ROR1 and BMI-1 proteins as potential predictors of the effectiveness of hormone therapy in luminal breast cancer
Автор: Tarakanova V.O., Krakhmal N.V., Patalyak S.V., Tarasov M.N., Babyshkina N.N., Vtorushin S.V.
Журнал: Сибирский онкологический журнал @siboncoj
Рубрика: Обзоры
Статья в выпуске: 3 т.21, 2022 года.
Бесплатный доступ
The purpose of the study was to generalize information regarding the molecular and biological mechanisms involved in the resistance to endocrine therapy with aromatase inhibitors in patients with luminal breast cancer. material and methods. the literature search was conducted using medline, cochrane library, elibrary and pubmed databases. results. the review highlights the results of international studies on molecular and biological characteristics of breast tumors and their relationship with the effectiveness of hormone therapy. particular attention was paid to the description of modern studies on RoR1 and Bmi-1 proteins and their contribution to the development of tumor resistance to treatment. conclusion. the analysis of the world literature confirms the relevance of studying the molecular and genetic characteristics of tumor tissue in patients with luminal breast cancer. the data obtained were compared to the clinical course and response to hormone therapy in order to standardize them for implementation in everyday practice as the “gold standard of diagnosis”.
Breast cancer, luminal subtypes, adjuvant hormone therapy
Короткий адрес: https://sciup.org/140295071
IDR: 140295071 | УДК: 618.19-006.6-08-037:615.357 | DOI: 10.21294/1814-4861-2022-21-3-135-142
Текст научной статьи ROR1 and BMI-1 proteins as potential predictors of the effectiveness of hormone therapy in luminal breast cancer
Breast cancer (BC) is the most commonly diagnosed cancer in women worldwide. According to the National Center for Health Statistics (NCHS), about 279,000 new cases of breast cancer were diagnosed in the United States in 2020, and about 42,000 women died from the disease [1]. About 70 % of all breast tumors are of the luminal Her2-negative type and characterized by the presence of estrogen/ progesterone receptor expression and the absence of Her2Neu protein expression [2]. Adjuvant endocrine therapy is one of the main therapeutic approaches for these types of tumors, as it reduces the risk of progression and recurrence by an average of 40 % [3]. The use of aromatase inhibitors in postmenopausal patients can achieve a favorable clinical outcome. However, most hormone-sensitive tumors develop resistance to therapy [4].
The role of various molecular genetic markers in the mechanisms involved in drug resistance in luminal breast cancer
Over the past years, various molecular biological characteristics of breast tumors have been described to understand the mechanisms of the development of resistance to therapy, including hormone therapy. Much attention is paid to the study of aromatase, which is responsible for the conversion of androgens into estrogens. Normally, the level of aromatase in the breast adipose tissue is low. The distal promoter 1.4 is used in the synthesis of aromatase, and the proximally located promoters 1.3 and II are practically not involved in the process. Malignant epithelial cells of breast tissue are able to secrete prostaglandin E2 (PGE 2), which shifts the promoter from 1.4 to 1.3/II, thereby initiating a sharp jump in aromatase synthesis [5], therefore, it is promising to study and create selective inhibitors of aromatase promoters.
Research is underway to find molecular characteristics of a tumor that can be used as targets to overcome emerging drug resistance. It is known that the TP53 gene mutation, the wild type of which is an anti-oncogene, is associated with the luminal B subtype of breast cancer and a higher Ki67 level, which, in turn, determines the low efficiency of hormone therapy and a less favorable prognosis of the disease. Also of interest is the contribution to the development of drug resistance of the protein kinase MAP3K1, which is a component of several signaling pathways that regulate inflammation and immune responses. The MAP3K1 gene mutation is associated with the luminal A subtype, low Ki67 values, and a more favorable tumor response to hormone therapy. A number of authors confirm the prospects for further study of the GATA3 transcription factor and its influence on the prognosis of breast cancer. It has been shown experimentally in vitro that GATA3 may be one of the key factors in the process of differentiation of tumor cells in luminal type of breast cancer. GATA3 is a regulator of a certain set of genes that directly affect the process of differentiation and proliferation of breast cancer cells [6, 7]. The mutation of the transcription factor GATA3 correlated with a greater suppression of Ki67 levels after treatment with aromatase inhibitors, but not with the initial Ki67 level, which can also be considered a predictor of a more favorable course of the disease [8]. Also, a number of authors note that high expression of GATA3 is most often associated with ER-positive tumors of low malignancy and a more favorable prognosis of the disease [9] The DNA-binding protein FOXA1 is of interest to many researchers. In breast cancer, the levels of the FOXA1 marker significantly correlate with the positive expression of ERα, GATA3, PR, which indicates its participation in the implementation of estrogen-mediated signaling pathways in tumor cells [10].
