Salicylic acid-induced biochemical changes in Swarna (MTU 7029) variety of rice under drought stress
Автор: Verma Preeti, Azad Chandra Shekhar, Singh Pramod Kumar
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 2 т.18, 2022 года.
Бесплатный доступ
The major population of the world is dependent on rice for food. Global warming creates drought conditions mostly in north eastern countries. It is a very challenging task to cultivate drought-sensitive variety in drought-prone areas. To overcome this problem we induced changes in the drought-sensitive variety of rice (Swarna MTU 7029) for drought tolerance. Drought condition was exposed for 7 days and 14 days to SA treated and untreated 56 days old rice plants. Rice seeds were presoaked with 0.5mM SA. The experiment was designed in four groups control (untreated), drought -SA, drought +SA, and SA control. On the 7th and 14th of drought stress, SA improved drought tolerance indicator proline, carotenoid, and total soluble sugar. Starch and protein content were augmented in salicylic acid-treated plants compared to untreated rice plants under drought stress. Antioxidants such as SOD, CAT, and APX levels drastically increased in salicylic acid-treated plants during both 7th and 14th days of drought stress. Therefore, salicylic acid improved antioxidative enzymes content in MTU 7029 rice variety after 7 and 14 days of drought stress.
Drought, rice crop, abiotic stress, salicylic acid, antioxidative enzymes, pre-soaking
Короткий адрес: https://sciup.org/143178802
IDR: 143178802
Текст научной статьи Salicylic acid-induced biochemical changes in Swarna (MTU 7029) variety of rice under drought stress
Rice ( Oryza sativa L.) is a prominent crop grown in India. Among four major crops (wheat, coarse cereals pulses, and rice) in Indian territory, Rice alone accounts for 40% of total food grains produced and accounts for one-quarter of total cultivated land (Kothakonda Krishniah, 2000). Rice provides sustenance to over 3.5 billion people in Asian countries (Muthayya et al., 2014). With the rise occurs in the world’s population, the demand for increased rice production to counteract the hunger problems. Several climatic conditions affect plant growth and development via ecological disturbances and create abiotic stresses in plants (Bellard et al. 2012). In rice, water stress (drought) has a profuse impact on crop yield (Kamoshita et al., 2008) (Palanog et al., 2014). Drought changes the working systems of plants at different levels such as morphological physiological, biochemical, and molecular levels ( haves et al., 2003) (Yousfi et al., 2016). Plants respond differently to stress depending on when the drought occurs, how long the plants are subjected to the stress, which organs or tissues are impacted by the stress, the intensity of the stress, and the stage of plant growth that is hampered by stress. (Dat et al., 2000) (Dourado et al., 2013).
Salicylic acid is a phenolic plant hormone that functions as a signal molecule, regulating physiological and biochemical processes. Endogenous SA administration has been shown to change gene regulation in response to biotic stressors (Raskin, 1992). SA has a substantial impact on plant physiology and increases agricultural yield by influencing plant growth and development (Hasanuzzaman et al., 2017). We hypothesized that pre-soaking SA treatment would strengthen water holding capacity at the late tillering stage. Long durational rice variety (MTU 1061) has more yield than the short durational variety (MTU 1010) but may face biotic stress (Rani et al., 2018). As a result, we chose a long-lasting rice variety called "Swarna (MTU 7029)" for our research. The current study investigated drought stress in rice by subjecting it to drought conditions for 7 and 14 days.
MATERIALS AND METHODS
Plant materials
The study took place in the Udai Pratap Botanical Garden in Varanasi, Uttar Pradesh, India. Rice ( Oryza sativa L.) seeds of the variety "Swarna (MTU 7029)" were received from the Institute of Agricultural Sciences (IAS), Banaras Hindu University, Varanasi, Uttar Pradesh, India.
Experimental design
The seeds were sorted for uniformity by selecting homogeneous and identical sizes and colors. The Seeds were sterilized by dipping in 5% sodium hypochlorite for 5 minutes followed by thrice washing with distilled water to remove chlorine traces and dried on blotting paper. Following sterilisation, the seeds were steeped in two beakers, one with distilled water (Untreated) and another with 0.5 mM of salicylic acid (treated) for 24 hours in dark. The pre-soaked seeds were sown separately in two pots for seedling growth. After 21 days (at the 4th leaf stage), young plants were transplanted in four pots (two for each untreated and treated) with three replications and termed as control ( ; well-watered without SA), drought without SA (D; water-stressed) drought with SA (D+SA; water-stressed with SA), SA control (SA; well watered treated with SA). Three hills were seeded, with three plants in each. Experimental conditions (water and drought) were provided after 35 days of transplantation. Thereafter, biochemical parameters were measured accordingly after 7 days and 14 days of drought condition (figure 1 and 2 respectively).
