh plants, raphanus sativus, culture of isolated microspores in vitro, regeneration in culture in vitro, rooting in vitro conditions, phytohormones, root-like structures (rls) with secondary tumors (st)
Короткий адрес: https://sciup.org/140250322
IDR: 140250322 | УДК: 573.6:635.152 | DOI: 10.18619/2072-9146-2020-5-3-15
Текст научной статьи Rooting technique of double haploids obtained in culture of microspore in vitro for European radish
B iotechnological methods of cell cultivation have made a breakthrough in various fields of science, opened up new horizons both for replenishing fundamental theoretical knowledge and for improving practical production. Thus, DH plants obtained in cell culture are an excellent material for genetic research and selection due to their complete homozygosity. Such plant material facilitates gene screening and genome sequencing, as well as the creation of mapping populations and collections of mutant forms. This increases the efficiency and speeds up the breeding process by increasing biodiversity, isolating rare forms of plants with recessive alleles, and quickly obtaining consistent material for further crosses (Forster, Thomas, 2005).
There are several haploid technologies that can be used to produce DHs. They are described in detail in reviews: Maluszynski et al., 2003; Dunwell, 2010; Asif, 2013. Isolated microspore culture in vitro (IMC) is an advanced haploid technology for producing DH – plants. It is based on the ability of cells of an immature male gametophyte (microspores) under the influence of external controlled conditions to change the path of development from gametophyte to sporophyte and, due to totipotency, form a full-fledged plant. Unlike other haploid technologies, somatic diploid cells are absent in microspore culture. This makes it possible to avoid the need for additional confirmation of the origin of the obtained tissues and to be sure that the obtained material is a product of embryogenesis of haploid microspore cells.
For a number of cultures, the IMC has been developed and is actively used in practical tasks. But due to the fact that the biodiversity of plants is extensive and has individual characteristics, the technology for obtaining DH-plants cannot be universal. Thus, the Brassicaceae family includes crops both with a high sensitivity to embryogenesis (rapeseed, white cabbage) and those for which an effective technology for the production of DH plants has not been developed. One of these crops is the European radish, which is considered the least responsive in the cabbage family, and the development of an effective technology for producing doubled haploids for this crop is of great practical and scientific interest.
There are separate articles in the literature describing attempts to obtain DH- plants of the genus Raphanus (Takahata et al., 1996; Chun et al., 2011; Han et al., 2014, 2018; Tuncer, 2017), but the full cycle of obtaining of doubled haploids in the culture of microspores in vitro ended only for the Chinese radish variety - daikon.
The method of obtaining doubled haploids in vitro microspore culture includes many stages, each of which significantly affects the final result. Therefore, the development of an effective technology is associated with a detailed study and development of each of them. The first successes of obtaining DH plants of European radish using IMC were described in our previous study (Kozar et al., 2020). In it, attention was paid to determining the most optimal parameters: the size of the buds, the duration of heat treatment, the composition of nutrient media for cultivation and regeneration. The influence of these factors and their interaction on the effectiveness of the technology was also analyzed. However, a high yield of DH- plants has not yet been achieved. In this regard, further study of the characteristics of the radish culture and the identification of the reasons for the low yield of regenerant plants remains relevant. In our previous work, the greatest losses of radish regenerant plants were noted at the stage of rooting. The study of the causes of disturbed root formation and the development of elements of this stage of technology will significantly increase the efficiency of the IMC technology for European radish.
Materials and methods
Plant material and growing conditions for donor plants In the work, we used samples of European radish from the collection of the laboratory of breeding and seed production of table root crops of the Federal State Budgetary Scientific Institution Federal Scientific Vegetable Center (FSBSI FSVC): "Rozovo-krasniy s belim konchikom" (DH-line No. 1,2), "Teplichny Gribovsky" (DH-line No. 3), and "Rhodes "(DH-line No. 4). Donor plants were grown in a growing chamber with additional illumination lamps (Osram plantstar 600 W) at a constant temperature of 19°C, illumination of 9000 lux, and a 16-hour photoperiod to stimulate flowering.
Culture of isolated microspores in vitro.
The induction of the androgenesis process and the production of embryoids in the culture of isolated microspores were carried out according to the protocol developed in the laboratory of reproductive biotechnology in the selection of agricultural plants for cultures of the Brassicaceae family (Domblides et al., 2016) with a modification of the rooting stage.
Root formation in vitro
For rooting, a solid (agar 7 g/L) hormone-free nutrient medium MS (Murashige, Skoog, 1962), MS was used with the addition of 3-indoleacetic acid (IAA) at concentrations of 0.5, 1, and 2 mg/L.
In experiments to study the process of root formation on a liquid medium, we used a nutrient medium MSm (Masuda et al., 1981) with the addition of 0.1 mg/L of kinetin. The cultivation was carried out in test tubes 20 cm in height and 2 cm in diameter on filter paper bridges placed in 10 ml of medium and covered with a foil lid.
The sucrose concentration for all regeneration media was 20 g/L.
We used three types of techniques for transplanting plant explants onto an agar hormone-free MS medium: planting micro-shoots with their basal part immersed by 2-3 mm into the medium; planting in a hole made in a nutrient medium using tweezers under sterile conditions; landing on the surface of the medium without deepening.
The cultivation was carried out on shelves with fluorescent lamps, with a photoperiod of 16 h, illumination of 2500 lux at a constant temperature of 25°C.