In the literature, there are also studies on the role of the estrogen receptor gene ESR1 in the development of resistance to hormone therapy with tamoxifen and aromatase inhibitors. It was noted that in the presence of the ESR1 mutation in tumor cells, hyperactivation of the UPR (Unfolded protein response) signaling pathway was observed, which contributed to the development of an endocrine-resistant phenotype in the tumor. Thus, UPR hyperactivation can be considered one of the predictors of resistance to hormone therapy. It was also shown that in the presence of the ESR1 mutation, patients responded to therapy with fulvestrant or targeted therapy with CDK 4/6. But to overcome endocrine-resistant forms, it is necessary to develop more selective estrogen receptor destructors and/or new targets [11].
In recent years, it has been promising to study the influence of immune responses on the development of malignant neoplasms. Chronic inflammation is often present in the tissues of breast tumors. In experimental models of breast cancer, it has been revealed that CD4 T-lymphocytes producing IL-4 regulate the activity of the so-called tumor-associated macrophages (TAMs) and promote the invasion of tumor cells into the surrounding healthy tissues, as well as the process of metastasis. TAMs, in turn, activate the EGFR signaling pathway, which is associated with a less favorable prognosis of the course of the disease and resistance to treatment, hormone therapy in particular [11].
It has been shown that chronic inflammation causes an increase in the level of prostaglandin E2 (PGE2), which can induce the expression of the FOXP3 protein in regulatory T cells (Treg), changing their phenotype and thereby reducing the effectiveness of the immune response in the tumor tissue, worsening the prognosis. Studies have shown that high infiltration of Treg cells in breast tumor tissue is associated with a more aggressive course of the disease and ineffectiveness of hormone therapy. It is interesting to note that with neoadjuvant hormone therapy, the levels of FOXP3 protein and Treg cells in the tumor decreased [12].
Currently, special attention is paid to the study of signaling pathways of tumor cells, including the WNT- intracellular cascade. The Wnt signaling pathway plays an important role in the processes of embryogenesis, cell differentiation, and the development of malignant tumors. The canonical Wnt/β-catenin signaling pathway and non-canonical signaling pathways such as Wnt/Ca2+ and Wnt/PCP (planar cell polarity) are distinguished. When Wnt proteins bind to ROR1/2 receptors, receptors of the Frizzled (FZD) family, and RYK (receptor like tyrosine kinase), the Wnt/Ca2+ signaling pathway is activated, which leads to the recruitment of G-proteins. G-proteins, in turn, activate phospholipase C (PLC), which catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). DAG remains in the cell membrane, promoting the activation of protein kinase C (PKC). IP3, by diffusion, enters the cytosol of the cell, where it binds to Ca2+ channels and triggers the process of Ca2+ ions entering the cytoplasm [13]. An increase in the concentration of Ca2+ inside the cell can stimulate the activation of calcineurin (Ca2+-dependent serine/ threonine phosphatase). This process leads to the accumulation of the nuclear factor associated with T-cells (NFAT), which enhances cell motility and promotes the initiation of the epithelial-mesenchymal transition [14]. I. Pacheco and R.J. Macleod showed that extracellular Ca2+ led to the activation of calciumsensitive receptors (CaSR), which was the reason for an increase in the production of Wnt5a protein.
Wnt5a protein binds to the transmembrane receptor protein kinase ROR1, causing the latter to dimerize, which leads to the activation of the PI3K/AKT signaling pathway. The active PI3K pathway causes phosphorylation of the BMI-1 protein, which leads to chromatin remodeling (fig. 1).