Measurement of biochemical parameters
By using a mortar and pestle, 2 gm of fresh leaves were crushed in 80% acetone. The homogenized mixture was filtered via Whatman filter paper. The amount of carotenoid was estimated from the absorbance at 664 nm. 0.5 ml of leaf extract was diluted in 4.5 ml of distilled water and incubated in an ice bath for 20 minutes followed by the addition of 10ml anthrone reagent. This mixture was heat shocked in boiling water for 7.5 minutes followed by an ice bath for cooling. Finally, starch content was calculated from the absorbance at 630 nm within 1 hr. Total soluble sugar was estimated by the ethanol-water extraction method according to (Dubois et al., 1956). The amount of sugar was determined at 620 nm by using the anthrone reagent. The proline content was estimated at 520 nm by using a ninhydrin reagent according to Bate’s rule (Bates et al, 1973). The protein content was estimated at 610 nm by using folin-ciocalteu reagent (F R) according to Lowry’s method (Lowry and Randall, 1951). Measurement of antioxidant enzymes
0.5 gm of fresh leaves were homogenized in 80% of acetone with the help of a mortar and pestle. The homogenized mixture was centrifuged at 30000g for 30 minutes at 4° . The supernatant was collected in the fresh tube and used as a crude reaction mixture for the determination of the following antioxidant enzymes.
Superoxide dismutase (SOD): The activity of SOD was done according to (Das et al., 2000). The SOD assay solution was composed of 1.1 ml of 50 mM phosphate buffer pH 7.4, 0.075 ml of 20 mM L-methionine, 1% of Triton X-100, 0.075 ml of 10 mM hydroxylamine hydrochloride, and 1 ml of 5 µM of EDTA. In this reaction mixture, 100 µl of all test samples were added and pre-incubated at 37° for 5 min. 0.08 ml of 50 µM riboflavin was added to all test tubes and then incubated for 8 min in a 40 W flourescent bulb in foil lined box. The absorbance of the reaction was recorded at 560 nm.
Catalase (CAT): The activity of catalase was done according to (Aebi, 1984). Solution of 1.5 ml of 100 mM sodium phosphate buffer (pH 7.0), and 0.1 ml of enzyme extract were reacted with 1 ml of 30 mM H 2 O 2 and a decrease in absorbance at 240 nm up to 1 min at every 15 sec was recorded.
Ascorbate peroxidase (APX): The enzyme extract 0.1 ml was taken in 1.5 ml of sodium phosphate buffer (100mM pH 7.0) containing 0.6 mM ascorbate, 0.12 mM EDTA, and 0.15 ml of H 2 O 2 solution were added. The change in absorbance was recorded for up to 60 seconds at every 15 seconds of interval. The ascorbate peroxidase activity was calculated at 290 nm according to (Nakano and Asada, 1981).
Statistical Analysis
The experiments were performed in triplicate. A oneway ANOVA (analysis of variance) was accomplished for data analysis. Means were compared using Duncan’s multiple comparison test for post hoc analysis (P≤0.05) by using the SPSS statistical analysis software version 16.0 (SPSS Inc., hicago, IL, USA).