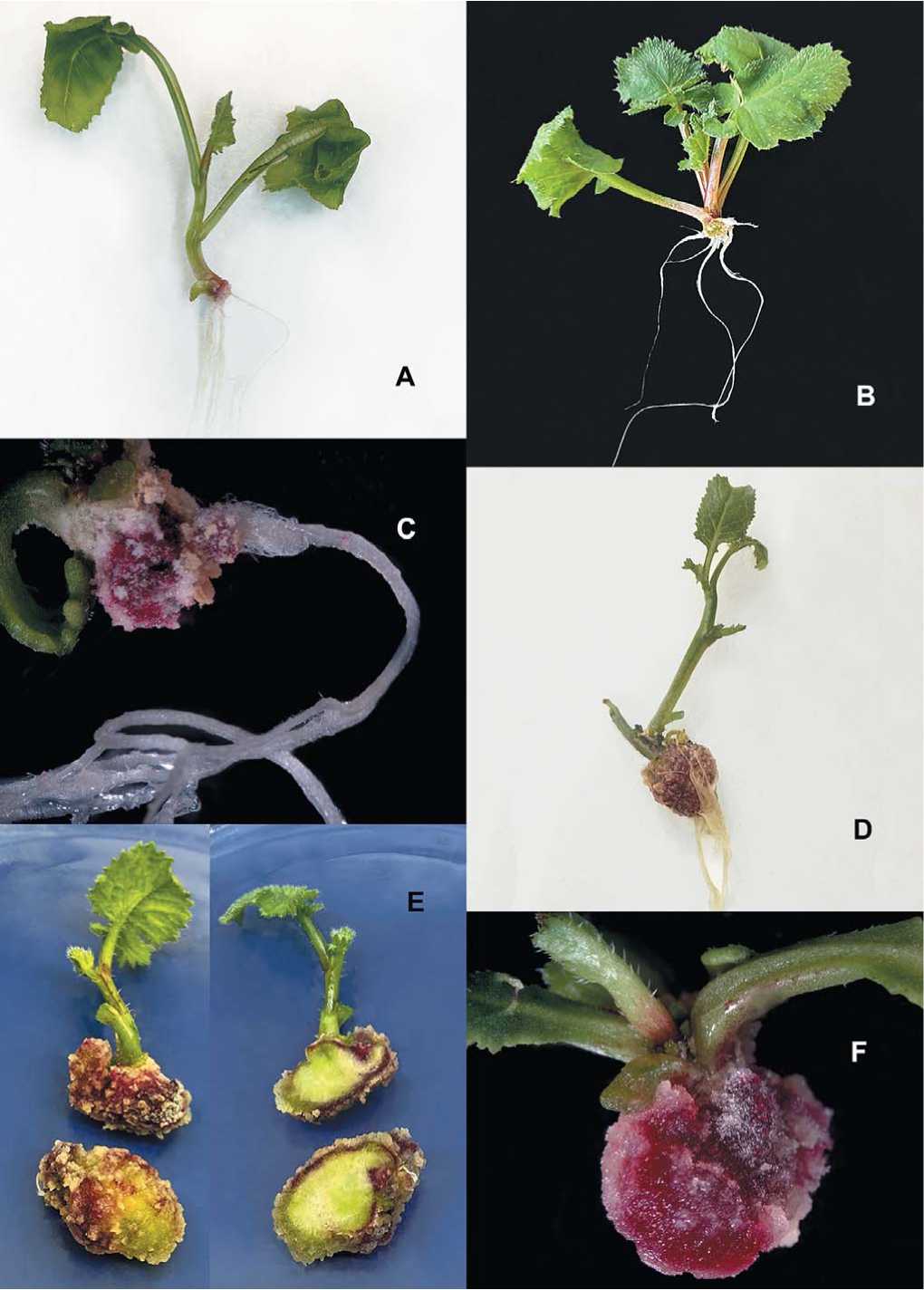
Fig. 1. Types of root system of DH-plants of European radish
A, B – normal root system – type I; C, D – root-like structures (RLSs) with secondary tumors (STs) with a weak root system – type II;
E, F – RLSs with STs without roots – type III
Рис. 1. Типы корневой системы DH-растений редиса европейского
A, B – нормальная корневая система – I тип; C, D – корнеплодоподобные структуры (КС) с вторичными опухолями (ВО) со слабой корневой системой – II тип; E, F – КС с ВО без корней – III тип
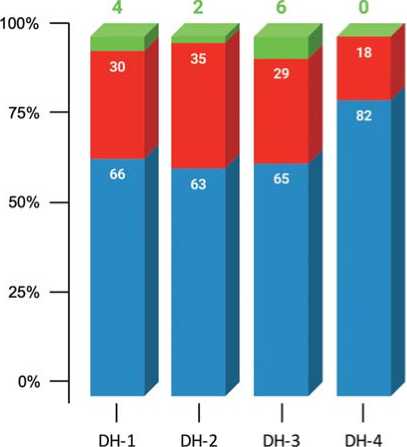
Fig. 2. Percentage of root system types in DH-lines of European radish
Type I – normal root system;
Type II – root-like structures (RLSs)
with secondary tumors (STs) with a root system;
Type III – RLSs with STs without roots
Рис.2. Процентное соотношение типов корневой системы у DH-линий редиса европейского
I тип – нормальная корневая система;
II тип – корнеплодоподобные структуры (КС) с вторичными опухолями (ВО) с корневой системой;
III тип – КС с ВО без корней
Growing of regenerant plants.
Plants with normally developed leaves and root systems were transferred to vegetation vessels filled with a mixture of peat and perlite (7: 3) and covered with perforated plastic cups for plant adaptation to in vivo conditions. After the appearance of two or three new leaves, the cups were removed. The regenerated plants were grown in a growing chamber at a constant temperature of 23°C, 8000 lux illumination and a 16 hour light period. After the beginning of flowering, the plants were placed under individual isolators. We carried out pollination in buds to obtain offspring from DH plants with control for self-incompatibility in flowers.
Results
According to the standard IMC protocols for cultures of the Brassicaceae family, hormone-free MS or B-5 media are used at the rooting stage, while the regenerated plants form a normal root system, after which they successfully adapt when transplanted into in vivo conditions (Custers, 2003; Ferrie A. 2003; da Silva Dias J.C., 2003; Shumilina, et al., 2020). However, in our experiments using the standard rooting technology, DH plants of European radish formed a normal root system (Fig. 1, type I) in a maximum of 6% of cases, depending on the genotype (Fig. 2, type I). In other cases, the basal part of the micro-shoots grew, forming abnormal structures that outwardly resemble an irregular root crop. In what fol-
Table 1. Results of rooting micro-shoots of European radish using the standard method on solid hormone-free MS medium
Таблица 1. Результаты укоренения micro-shoots редиса европейского при стандартной методике на твердой безгормональной среде MS
Such low rates are associated with the fact that tumors and the areas of RLSs covered by them, during growth, form spiral, wavy, unevenly distributed vessels (data not shown; consistent with data from other researchers – Ilina et al. 2006; Lebedeva et al. 2015). Therefore, plant nutrition through roots formed on the surface of RLSs with STs is impaired and is unable to provide regenerant plants with everything necessary when transplanted into soil under in vivo conditions. This means that for the successful rooting of regenerated plants of European radish, it is necessary to induce the process of root formation by passing or minimizing the formation of RLSs and tumors, since their tissues are a buffer zone that prevents direct contact between the cells of the vascular system of the roots and the basal part of micro-shoots of regenerants.
Table 1 shows the results of rooting four DH-lines of radish obtained in IMC culture. For them, the percentage of regenerated plants that could be adapted in vivo conditions from the total amount of microplants in the experiment varied from 6 to 14%. The remaining DH radish lines were lost due to problems in the in vitro rooting stage, so no statistical data could be obtained on them.
Since the control of cell division and differentiation is directly or indirectly affected by the balance of phytohormones, usually the solution to problems at all stages of regeneration of DH-plants comes down to modifying solid media for regeneration by adding auxins, cytokinins and /or gibberellins. It is known that the induction of shoot formation is observed when the balance of phytohormones shifts towards cytokinins, and induction of root formation - towards auxins. In our experiments, the roots of DH-plants were rarely formed, while the formation of shoots was quite intensive. From this, it can be concluded that the balance of phytohormones in our case is shifted towards cytokinins and an increase in the concentration of auxins is required to induce root formation.