A number of authors associate this process with the development of subsequent genetic instability of the tumor cell and the possibility of the development of
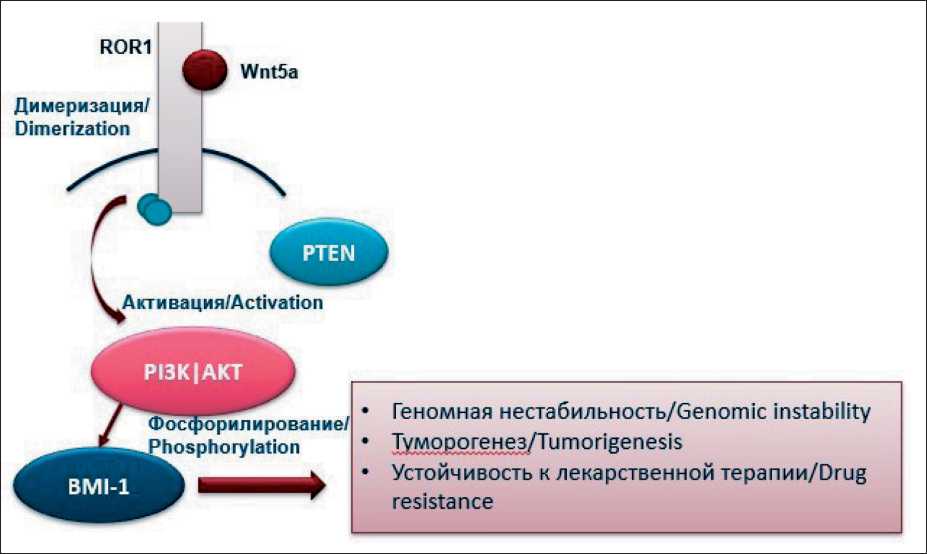
Fig. 1. Wnt signaling pathway. ROR1 and BMI-1 activation mechanism Рис.1. Wnt-сигнальный путь. Механизм активации ROR1 и BMI-1
drug resistance [15, 16]. The described mechanism is being actively studied in malignant neoplasms of various localization (lungs, prostate gland, colon), but its role in the development of the ineffectiveness of the drug treatment of breast cancer (hormone therapy in particular) has not been sufficiently studied.
Characteristics and role of the ROR1 protein in the development of malignant neoplasms
ROR1 (Receptor Tyrosine Kinase Like Orphan Receptor 1) is a protein of the receptor tyrosine kinase family that is encoded in humans by the ROR1 gene. High expression of this protein is typical for most body tissues during embryogenesis, but practically does not occur in healthy tissues of an adult [17, 18]. However, under physiological conditions, the protein can be expressed in the parathyroid glands, islets of the pancreas, in the esophagus, stomach and duodenum [19]. Increased expression of ROR1 in tissues is associated with the development of malignant neoplasms of various localizations. High levels of expression of the marker are especially characteristic in chronic lymphocytic leukemia and some types of solid tumors, including cancer of the lungs, ovaries, and breast [20, 21]. It has been shown that high rates of ROR1 expression in patients with chronic lymphocytic leukemia are associated with a decrease in overall survival [22]. When studying the role of this protein in ovarian cancer, it was found that tumor cells with a high level of ROR1 expression had a more pronounced genetic similarity with tumor stem cells, which was manifested in high values of CD133, CD44 expression on the cell surface, as well as in high levels of ALDH1. Also, ovarian tumors with high ROR1 expression were prone to more rapid onset of distant metastases, which gives grounds to associate high levels of this protein with a decrease in life expectancy and an unfavorable prognosis of the disease [20].
Studies have found that high levels of ROR1 expression in breast cancer are associated with a later stage of the disease, an aggressive course of the tumor process, more frequent relapses after treatment, and a generally poor prognosis [16]. It was also noted that high levels of expression of this protein in breast neoplasms can promote the growth and proliferation of tumor cells, avoidance of apoptosis, and epithelial-mesenchymal transition [23].
One of the key components associated with the epithelial-mesenchymal transition is the Twist group of transcription factors. Twist1 and Twist2 are key regulators of embryonic development and organogenesis. Twist1 is involved in angiogenesis, dissemination and chemoresistance, and also promotes the acquisition of stem properties by tumor cells in carcinomas, sarcomas of various localizations and hematological malignant neoplasms. At the same time, the biological functions of Twist2 in tumors are still very controversial and poorly understood. According to a study by J. Cao et al. [24] the transcription factor Twist directly activates
ROR1 transcription and triggers a chain of events that can lead to epithelial-mesenchymal transition, invasion, and metastasis.