RESULTS AND DISCUSSION
Effect of SA on Biochemical parameters in Rice Plants under Drought Stress
In the present study, we analyzed the effect of SA on certain biochemical parameters. Total soluble sugar carotenoid, and proline content were increased under drought stress in untreated plants and the level was augmented in the salicylic acid-treated plants. Starch and protein content were decreased under drought stress in untreated plants and the level was augmented in salicylic acid-treated plants (figure 3). arotenoid content of untreated plants under drought conditions increased by 8.1% and 29.3% respectively at 7 days and 14 days while in treated plants, it was increased by 19.1% and 68% respectively. Total soluble sugar of untreated plants under drought conditions increased by 12.3% and 22.2% respectively at 7 days and 14 days while in the treated plant, it was increased by 21% and 33.8% respectively. Starch content of untreated plants under drought conditions was reduced by 7.5% and 22.2% respectively at 7 days and 14 days while in the treated plants is reduced by 4.5% and 18.5% respectively. The protein content of untreated plants under drought conditions was reduced by 17.9% and 38.1% respectively at 7 days and 14 days while in the treated plants is reduced by 10% and 33.4% respectively. Proline content of untreated plants under drought conditions increased by 58.6% and 143.6% respectively at 7 days and 14 days while in the treated plants, it increased by 83.1% and 182.8% respectively. SA is a phytohormone found in plants and plays an important role in defense mechanisms under abiotic stress (Maruri-López et al., 2019). Drought stress alters the chlorophyll and carotenoid content (Moursy and Hussein, 2008). arotenoid actively participated in the prevention of superoxide formation and restrain ROS accumulation via photoprotection of the photosystem in the chloroplast (Reddy et al., 2004) and enhanced drought tolerance mechanisms (Hayat et al., 2008). In our analysis, we found an increment in the level of carotenoid content in SA-treated plants than the untreated plant both after 7 days and 14 days of drought stress. The increased carotenoid content was correlated with the decrease in leaf area and may involve in a defensive mechanism to prevent plants from harmful effects of stress (Farooq et al., 2009). A study similarly reported an increment in carotenoid level and chlorophyll content during drought conditions in chickpea (Cicer arietinum L.) (Rahbarian et al., 2011) while some studies reported a reduction in chlorophyll content during drought stress in cotton (Massacci et al., 2008) and Catharanthus roseus (Jaleel et al., 2008). Starch is the complex form of carbohydrate which is stored in sink organs as a carbohydrate source and degraded into sugars under environmental stresses (Dien et al., 2019). Therefore, during drought stress, the starch content was decreased. During our analysis, we found a decrement in the level of starch in stressed plants in comparison to control plants but after 7 and 14 days of drought stress, starch content was slightly maintained in SA-treated plants. SA might be deactivated the enzymes for the degradation of starch this shown SA treated plants are less stressed than untreated plants. As the drought continued, starch content decreased might be due to the breaking of starch into simpler forms for absorbing and translocating nutrients easily. The value of starch in control of treated rice plants was slightly higher than untreated rice plants. SA might be maintained the rate of photosynthesis up to a certain extent compared to untreated rice plants. The trend of changes in sugar content under drought was different from starch content. In our study, we found an increased level of total soluble sugar in drought stress which increased with the drought duration (7-14 days). These levels were enhanced more in SA-treated plants than untreated plants under drought stress. Plants store starch for cell metabolism during unfavorable conditions. Whenever plants come under stress, the stored starch is converted into sugar and fulfills the energy requirement of plants. haves and their colleagues reported the relationship between soluble sugars and plant hormones which act as signaling molecules. These signaling molecules may regulate all cell functions in coping with drought stress conditions ( haves et al., 2009). In the analysis of our study, we found a lower deterioration in protein content in SA-treated rice plants than in untreated rice plants under drought stress. In the present study, we found increased proline level in drought stress which was more enhanced in SA treated plants during 7 days to 14 days of drought stress condition. Proline maintains the structure and functions of the cell membrane and weakens the effect of oxidative damage during stresses (Matysik et al., 2002).
Effect of SA on Antioxidative enzymes in Rice Plants under Drought Stress
In the present study, we also analyzed the effect of SA on antioxidative enzymes. Under drought stress, the SOD, AT, and APX levels increased, which is augmented with the increment of stress time (7-14 days). These levels were drastically improved by the use of salicylic acid (figure 4). SOD, AT, and APX were increased by 24%, 33.4%, and 7% respectively at 7 days and 42.6%, 43.5%, and 12.5% at 14 days in untreated control plants under drought conditions while these increments were dramatically augmented to 62.4%, 42.3%, and 16.2% respectively at 7 days and 91.6%, 55.7%, and 20.3% at 14 days in salicylic acid-treated plants under drought condition.
In the analysis, we found a higher level of SOD
AT, and APX in the SA-treated rice plants compared to untreated rice plants under 7 days and 14 days of drought stress. Several studies supported our findings by reporting similar results in bluegrass for heat stress (He et al., 2005), in maize, cucumber, banana, and rice for chilling stress (Janda T et al., 1999) (Kang and Saltveit, 2002) (Kang Guo-Zhang, Wang Zheng-Xun 2003). To minimize the effect of excess reactive oxygen species (ROS) under drought, the plant uses its enzymatic molecules like SOD, AT, and APX (Noctor et al., 2018), and the activity of such enzymes increases. ROS disturbs the structure of lipid that is an integral part of cell walls and organelles, it also deteriorates protein structure, DNA, RNA, and protein forming enzymes (Gill and Tuteja, 2010). Among them SOD was the first antioxidative enzyme that was produced under drought stress which forms oxygen and hydrogen peroxide from free radicals. In many steps, the free radical of oxygen (highly reactive) is changed to a non-reactive form, water, and for this AT and APX play an important role ( ruz De arvalho, 2008). SOD and
AT mostly contributed to tolerance to drought (Sarker and Oba, 2018).