For these reasons, an experiment was conducted on the cultivation of micro-shoots on MS / B-5 media with the addition of IAA and NAA at concentrations of 0.5, 1, and 2 mg/l. Unfortunately, no significant differences from the cultivation of regenerant plants on hormone-free media were found (data not shown). In view of this, it became necessary to find other ways to solve the problem of induction of root formation.
After numerous experiments, we were able to notice that in some micro-shoots the basal part was bent during growth, so that its area of contact with the nutrient medium rose above the surface of the medium. As a result, the root part of the micro-shoots was exposed to air, and the tissues in this area dried up locally. In such micro-shoots, an increase in RLS with ST was less often observed, and a normally developed root system was more often formed. In this regard, we hypothesized that the area of contact with the nutrient medium, good aeration, and local drying of tissues in the root part of micro-shoots may play an important role in the successful induction of root formation during the regeneration of European radish explants.
To test our hypothesis, we conducted an experiment using various micro-shoots transplantation techniques. We also drew attention to the method of rooting regenerated plants on liquid media with bridges, which is used for carrots. On the one hand, carrots are also a root crop, and on the other hand, cultivation of micro-shoots on bridges provides the necessary conditions - a small area of contact of explants with a nutrient medium, good aeration of the basal zone of micro-shoots, and the possibility of partial drying of its surface. Experiments on various techniques for transplanting European radish and cultivating micro-shoots on bridges in a liquid nutrient medium were carried out in parallel. Below are the results for each series of experiments.
Influence of different techniques of transplanting micro-shoots of European radish when cultivated on solid hormone-free MS media.
In the experiment, three variants of transplantation were used: planting micro-shoots with the immersion of
VW
Fig. 3. Variants of transplanting micro-shoots of European radish on solid hormone-free MS medium
1– planting micro-shoots with a 2-3mm hypocotyl immersion in solid MS medium;
-
2 – planting in a well made in a nutrient medium using tweezers under sterile conditions;
-
3 – planting on the surface of a solid nutrient medium for regeneration without deepening
1
1.2±0.2
0.0±0.0
0.0±0.0
1.6±0.6
1.4±0.4
0.0±0.0
6.1±0.7
3.0±0.2
0.0±0.0
2
0.0±0.0
0.0±0.0
0.0±0.0
1.3±0.2
1.0±0.1
0.0±0.0
7.4±0.6
1.6±0.6
0.0±0.0
3
0.0±0.0
1.4±0.1
0.0±0.0
0.0±0.0
1.5±0.2
0.0±0.0
8.8±0.8
1.2±0.4
0.0±0.0
average, pcs.
0.4±0.7
0.5±0.6
0.0±0.0
1.0±0.6
1.3±0.3
0.0±0.0
7.4±1.2
1.9±1.1
0.0±0.0
average, %
4.0
4.7
0.0
9.7
13.0
0.0
74.7
19.3
0.0
Рис.3. Варианты пересадки micro-shoots редиса европейского на твердую безгормональную среду MS
1 – посадка micro-shoots с погружением гипокотиля на 2-3 мм в твердую питательную среду MS; 2 – посадка в лунку, сделанную в питательной среде с помощью пинцета в стерильных условиях; 3 – посадка на поверхность твердой питательной среды для регенерации без заглубления the hypocotyl by 2-3 mm in solid nutrient medium MS (Fig. 3 – 1 variant); planting in a hole made in a nutrient medium using tweezers under sterile conditions (Fig. 3 – 2 variant); and planting on the surface of a solid nutrient medium for regeneration without deepening (Fig. 3 – 3 variant). For the experiment, we used three DH-lines of European radish (No. 1,2,3) with intensive shoots formation, which made it possible to micro-propagate microshoots of these lines in the amount required for the experiment. For each variant of the experiment, 10 microshoots of each DH-line were taken. The experiment was carried out in three replications, which were prolonged in time (autumn, winter, spring).
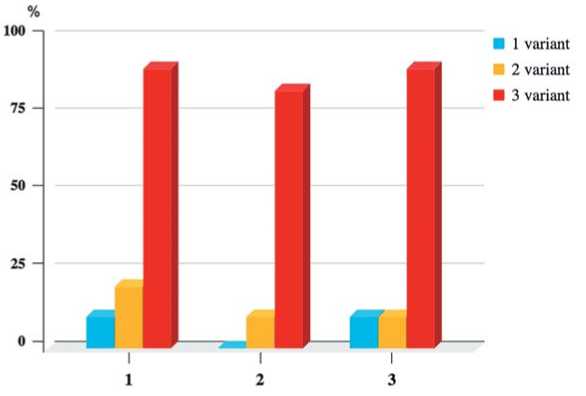
As a result of the study, it was shown that in the first and second variants of micro-shoots transplantation into the MS culture medium, the formation of RLSs with TSs was observed in 96% and 90.3% of cases, respectively (Table 2).
The third method of transplanting promoted the formation of a powerful root system directly from the basal part of microshoots in 60-80% of plants, depending on the genotype (Figure 4 - A, B, C, E). Moreover, in the latter variant of the transplantation of regenerant plants, in which abnormal development of the basal part was observed, RLSs with TSs were formed of a small size (up to 3-4 mm in diameter, Fig. 4 - D) and roots on their surface were formed more vigorously than in similar structures with a large size in other variants of the experiment (Fig. 1 - С, D; 4 - F). Accordingly, plants with small RLSs with TSs and a more developed root system were more likely to take root after planting in the soil. Thus, the third method of transplanting micro-shoots of European radish increased the proportion of plants that successfully underwent adaptation and rooted in vivo to 80-90% (Fig. 5), which means that it can be argued that the technique of transplanting microshoots plays a decisive role in the induction of root formation in European radish.
Table 2. Types of root formation for different variants of in vitro micro-shoots transplantation. Plant survival under in vivo conditions depending on the type of root formation (average over three independent series of experiments) Таблица 2. Типы корнеобразования при различных вариантах пересадки micro-shoots in vitro и приживаемость растений в условиях in vivo в зависимости от типа корнеобразования (среднее по трем независимым сериям опытов)
Note: Type I – normal root system;
Type II – root-like structures (RLSs) with secondary tumors (STs) with a root system;
Type III – RLSs with STs without roots.
Примечание: I тип- нормальная корневая система; II тип - корнеплодоподобные структуры (КС) с вторичными опухолями (ВО) с корневой системой; III тип - КС с ВО без корней

Fig. 4. Root systems of DH-plants of European radish in the third variant of transplantation on solid hormone-free medium MS.