The researchers found that the mechanism of tumor migration and metastasis occurred due to the phosphorylation of the cortactin protein by ROR1 kinase. Cortactin (also known as EMS1 or CTTN) is expressed by neoplastic cells of various cancers and appears to be involved in the migration of tumor cells during metastasis. Upon external stimulation, cortactin undergoes phosphorylation by tyrosine, which leads to an ordered rearrangement and polymerization of the actin cytoskeleton. This process is also required to start the processes of cell migration [25]. It is interesting to note that a relationship between ROR1 activation and FGFR activation has been shown in models of basal cell BC, which is associated with a poorer prognosis [26].
Several studies reported a high level of ROR1 expression in the surviving tumor cells using models of breast cancer xenografts in mice after a course of chemotherapy with paclitaxel. As a second line of therapy, in such cases, therapy was prescribed with the targeted anti-ROR1 drug сirmtuzumab, which slowed down the processes of metastasis. It was noted that in the group that was initially treated with the combination of paclitaxel+сirmtuzumab, the effectiveness of therapy was higher than in the group where the treatment was started with paclitaxel monotherapy [16]. This study also revealed a relationship between high expression of ROR1 with the activation of the Hippo signaling pathway (namely, the YAP/TAZ gene, which carries the role of an oncogene and promotes the transformation of tumor cells into tumor stem cells) and BMI-1. Targeted blocking of the ROR1 receptor with сirmtuzumab blocked Wnt5a ligand-mediated activation of the Hippo-YAP/TAZ signaling pathway and BMI-1, and was therefore associated with a better prognosis.
One of the mechanisms leading to an increase in the effectiveness of chemotherapy in ROR1 knockdown is associated with the involvement of glycoproteins in the process. ROR1 indirectly through MAPK/ERK increases the expression of the so-called «drug pump» ABCB1, which is a P-glycoprotein, which, in turn, removes chemotherapy drugs outside the cell. Functional ABC transporters are large integral membrane proteins containing two transmembrane domains (TMD) and two nucleotide binding domains (NBD). The molecular mechanism of transport occurs due to the hydrolysis of adenosine triphosphate (ATP), which leads to a series of conformational changes covering the molecule from cytoplasmic ATP-binding units to TMD helices, which form a transmembrane pore through which a particular substance is excreted [27].
ABC transporters are not limited to removing only chemotherapy drugs, but also utilize a whole range of different groups of drugs from the cell, including NSAIDs, antibiotics, and others. ABCB1 predominantly removes hydrophobic compounds. The associa- tion was reliably identified between ROR1 inhibition and increased DNA damage by anthracyclines and platinum-based drugs [27].
Dimerization of the ROR1 receptor by the Wnt5a ligand of the Wnt signaling pathway leads to the recruitment of various adapter proteins that trigger unwanted activation of the PI3K/AKT pathway [28, 29]. PI3K/AKT pathway is an intracellular signaling pathway, the central components of which are the enzymes phosphoinositide 3-kinase (PI3K), AKT and mTOR kinases. This pathway is one of the universal signaling pathways characteristic of most cells of the human body. Its activation is responsible for cell escape from apoptosis, promotes cell growth and proliferation. A number of clinical studies have confirmed that mutations in the alpha catalytic subunit p110αPI3K (PI3KCA) are present in 40 % of cases of luminal Her2Neu-negative breast cancer. It was determined that these genetic disorders lead to PI3K/ AKT hyperactivation, which, in turn, leads to uncontrolled cell proliferation and is associated with a more unfavorable prognosis of the course of luminal breast cancer [30, 31]. One of the mechanisms of action of the active PI3K/AKT pathway is through phosphorylation of the BMI-1 protein.
Characteristics and role of BMI-1 protein in the development of malignant neoplasms
BMI-1 is a polycomb family protein that is an epigenetic suppressor through heterodimerization by Ring1B/Rnf2. Ring1B/Rnf2 stimulates the activity of BMI-1 ubiquitin ligase against lysine 119 of histone 2A (H2A-K119) [32]. The latter determines the expression pattern of genes involved in various cellular processes such as proliferation, growth, DNA repair, apoptosis, and aging. It is known that BMI-1 plays an important role in modulating the potential of hematopoietic and neuronal stem cells for self-renewal and differentiation. However, this protein also promotes self-renewal and differentiation of tumor stem cells.