Figure 1. Effect of salicylic acid on rice plants at 7days after drought stress

Figure 2. Effect of salicylic acid on rice plants at 14 days after drought stress
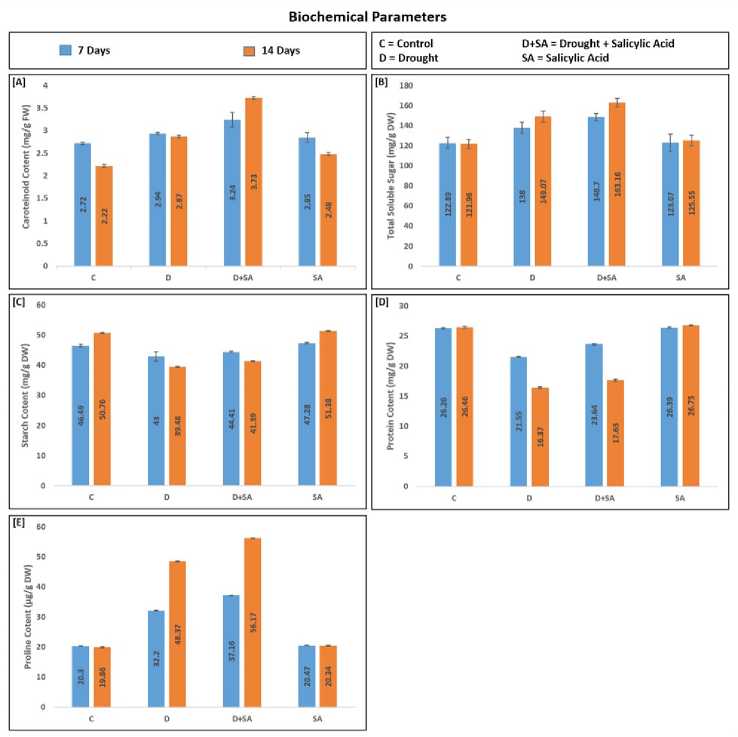
Figure 3. Effect of salicylic acid (SA) on (A) carotenoid content; (B) total soluble sugar; ( ) starch content; (D) protein content; and (E) proline content. Data are presented as mean ± standard deviation. – control; D – drought; D+SA – drought with salicylic acid treatment; SA – salicylic acid treatment; FW – fresh weight; DW – dry weight
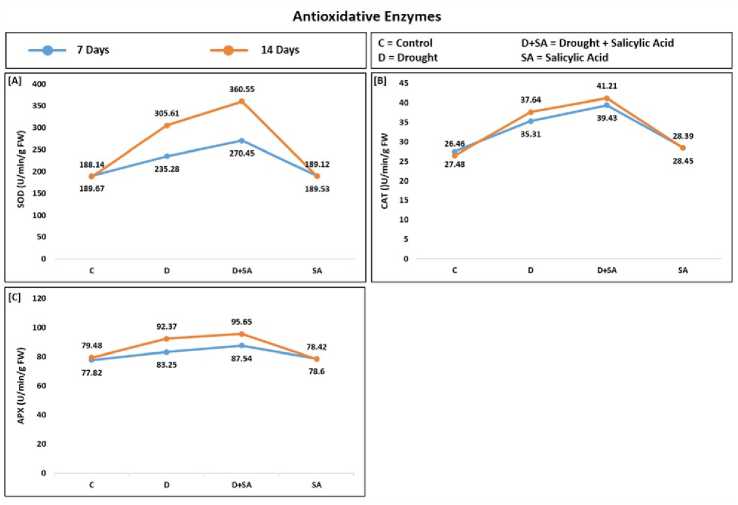
Figure 4. Effect of salicylic acid (SA) on (A) superoxide dismutase (SOD); (B) catalase ( AT); and ( ) ascorbate peroxidases (APX). Data are presented as mean ± standard deviation. – control; D – drought; D+SA – drought with salicylic acid treatment; SA – salicylic acid treatment; FW – fresh weight; DW – dry weight
CONCLUSION
The present study revealed that salicylic acid improves enzymatic activities in SA-treated rice plants after 7 and 14 days of drought stress. This research contributes to sustainable development in agriculture under drought stress conditions. With certain limitations the experiment was performed only on a long variety of rice (Swarna; MTU-7029) therefore, it is necessary to conduct the experiments on other varieties of rice under different stress conditions.