A, B, C, E – normal root system; D – structure of RLSs with TSs of small size 3-4 mm in diameter, with a developed root system, suitable for in vivo adaptation; F – structure of RLSs with TSs larger than 4 mm in diameter with an underdeveloped root system that is not suitable for survival in vivo; G – complete dissection of RLSs with TSs before replanting
Рис.4. Корневые системы DH-растений редиса европейского при третьем варианте пересадки на твердую безгормональную среду MS
-
A, B, C, E – нормальная корневая система; D – структура КС с ВО маленького размера 3-4 мм в диаметре, с развитой корневой системой, пригодной для адаптации in vivo; F– структура КС с ВО размером более 4 мм в диаметре со слаборазвитой корневой системой, не пригодной для выживания в in vivo; G – полная диссекция КС с ВО перед повторной пересадкой
number of DH-linc
Fig.5. Percentage of regenerants of DH-lines established in vivo depending on the variant of in vitro micro-shoots transplantation (average over three independent series of experiments)
1 – planting micro-shoots with a 2-3mm hypocotyl immersion in solid MS medium;
2 – planting in a well made in a nutrient medium using tweezers under sterile conditions;
3 – planting on the surface of a solid nutrient medium for regeneration without deepening
Рис.5. Процент укоренившихся регенерантов DH-линий в условиях in vivo в зависимости от варианта пересадки микропобегов in vitro (среднее по трем независимым сериям опытов)
1 – посадка микропобегов с погружением базальной части на 2-3мм в твердую питательную среду MS; 2 – посадка в лунку, сделанную в питательной среде с помощью пинцета в стерильных условиях; 3 – посадка на поверхность твердой питательной среды для регенерации без заглубления
Nevertheless, even with the third type of transplantation, the proportion of micro-shoot remained, which formed rather large RLSs with TSs with weak or no roots. In view of this, in order to increase the efficiency of root formation, it was of interest to develop a technique for rooting regenerated plants with already formed RLSs and TSs, including those obtained during transplantation by two other methods. Since, even during long-term cultivation, no changes in the power or induction of the root system were observed in the regenerated plants with RLSs and TSs, we assumed that complete (Fig. 4 - G), dissection of abnormal structures is necessary for a repeated attempt to initiate root formation. For this, we removed plant explants with abnormal development from incubation vessels and under sterile conditions dissected RLSs and TSs partially or completely (Fig. 4-F), leaving 1-2 mm of the basal part of micro-shoots below the growth point. Thereafter, the plant explants were placed on the surface of the induction medium using the developed method without deepening (3rd variant).
As a result of the experiments, it was noted that with partial dissection, the tissues of RLSs continued to grow without changing their specification; no root formation occurred. In plant explants, where the tissues of RLSs with STs were completely removed, the formation of a root system was observed in about 50% of cases, regardless of which method they were transplanted onto nutrient media before (Fig. 6).
For plants in which RLSs with TSs were re-formed, the procedure was repeated for the complete excision of abnormal structures with further cultivation on solid nutrient medium in the third way. It was found that in plants in which the basal part of the micro-shoots was prone to regrowth, multiple dissection of RLS and TSs with transplantation to the surface of a hormone-free nutrient medium still led to the formation of a normal root system. The number of transplantations varied from one to six, depending on the genotype and the individual plant explant (if, after the sixth transplantation, the formation of abnormal structures was repeated, then such explants were classified as non-responsive to root formation and were excluded from the work).
As a result of such transplants, about 90% of all plant explants prone to basal part overgrowth have formed a powerful root system and have been successfully rooted in vivo conditions (Fig. 6). Thus, the developed transplantation technique, together with dissection, improved root formation in micro-shoots of European radish and increased the percentage of plants that successfully underwent adaptation in vivo from 12-14% to 95-98%. The proposed transplantation technique, together with dissection, was also effective for the fourth DH-line, which did not participate in a series of experiments on the development of technological stages of rooting. Nine out of eleven plants were able to root and adapt in vivo conditions. For DH-lines, previously lost due to rooting prob-
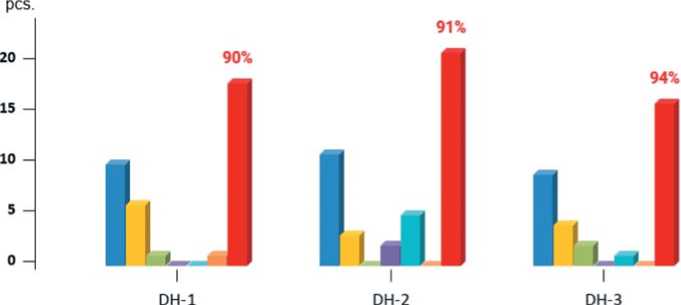
|
total amount of micro-shoots |
20 |
23 |
17 |
|
■ 1 transplantation |
10 |
11 |
9 |
|
2 transplantation |
6 |
3 |
4 |
|
■ 3 transplantation |
1 |
0 |
2 |
|
■ 4 transplantation |
0 |
2 |
0 |
|
■ 5 transplantation |
0 |
5 |
1 |
|
■ 6 transplantation |
1 |
0 |
0 |
|
■ total amount of micro-shoots rooted in vivo |
18 |
21 |
16 |
-
Fig.6. The number of micro-shoots with a normally developed root system of DH lines in vivo, depending on the number of transplantations after successive dissections of regenerants with RLSs and STs structures (the total number of rooted plants in the sample after six successive transplants is indicated as a percentage)
Рис.6. Число микропобегов с нормально развитой корневой системой DH-линий в условиях in vivo в зависимости от числа пересадок после проведения последовательных диссекций регенерантов с КС с ВО структурами (в процентах указано общее число укоренившихся растений в образце после шести последовательных пересадок)
lems, it was not possible to test the effectiveness of our modifications. Therefore, the likelihood of both an increase and a decrease in the effectiveness of the developed technique of transplanting DH-regenerants in other genotypes of European radish is not excluded.
Rooting of micro-shoots of European radish on paper bridges in MSMm liquid medium.
Micro-shoots of European radish were cultivated on paper bridges in test tube with a liquid MSm medium containing 0.1 mg/L kinetin, recommended for the regeneration and rooting of carrots (Tyukavin et al., 1999; Vjurtts et al., 2017). The present study involved four DH-lines of European radish. It was shown that cultivation of microshoots on paper bridges in liquid medium actually reduced the percentage of RLSs and TSs formation towards normal root formation (Table 3).
Moreover, RLSs with TSs on the bridges were mostly small, often formed a developed root system (Fig. 7, A), due to which they were able to adapt in vivo . The effects that we obtained when cultivating on the bridges were similar to the results that we obtained when we cultivated micro-shoots on solid hormone-free MS medium with transfer in the recommended way (on the surface of the nutrient medium without burying).