High levels of BMI-1 expression are found in malignant neoplasms of various localizations: cancer
Список литературы ROR1 and BMI-1 proteins as potential predictors of the effectiveness of hormone therapy in luminal breast cancer
- Siegel R.L., Jakubowski C.D., Fedewa S.A., Davis A., Azad N.S. Colorectal cancer in the young: epidemiology, prevention, management. Am Soc Clin Oncol Educ Book. 2020; 40: 1-14. doi: 10.1200/ EDBK_279901.
- S0rlie T., Perou C.M., TibshiraniR., Aas T., Geisler S., JohnsenH., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., Thorsen T., Quist H., Matese J.C., Brown P.O., Botstein D., L0nning P.E., B0rresen-Dale A.L. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001; 98(19): 10869-74. doi: 10.1073/pnas.191367098.
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis ofthe randomised trials. Lancet. 2015; 386(10001): 1341-52. doi: 10.1016/S0140-6736(15)61074-1.
- Araki K., Miyoshi Y. Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer. 2018; 25(4): 392-401. doi: 10.1007/s12282-017-0812-x.
- ZhaoH., ZhouL., ShangguanA.J., BulunS.E. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol. 2016; 57(1): 19-33. doi: 10.1530/JME-15-0310.
- Gustin J.P., Miller J., Farag M., Rosen D.M., Thomas M., Scharpf R.B., Lauring J. GATA3 frame shift mutation promotes tumor growth in human luminal breast cancer cells and induces transcriptional changes seen in primary GATA3 mutant breast cancers. Oncotarget. 2017; 8(61): 103415-427. doi: 10.18632/oncotarget.21910.
- EmmanuelN., LofgrenK.A., PetersonE.A., Meier D.R., JungE.H., Kenny P.A. Mutant GATA3 Actively Promotes the Growth of Normal and Malignant Mammary Cells. Anticancer Res. 2018; 38(8): 4435-41. doi: 10.21873/anticanres.12745.
- Ellis M.J., Ding L., Shen D., Luo J., Suman V.J., Wallis J.W., Van TineB.A.,HoogJ., GoiffonR.J., GoldsteinT.C., NgS., LinL., CrowderR., Snider J., Ballman K., Weber J., Chen K., Koboldt D.C., Kandoth C., Schierding W.S., McMichael J.F., Miller C.A., Lu C., Harris C.C., McLellan M.D., Wendl M.C., DeSchryver K., Allred D.C., Esserman L., Unzeitig G., Margenthaler J., Babiera G.V., Marcom P.K., Guenther J.M., Leitch M., Hunt K., Olson J., Tao Y., Maher C.A., Fulton L.L., Fulton R.S., Harrison M., Oberkfell B., Du F., Demeter R., Vickery T.L., ElhammaliA., Piwnica-WormsH., McDonaldS., WatsonM., DoolingD.J., Ota D., Chang L.W., Bose R., Ley T.J., Piwnica-Worms D., Stuart J.M., Wilson R.K., Mardis E.R. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012; 486(7403): 353-60. doi: 10.1038/nature11143.
- Gonzalez R.S., Wang J., Kraus T., Sullivan H., Adams A.L., Cohen C. GATA-3 expression in male and female breast cancers: comparison of clinicopathologic parameters and prognostic relevance. Hum Pathol. 2013; 44(6): 1065-70. doi: 10.1016/j.humpath.2012.09.010.
- Hurtado A., Holmes K.A., Ross-Innes C.S., Schmidt D., Carroll J.S. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011; 43(1): 27-33. doi: 10.1038/ng.730.
- Ma C.X., Reinert T., Chmielewska I., Ellis M.J. Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer. 2015; 15(5): 261-75. doi: 10.1038/nrc3920.
- GeneraliD, Bates G., BerrutiA., BrizziM.P., CampoL., Bonardi S., Bersiga A., Allevi G., Milani M., Aguggini S., Dogliotti L., Banham A.H., Harris A.L., Bottini A., Fox S.B. Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin Cancer Res. 2009; 15(3): 1046-51. doi: 10.1158/1078-0432.CCR-08-1507.
- De A. Wnt/Ca21 signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai). 2011; 43(10): 745-56. doi: 10.1093/ abbs/gmr079.