For plants that did not show the development of a root system suitable for adaptation in vivo , as well as plants with RLSs and TSs without roots, we also used the dissection technique with re-cultivation, until a normal root system was formed (Fig. 7, B). However, in micro-shoots on liquid media with paper bridges, a pronounced apical dominance and elongation of internodes was observed (Fig.7, C). Therefore, during dissection, not only the overgrown basal part of the micro-shoots was removed, but also all parts of the plant explant 2-3 mm below the growth point (Fig. 7, C). As a result of multiple dissections (up to six times), ten out of twelve regenerant plants were able to root, which initially formed RLSs and TSs with weak or no roots. Thus, the efficiency of rooting of regenerated plants of European radish during cultivation using multiple dissection, both on solid hormone-free media with the technique of planting regenerated plants without burying, and on paper bridges in a liquid medium, reaches 95-98%, depending on the genotype of the donor plant.
Advantages and disadvantages of cultivating microshoots of European radish on MS agar medium with micro-shoots planting on the surface of the medium without burying and on paper bridges in test tube with liquid MSm medium.
Table 3. Types of root formation during in vitro micro-shoots cultivation on paper bridges in test tube with a liquid MSm medium containing 0.1 mg / L of kinetin and plant survival in vivo, depending on the type of root formation
|
1 |
19 |
10 |
52.6 |
5 |
26.3 |
0 |
0.0 |
78.9 |
|
2 |
20 |
10 |
50.0 |
5 |
25.0 |
0 |
0.0 |
75.0 |
|
3 |
15 |
8 |
53.3 |
6 |
40.0 |
0 |
0.0 |
93.3 |
|
4 |
8 |
5 |
62.5 |
1 |
12.5 |
0 |
0.0 |
75.0 |
|
total |
62 |
33 |
53.2 |
17 |
27.4 |
0 |
0.0 |
80.6 |
Note: Type I – normal root system; Type II – root-like structures (RLSs) with secondary tumors (STs) with a root system; Type III – RLSs with STs without roots
Примечание: I тип – нормальная корневая система; II тип – корнеплодоподобные структуры (КС) с вторичными опухолями (ВО) с корневой системой; III тип – КС с ВО без корней
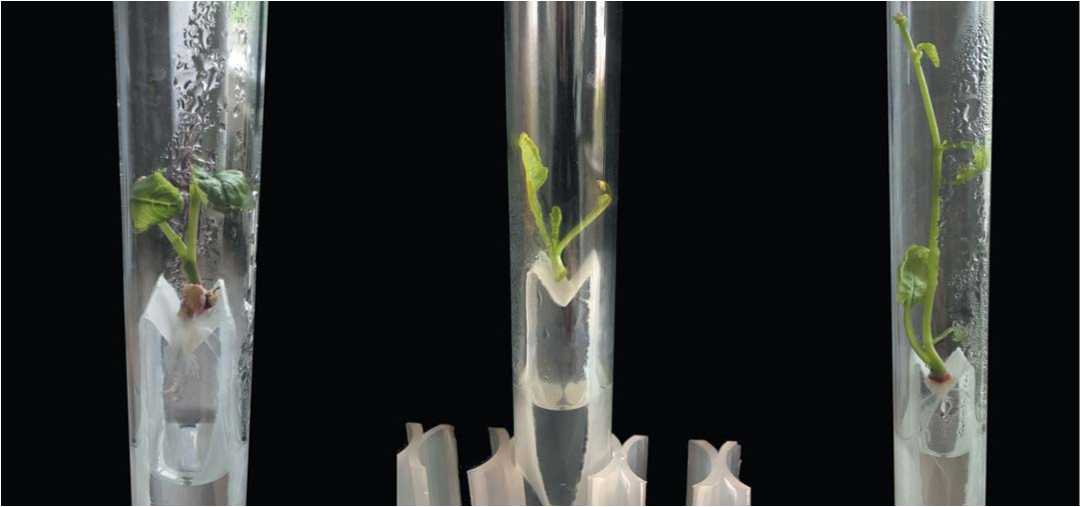
ABC
Fig.7. Root systems of European radish DH-plants on paper bridges in test tube with a liquid MSm medium containing 0.1 mg / L of kinetin
A – structure of RLSs with small TSs with well developed root system;
B – micro-shoots after dissection of all parts of the plant explant 2-3 mm below the growth point;
C – regenerant plant with pronounced apical dominance and elongation in internodes
Рис.7. Корневые системы DH-растений редиса европейского в пробирках на мостиках из фильтровальной бумаги в жидкой среде MSm c 0,1мг/л кинетина
А – структура КС с ВО маленького размера с хорошо развитой корневой системой;
B – микропобег после диссекции всех частей растительного экспланта ниже точки роста на 2-3 мм;
С – растение-регенерант с ярко выраженным апикальным доминированием и удлиненными междоузлиями
As we noted earlier, these two ways of cultivating microshoots of European radish were comparable in the efficiency of root formation of regenerant plants and allow us to solve the main problem – rooting of DH plants. Nevertheless, they have their own characteristics that deserve attention, starting from the stage of preparing environments. Working with a solid medium is not as laborious as with a liquid medium, where the process of cutting bridges from filter paper and placing them in test tubes takes a lot of time. However, as we assume, on liquid media with bridges, plant nutrition occurs more evenly. Nutrients are in a more accessible liquid form for plants, and due to their gradual rise along filter paper, there is no local decrease in the concentration of nutrients in the area of contact between the medium and the basal part of the plant. This makes it possible to cultivate plants in one test tube without transplanting for up to three months. In a solid medium, nutrients are not so mobile and are in a less accessible form, and are also absorbed by the plant only in the immediate vicinity of it. As a result, the concentration of nutrients around plants is locally reduced, which can lead to starvation and a general deterioration in the condition of plants. Therefore, cultivation of regenerated plants on a solid medium without transplanting is possible for no more than three to four weeks.
Another significant difference between the analyzed cultivation methods is their effect on the ability of DH plants to form shoots. Thus, on solid media, a more intensive secondary shoot formation was noted, due to which regenerant plants can be effectively propagated microclonally and a large number of identical explants can be obtained. It is convenient for carrying out various experiments and is also necessary for the applied use of DH-plants in the breeding process. Cultivation in a liquid medium does not allow the multiplication of plants in large quantities, due to the more pronounced apical dominance.
It is also worth noting the differences between agar and liquid media in terms of damage to the root system of plants during transplantation, including in vivo . When the agar is removed from the nutrient medium, the roots are damaged more, especially in the absorption zone, which causes stress in the plant, and after transplantation, it takes more time for adaptation. In addition, during in vivo rooting, if the roots are poorly cleaned of agar / phytogel, problems may arise due to poor contact of the roots with the soil. And also with the fact that the remains of the nutrient medium serve as a favorable substrate for the intensive development of soil microbiota, including the pathogens present in its composition. As a result, plants can be affected by root rot and other diseases. In the case of liquid media, these problems are not observed. Even if the root system of the plant has grown into the lintel, the plant can be planted without separating it from the filter paper, since this does not affect the success of rooting and adaptation.