- Cai X., Yao Z., Li L., Huang J. Role of DKK4 in Tumorigenesis and Tumor Progression. Int J Biol Sci. 2018; 14(6): 616-21. doi: 10.7150/ ijbs.24329.
- Hasan K., Widhopf 2nd G.F., Zhang S., Lam S.M., Shen Z., Briggs S.P., Parker B.A., Kipps T.J. Wnt5a induces ROR1 to recruit cortactin to promote breast-cancer migration and metastasis. NPJ Breast Cancer. 2019; 5: 35. doi: 10.1038/s41523-019-0131-9.
- Zhang S., Zhang H., Ghia E.M., Huang J., Wu L., Zhang J., Lam S., Lei Y., He J., Cui B., Widhopf 2nd G.F., Yu J., Schwab R., Messer K., Jiang W., Parker B.A., Carson D.A., Kipps T.J. Inhibition of chemotherapy resistant breast cancer stem cells by a ROR1 specific antibody. Proc Natl Acad Sci USA. 2019; 116(4): 1370-7. doi: 10.1073/pnas.1816262116.
- Karvonen H., Barker H., Kaleva L., Niininen W., Ungureanu D. Molecular Mechanisms Associated with ROR1-Mediated Drug Resistance: Crosstalk with Hippo-YAP/TAZ and BMI-1 Pathways. Cells. 2019; 8(8): 812. doi: 10.3390/cells8080812.
- BorcherdingN., KusnerD., Liu G.H., Zhang W. ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell. 2014; 5(7): 496-502. doi: 10.1007/s13238-014-0059-7.
- Balakrishnan A., Goodpaster T., Randolph-Habecker J., Hoffstrom B.G., Jalikis F.G., Koch L.K., Berger C., Kosasih P.L., Rajan A., Sommermeyer D., Porter P.L., Riddell S.R. Analysis of ROR1 Protein Expression in Human Cancer and Normal Tissues. Clin Cancer Res. 2017; 23(12): 3061-71. doi: 10.1158/1078-0432.CCR-16-2083.
- Zhang S., Zhao X., Zhang D. Cellular and molecular immunop-athogenesis of ulcerative colitis. Cell Mol Immunol. 2014; 11(3): 314. doi: 10.1038/cmi.2014.18.
- Saleh R.R., Antrás J.F., Peinado P., Pérez-Segura P., Pandiella A., AmirE., Ocaña A. Prognostic value of receptor tyrosine kinase-like orphan receptor (ROR) family in cancer: A meta-analysis. Cancer Treat Rev. 2019; 77: 11-9. doi: 10.1016/j.ctrv.2019.05.006.
- Gonzalez-Angulo A.M., Timms K.M., Liu S., Chen H., Litton J.K., Potter J., Lanchbury J.S., Stemke-Hale K., Hennessy B.T., Arun B.K., Hortobagyi G.N., Do K.A., Mills G.B., Meric-Bernstam F. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011; 17(5): 1082-9. doi: 10.1158/1078-0432.CCR-10-2560.
- Li C., Wang S., Xing Z., Lin A., Liang K., Song J., Hu Q., Yao J., Chen Z., Park P.K., Hawke D.H., Zhou J., Zhou Y., Zhang S., Liang H., HungM.C., GallickG.E., HanL., Lin C., YangL. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017; 19(2): 106-19. doi: 10.1038/ncb3464.
- Cao J., Wang X., Dai T., Wu Y., Zhang M., Cao R., Zhang R., Wang G., Jiang R., Zhou B.P., Shi J., Kang T. Twist promotes tumor metastasis in basal-like breast cancer by transcriptionally upregulating ROR1. Theranostics. 2018; 8(10): 2739-51. doi: 10.7150/thno.21477.
- Hammer A., Laghate S., DiakonovaM. Src tyrosyl phosphorylates cortactin in response to prolactin. Biochem Biophys. Res. Commun. 2015; 463: 644-9. doi: 10.1016/j.bbrc.2015.05.116.
- Pandey G., Borcherding N., Kolb R., Kluz P., Li W, Sugg S., Zhang J., Lai D.A., Zhang W. ROR1 Potentiates FGFR Signaling in Basal-Like Breast Cancer. Cancers (Basel). 2019; 11(5): 718. doi: 10.3390/ cancers11050718.