Considering the above, we suggest combining the two types of cultivation to optimize the rooting stage. At the first stage of rooting, we recommend planting plants on solid nutrient media in order to be able to propagate them micro-clonally. Rooted plants should then be planted on bridges with liquid media to reduce the number of transplants before the plant is ready for rooting in vivo and to reduce mechanical damage and damage to the root system when the regenerant plant is transferred to the soil.
Discussion
Our research demonstrates that when developing biotechnology methods for previously unresponsive crops, it is possible to encounter their diverse and truly amazing features. In view of what, difficulties arise at those stages at which other cultures have long been worked out and do not present surprises. The search for solutions to overcome problems within a specific species, in the early stages, most often occurs empirically and the results obtained have yet to be studied to understand the mechanisms of their action.
Earlier, we reported that for the first time we were able to complete the full cycle of obtaining DH-plants of European radish in the culture of isolated microspores in vitro (Kozar et al., 2020); nevertheless, the effectiveness of the technology remains low and requires significant improvement. The stage of rooting of regenerated plants of European radish was noted as one of the most difficult (Kozar et al., 2020). This is due to the physiological characteristics of the radish. Moreover, the point is not only that radish is a root crop, unlike the bulk of the Brasseaceae family crops (rapeseed, white cabbage, etc.), for which the IMC technology has already been developed, but also that the wild ancestors of radish did not have a root crop. This means that the edible root crop of the cultivated radish is a rather young trait that appeared as a result of the selection of mutant forms with impaired control of cell division (Buzovkina, Lutova, 2007). Accordingly, all cultivars and hybrids of radish are saturated with various mutations, and during inbreeding, and even more so in DH-plants, the manifestation of unexpected deviations in development of the phenotype is quite natural.
The first records of root anomalies in inbred radish lines date back to 1967, when spontaneous formation of tumors on the roots was noted after 2-3 generations of inbreeding (Narbut, 1967). Subsequently, the rarity of such an anomaly for the Brasseaceae family made radish a convenient model culture for studying the genetic regulation of cell differentiation and proliferation. In this regard, at the moment there is information and hypotheses about possible mechanisms of induction of such formations, which are described in reviews: Lutova, Dodueva, 2019; Dodueva et al., 2020. Interpretation of our data in this aspect is somewhat complicated by the fact that, as a result of studying the material presented in the literature, we did not come to a consensus on what criteria can be used to visually distinguish RLS from TS formed in structures in vitro. In some articles, structures that outwardly resemble those that we observed in our studies were described as RLS with secondary TSs, while in other sources they were described as RLS structures with- out indicating the presence of secondary TSs on their surface. Interestingly, there are no data in the literature on the spontaneous formation of RLS or TS on hormone-free media in vitro. The formation of such structures was noted only as a response to media with high concentrations of cytokinins (Buzovkina et al., 1993; Il'ina et al., 2006; Buzovkina and Lutova, 2007). Only the formed TS, cut off from the donor plant, were capable of hormone-independent growth in a hormone-free environment. This means that the induction and subsequent growth of RLS and / or TS occur under different conditions, and the abnormalities that we observed in DH plants of European radish when cultivated on a hormone-free medium are described for the first time.
According to a number of researchers, the formation of RLSs and TSs structures is regulated by different genes (Buzovkina et al., 1993). Nevertheless, both of these processes are associated with impaired differentiation and proliferation of cells against the background of an imbalance of phytohormones, therefore, all hypotheses about the causes of such formations that we proposed and described in the literature can be considered suitable for both types of anomalies.
Several possible reasons for the spontaneous formation of tumors and other abnormal structures in radishes are described in the literature (Ilyina et al., 2006; references). Among them, the most common version is an imbalance of endogenous hormones with a shift towards cytokinins, as well as their high absolute content (Matveeva et al., 2004). We are also inclined to such assumptions about the reasons for the formation of RLS and tumors in our experiments. In accordance with the theory set forth by Ilyina and other researchers (2006), if the level of endogenous hormones in plants is increased, then when cytokinins are added to the nutrient medium, necrosis of cotyledonous explants occurs, which we observed in our earlier studies, where the regeneration of embryoids on a medium with TDZ led to their necrosis within 3-5 days (Kozar et al., 2020). Moreover, in our experiments, in micro-shoots of radish obtained in IMC culture, RLS with tumors were formed on hormone-free media, which means that endogenous imbalances in the phytohormones were so pronounced that exogenous intervention was not required. Accordingly, for the formation of a normal root system, it is necessary to shift the balance of phytohormones towards auxins. Based on this, we were able to put forward some ideas about the possible mechanisms of the influence of the technological methods and modifications developed by us on the effect of rooting of regenerated plants.
As a result of our experiments, it was found that the normal rooting of DH-plants under in vitro conditions is facilitated by planting micro-shoots strictly on the surface of a hormone-free nutrient medium, without burying them. In this regard, it is possible to distinguish three necessary parameters for the successful rooting of radish micro-shoots: a small area of contact of the basal part with the surface of a solid nutrient medium; good aeration of the tissues of the basal part of the micro-shoots; as well as partial drying of the basal part of the micro-shoots. It is not yet known what exactly, how, and to what extent each of them affects the root formation processes of DH-plants of radish, but together they ensure the successful implementation of the rooting of doubled haploids obtained in the IMC culture.
Cytokinin is known to be the root hormone of well-being, which means that it signals the body that the root system is working well and does not require reorganization. In our case, due to a decrease in the area of contact of the root part of microplants with a solid nutrient medium, a deficiency of water and nutrients is likely to occur in the tissues of regenerated plants. Such conditions can contribute to the suppression of cytokinin synthesis as a natural response to deterioration of conditions. Accordingly, the balance of phytohormones shifts towards auxins, which contributes to the induction of root formation. On the contrary, with a large area of contact between the basal part of micro-shoots and the environment, its tissues can be oversaturated with nutrients, against which the cells begin to divide chaotically and form RLS with TS.
The second condition for rooting is good aeration of the tissues of the basal part of micro-shoots. When the basal part is immersed in a nutrient medium (method 1 of transplantation) or in a hole (method 2 of transplantation), hypoxic conditions may form locally around the tissues. Whereas, the position of the basal part on the surface of the nutrient medium without deepening into it contributes to an increase in the level of exogenous oxygen around the tissues, which can indirectly increase the level of oxygen in the tissues of the basal part. If we consider the problem of the formation of RLSs with tumors as a result of an imbalance in phytohormones, it can also be assumed that the position of the basal part of the micro-shoot on the surface of the nutrient medium, due to an increase in the level of aeration, enhances auxin signals, thereby somewhat leveling the phytohormon-al imbalance. These assumptions are based on recent studies on the effect of hypoxic conditions on secondary root formation, where it was shown that the level of exogenous and endogenous oxygen can influence the transmission of auxin signals. This is due to the oxygen-sensitive ERF-VII transcription factors, which, under hypoxic conditions, bind to the auxin-induced genes LBD16 and LBD18 and suppress their expression, thereby inhibiting the initiation of root pri-mordia formation (Licausi et al., 2011; Gibbs et al. , 2015; Shukla et al., 2019).
And finally, an element of partial drying of the basal part of the micro-shoots. It is known that auxin is synthesized in the apical meristems of the shoots and transported from top to bottom. Then it can be assumed that due to the drying out of the basal part, between living cells and the environment, a barrier is formed from a layer of dead tissue. When moving from top to bottom, auxin can accumulate locally in tissues adjacent to such a barrier, without being able to diffuse into the external environment, which in turn contributes to a shift in the balance of phytohormones towards auxins.
All conclusions and assumptions are similar for the cultivation of micro-shoots of radish, with regeneration on liquid MSm medium with bridges.
As a result, the following logical chain can be built: a small area of contact with the nutrient medium reduces the production of cytokinins and “causes” the influx of auxins from the apical meristem of the shoots. Drying of tissues promotes local accumulation of auxins in the basal part of micro-shoots. And good aeration enhances the gene response to auxins, so that even small concentrations have a tangible effect. As a result of the combination of all these factors, the balance of phytohormones shifts towards auxins and which induces root formation.
As for the efficiency of complete dissection of RLSs for rooting of regenerated plants, there is evidence in the literature that during tissue dissection in the area of injury due to the self-organizing interaction of auxin and cytokinin, the settings for the identity of wound cells can be “reset” and new positional signals can be given to the remaining dividing cells (Efroni at el., 2016). Also, during dissection, a wound surface is formed, in the cells of which, in response to stress, jasminic acid (JA) begins to actively produce. JA is known to promote root formation in Arabidopsis leaf explants (Zhang et al., 2019). JA is activated within 10-30 minutes after injury, after which the ERF109 gene is detected, which is identified as a key factor in de novo root regeneration. In addition, the ERF109 gene activates ANTHRANILATE SYNTHASE α 1 (ASA1), which in turn encodes one of the enzymes involved in tryptophan biosynthesis, and tryptophan is a precursor of auxin (Liu et al., 2014; Zhang et al., 2019; Zhou et al., 2019). Which means that wounding by dissecting RLSs with TSs increases the synthesis of auxin and thereby brings us closer to the main task of restoring the balance of phytohormones. It is also worth noting that, when analyzing the content of cytokinins in tumor and non-tumor lines of radish, it was found that the greatest differences in the concentration of cytokinins were in plant roots (Matveeva et al., 2004). This suggests that cutting off parts with an increased cytokinin content may have a positive effect, temporarily reducing their concentration in the basal part of micro-shoots.
Thus, as a result of the work carried out, the stage of regeneration of microspores in vitro obtained in the culture of regenerated plants of European radish was worked out. As a further direction of research, it would be interesting to determine the endogenous content of hormones in abnormal formations of RLS with TS, depending on the method of their cultivation. This will help to better understand the mechanism of the influence of the proposed modifications on the formation of roots in vitro and rooting in vivo . This will also give an idea of what endogenous content and balance of phytohormones corresponds to the appearance of deviations in the plant phenotype in the form of RLS with TS. Among other things, our main global task is still the development of an effective technology for obtaining doubled haploids of European radish in the culture of IMC, therefore, it is necessary to continue research on the characteristics of the radish culture and to work out other problematic stages of the technology in accordance with them.
Об авторах:
Козарь Елена Викторовна – м.н.с. лаб.
репродуктивной биотехнологии в селекции с.-х. растений,
Elena V. Kozar, Еlena G. Kozar, Alexey V. Soldatenko, Elena A. Domblides
Elena V. Kozar – Junior Researcher of Laboratory of Reproductive Biotechnology in Crop Breeding,
Еlena G. Kozar – Ph.D. in Agriculture, leading researcher head of the laboratory of immunity and plant protection,
Alexey V. Soldatenko – Dc. Sci. (Agriculture), corresponding member RAS, chief scientist,
Elena A. Domblides – Ph.D. in Agriculture, Head of Laboratory of Reproductive Biotechnology in Crop Breeding
Список литературы Rooting technique of double haploids obtained in culture of microspore in vitro for European radish
- Asif M. Progress and Opportunities of Doubled Haploid Production. Springer. 2013. DOI: 10.1007/978-3-319-00732-8_1
- Buzovkina I.S., Lutova L.A. Genetic collection of inbred lines of radish: history and prospects. Russian Journal of Genetics. 2007;(4):1411-1423.
- Buzovkina, I. S., Kneshke, I., and Lutova, L.A. In vitro modeling of tumor formation in radish lines and hybrids. Genetika. 1993;29(6):1002-1008.
- Chun C., Park H., Na H. Microspore-derived embryo formation in radish (Raphanus sativus L.) according to nutritional and environmental conditions. Hort. Environ. Biotechnol. 2011;52(5):530-535. DOI: 10.1007/s13580-011-0080-1
- Custers J.B.M. Microspore culture in rapeseed (Brassica napus L.). In: Maluszynski M., Kasha K.J., Forster B.P., Szarejko I. (eds). Doubled Haploid Production in Crop Plants. 2003:185-186. DOI: 10.1007/978-94-017-1293-4_29
- da Silva Dias J.C. Protocol for broccoli microspore culture. In: Maluszynski M., Kasha K.J., Forster B.P., Szarejko I. (eds). Doubled Haploid Production in Crop Plants. 2003:195-204.
- DOI: 10.1007/978-94-017-1293-4_30
- Dodueva, I.E., Lebedeva, M.A., Kuznetsova, K.A., Gancheva, M.S., Paponova, S.S., Lutova, L.L. Plant tumors: a hundred years of study. Planta. 2020;251(4):82.
- DOI: 10.1007/s00425-020-03375-5
- Domblides E.A., Shmykova N.A., Shumilina N.A., Zayachkovskaya T.V., Mineykina A.I., Kozar E.V., Ahramenko V.A., Shevchenko L.L., Kan L.Ju., Bondareva L.L., Domblides A.S. A technology for obtaining doubled hap-loids in microspore cultures of the Brassicaceae family (guidelines). Moscow: VNIISSOK Publ., 2016. (In Russ)
- Dunwell J.M. Haploids in flowering plants: origins and exploitation. Plant Biotechnol. J. 2010;(8):377-424. 10.1111/j.1467-7652. 2009.00498.x.