- Fultang N., Illendula A., Lin J., Pandey M.K., Klase Z., Peetham-baranB. ROR1 regulates chemoresistance in Breast Cancer via modulation of drug efflux pump ABCB1. Sci Rep. 2020; 10(1): 1821. doi: 10.1038/ s41598-020-58864-0.
- Yu J., Chen L., Cui B., Widhopf G.F. 2nd, Shen Z., Wu R., Zhang L., Zhang S., Briggs S.P., Kipps T.J. Wnt5a induces ROR1/ROR2 heteroo-ligomerization to enhance leukemia chemotaxis and proliferation. J Clin Invest. 2016; 126(2): 585-98. doi: 10.1172/JCI83535.
- Faiao-FloresF., EmmonsM.F., DuranteM.A., KinoseF., SahaB., Fang B., Koomen J.M., Chellappan S.P., Maria-Engler S.S., Rix U., Licht J.D., Harbour J. W., Smalley K.S.M. HDAC Inhibition Enhances the In Vivo Efficacy of MEK Inhibitor Therapy in Uveal Melanoma. Clin Cancer Res. 2019; 25(18): 5686-5701. doi: 10.1158/1078-0432.CCR-18-3382.
- Hoeflich K.P., Guan J., Edgar K.A., O'Brien C., Savage H., Wilson T.R., Neve R.M., Friedman L.S., Wallin J.J. The PI3K inhibitor taselisib overcomes letrozole resistance in a breast cancer model expressing aromatase. Genes Cancer. 2016; 7(3-4): 73-85. doi: 10.18632/ genesandcancer.100.
- Baselga J., Campone M., Piccart M., Burris H.A., Rugo H.S., Sahmoud T., Noguchi S., GnantM., PritchardK.I., LebrunF., Beck J.T., Ito Y., Yardley D., Deleu I., Perez A., Bachelot T., Vittori L., Xu Z., Mukho-padhyay P., LebwohlD., Hortobagyi G.N. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012; 366(6): 520-9. doi: 10.1056/NEJMoa1109653.
- Gray F, Cho H.J., Shukla S., He S., Harris A., Boytsov B., Jaremko L., Jaremko M., Demeler B., Lawlor E.R., Grembecka J., Cierpicki T. BMI1 regulates PRC1 architecture and activity through homo- and hetero-oligomerization. Nat Commun. 2016; 7: 13343. doi: 10.1038/ ncomms13343.
- Claude-Taupin A., Boyer-Guittaut M., Delage-Mourroux R., Hervouet E. Use of epigenetic modulators as a powerful adjuvant for breast cancer therapies. Methods Mol Biol. 2015; 1238: 487-509. doi: 10.1007/978-1-4939-1804-1_25.
- Kreso A., van Galen P., Pedley N.M., Lima-Fernandes E., Frelin C., Davis T., CaoL., BaiazitovR., Du W., SydorenkoN., Moon Y.C., GibsonL., Wang Y., Leung C., Iscove N.N., Arrowsmith C.H., Szentgyorgyi E., Gallinger S., Dick J.E., O'Brien C.A. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014; 20(1): 29-36. doi: 10.1038/ nm.3418.
- Bolomsky A., Schlangen K., Schreiner W., Zojer N., Ludwig H. Targeting of BMI-1 with PTC-209 shows potent anti-myeloma activity and impairs the tumour microenvironment. J Hematol Oncol. 2016; 9: 17. doi: 10.1186/s13045-016-0247-4.
- Darwish N.H., Sudha T., Godugu K., Elbaz O., Abdelghaf-far H.A., Hassan E.E., Mousa S.A. Acute myeloid leukemia stem cell markers in prognosis and targeted therapy: potential impact of BMI-1, TIM-3 and CLL-1. Oncotarget. 2016; 7(36): 57811-20. doi: 10.18632/ oncotarget.11063.
- SahasrabuddheA.A. BMI1: A Biomarker of Hematologic Malignancies. Biomark Cancer. 2016; 8: 65-75. doi: 10.4137/BIC.S33376.
- Althobiti M., Muftah A.A., Aleskandarany M.A., Joseph C., Toss M.S., Green A., Rakha E. The prognostic significance of BMI1 expression in invasive breast cancer is dependent on its molecular subtypes. Breast Cancer Res Treat. 2020; 182(3): 581-9. doi: 10.1007/s10549-020-05719-x.