- DOI: 10.1111/j.1467-7652.2009.00498.x
- Efroni I., Mello A., Nawy T., Ip P.L., Rahni R., Delrose N., Powers A., Satija R., Birnbaum K.D. Root regeneration triggers an embryo-like sequence guided by hormonal interactions. Cell. 2016;(165):1721-1733. [CrossRef]
- DOI: 10.1016/j.cell.2016.04.046
- Ferrie A. Microspore culture of Brassica species. In: Maluszynski M., Kasha K.J., Forster B.P., Szarejko I. (eds). Doubled Haploid Production in Crop Plants. 2003:205-215.
- DOI: 10.1007/978-94-017-1293-4_31
- Forster B.P., Thomas W.T.B. Doubled haploids in genetics and plant breeding. In: Janick J. (Ed.). Plant Breeding Reviews. 2005;(25):57-88.
- DOI: 10.1002/9780470650301
- Gibbs D.J., Conde J.V., Berckhan S., Prasad G., Mendiondo G.M., Holdsworth M.J. Group VII ethylene response factors coordinate oxygen and nitric oxide signal transduction and stress responses in plants. Plant Physiology. 2015;(169):23-31.
- DOI: 10.1104/pp.15.00338
- Han N., Kim S.U., Park H.Y., Na H. Microspore-derived embryo formation and morphological changes during the isolated microspore culture of radish (Raphanus sativus L.). Kor. J. Hort. Sci. Technol. 2014;32(3):382-389.
- DOI: 10.7235/hort.2014.13170
- Han N., Na H., Kim J. Identification and variation of major aliphatic glu-cosinolates in doubled haploid lines of radish (Raphanus sativus L.). Kor. J. Hort. Sci. Technol. 2018;36(2):302-311.
- DOI: 10.12972/kjhst.20180030
- Il'ina, E.L., Dodueva, I.E., Ivanova, N.M. et al. The effect of cytokinins on in vitro cultured inbred lines of Raphanus sativus var. radicula Pers. with genetically determined tumorigenesis. Russian Journal of Plant Physiology. 2006;(53):514-522.
- DOI: 10.1134/S1021443706040133
- Kozar E.V., Domblides E.A., Soldatenko A.V. Factors affecting DH plants in vitro production from microspores of European radish. Vavilov Journal of Genetics and Breeding. 2020;24(1):31-39.
- DOI: 10.18699/VJ20.592
- Lebedeva M.A, Tvorogova V.E., Vinogradova A.P., Gancheva M.A., Azarakhsh M., Ilina E.L., Demchenko K.N., Dodueva I.E., Lutova L.A. Initiation of spontaneous tumors in radish (Raphanus sativus): cellular, molecular and physiological events. Journal of Plant Physiology. 2015;(173):97-104.
- DOI: 10.1016/jJplph.2014.07.030
- Licausi F., Kosmacz M., Weits D.A., Giuntoli B., Giorgi F.M., Voesenek L. ACJ, Perata P., van Dongen J.T. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature. 2011;479(7373):419-422.
- DOI: 10.1038/nature10536
- Liu J., Sheng L., Xu Y., Li J., Yang Z., Huang H., Xu L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell. 2014;(26):1081-1093. [CrossRef] [PubMed]
- DOI: 10.1105/tpc.114.122887
- Lutova L., Dodueva I. Genetic control of regeneration processes of radish plants in vitro: from phenotype to genotype. Bio. Comm. 2019;64(2):124-132.
- DOI: 10.21638/spbu03.2019.204
- Maluszynski M., Kasha K.J., Forster B.P., Szarejko I. Doubled Haploid Production in Crop Plants: A Manual. Springer Science Business Media. 2003:141-150.
- DOI: 10.1007/978-94-017-1293-4
- Masuda K., Kikuta Y., Okazava Y. A Revision of the Medium for Somatic Embryogenesis in Carrot Suspension Culture. J. Fac. Agr. Hokkaido Univ. 1981;(60):183-193.
- Matveeva T.V., Frolova N.V., Smets R., Dodueva I.E., Buzovkina I.S., Van Onckelen H., Lutova L.A. Hormonal control of tumor formation in radish. Journal of Plant Growth Regulation. 2004;(23):37-43.
- DOI: 10.1007/s00344-004-0004-8
- Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum. 1962;15(3):473-497.
- Narbut S.I. Genetic tumor generated during inbreeding in radish. Vestn. Leningr. Univ. 1967;(15):144-149. (In Russ)
- Shukla V., Lombardi L., Iacopino S., Pencik A., Novak O., Perata P., Giuntoli B., Licausi F. Endogenous Hypoxia in Lateral Root Primordia Controls Root Architecture by Antagonizing Auxin Signaling in Arabidopsis. Molecular Plant. 2019;(12):538-551. https://doi.org/10.1016Zj.molp.2019.01.007
- Shumilina D., Kornyukhin D., Domblides E., Soldatenko A., Artemyeva A. Effects of Genotype and Culture Conditions on Microspore Embryogenesis and Plant Regeneration in Brassica rapa ssp. rapa L. Plants. 2020;9(2):278.
- DOI: 10.3390/plants9020278
- Takahata Y., Komatsu H., Kaizuma N. Microspore culture of radish (Raphanus sativus L.): influence of genotype and culture conditions on embryogenesis. Plant Cell Rep. 1996;16(3-4):163-166.
- DOI: 10.1007/BF01890859
- Tuncer B. Callus formation from isolated microspore culture in radish (Raphanus sativus L.). J. Anim. Plant Sci. 2017;27(1):277-282.
- Tyukavin, G.B., Shmykova, N.A., Mankhova, M.A. Cytological study of embryogenesis in cultured carrot anthers. Russian Journal of Plant Physiology. 1999;46(6):876-884. (In Russ)
- Vjurtts T.S., Domblides E.A., Shmykova N.A., Fedorova M.I., Kan L.Ju., Domblides A.S. Production of DH-plants in culture of isolated microspore in carrot. Vegetable crops of Russia. 2017;(5):25-30. (In Russ.)
- DOI: 10.18619/2072-9146-2017-5-25-30
- Zhang G., Zhao F., Chen L., Pan Y., Sun L., Bao N., Zhang T., Cui C.X., Qiu Z., Zhang Y. Jasmonate-mediated wound signalling promotes plant regeneration. Nat. Plants. 2019;(5):491-497. [CrossRef] [PubMed]
- DOI: 10.1038/s41477-019-0408-x
- Zhou W., Lozano-Torres J.L., Blilou I., Zhang X., Zhai Q., Smant G., Li C., Scheres B. A jasmonate signaling network activates root stem Cells and promotes regeneration. Cell. 2019;(177):942-956.e14. [CrossRef] [PubMed]
- DOI: 10.1016/j.cell.2019.03.006