ACKNOWLEDGMENTS
We are thankful to Institute of Agricultural Sciences (IAS), Banaras Hindu University, Varanasi, UP, India for seed availability. We are also thankful for all the teaching and technical staff involved in the study.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.
Список литературы Salicylic acid-induced biochemical changes in Swarna (MTU 7029) variety of rice under drought stress
- Aebi, H. (1984). Catalase in vitro. Methods Enzymol. 105, 121-126. doi:10.1016/s0076-6879(84)05016-3.
- Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W., and Courchamp, F. (2012). Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365-377. doi:10.1111/j.1461-0248.2011.01736.x.
- Chaves, M. M., Flexas, J., and Pinheiro, C. (2009). Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551-560. doi:10.1093/aob/mcn125.
- Chaves, M. M., Maroco, J. P., and Pereira, J. S. (2003). Understanding plant responses to drought - From genes to the whole plant. Funct. Plant Biol. 30, 239-264. doi:10.1071/FP02076.
- Cruz De Carvalho, M. H. (2008). Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 3, 156-165. doi:10.4161/psb.3.3.5536.
- Das, K., Samanta, L., and Chainy, G. B. N. (2000). A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J. Biochem. Biophys. 37, 201204.
- Dat, J., Vandenabeele, S., Vranova, E., Van Montagu, M., Inze, D., and Van Breusegem, F. (2000). Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 57, 779-795. doi:10.1007/s000180050041.
- Dien, D. C., Mochizuki, T., and Yamakawa, T. (2019). Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 22, 530-545. doi:10.1080/1343943X.2019.1647787.
- Dourado, M. N., Martins, P. F., Quecine, M. C., Piotto, F. A., Souza, L. A., Franco, M. R., et al. (2013). Burkholderia sp. SCMS54 reduces cadmium toxicity and promotes growth in tomato. Ann. Appl. Biol. 163, 494-507. doi:10.1111/aab.12066.
- Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 28, 350-356. doi:10.1021/ac60111a017.
- Farooq, M., Wahid, A., Kobayashi, N., Fujita, D., and Basra, S. M. A. (2009). Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 29, 185-212
- Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909-930. doi:10.1016/j.plaphy.2010.08.016.
- Hasanuzzaman, M., Nahar, K., Bhuiyan, T. F., Anee, T. I., Inafuku, M., Oku, H., et al. (2017). Salicylic Acid: An All-Rounder in Regulating Abiotic Stress Responses in Plants. In: Phytohormones -Signaling Mechanisms and Crosstalk in Plant Development and Stress Responses Edited by Mohamed El-Esawi. doi:10.5772/intechopen.68213.
- Hayat, S., Hasan, S. A., Fariduddin, Q., and Ahmad, A. (2008). Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J. Plant Interact. 3, 297-304. doi:10.1080/17429140802320797.
- He, Y., Liu, Y., Cao, W., Huai, M., Xu, B., & Huang, B. (2005). Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in Kentucky bluegrass. Crop science, 45, 988-995. doi:10.2135/cropsci2003.0678.
- Jaleel C.A., Manivannan P., Lakshmanan G.M., Gomathinayagam M., Panneerselvam R. (2008) Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. Colloids Surf B Biointerfaces. 61(2), 298-303. doi:10.1016/j.colsurfb.2007.09.008.
- Janda T. A4 - Szalai, G. A4 - Tari, I. A4 - Paldi, E., T. A.-J. (1999). Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 208(2). doi:10.1007/s004250050547.
- Kamoshita, A., Babu, R. C., Boopathi, N. M., and Fukai, S. (2008). Phenotypic and genotypic analysis of drought-resistance traits for development of rice cultivars adapted to rainfed environments. F. Crop. Res. 109, 1-23. doi:10.1016/j.fcr.2008.06.010.
- Kang Guo-Zhang, Wang Zheng-Xun, S. G.-C. (2003). Participation of H2O2 in Enhancement of Cold Chilling by Salicylic Acid in Banana Seedlings. J Integr Plant Biol. 45(5), 567-573.
- Kang, H., and Saltveit, M. E. (2002). Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiologia Plantarum, 115(4), 571-576.
- Kothakonda Krishniah, N. S. R. (2000). New avenues for augmenting and sustaining rice exports from India. Int. Rice Comm. Newsl. Year 49, 42-51. Available at: https://www.phtnet.org/research/view-abstract.asp?research_id=wq562.
- Bates L. S., R. P. Waldren, I. D. T. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205-207. Available at: https://doi.org/10.1007/BF00018060.
- Lowry, O. H., and Randall, R. J. (1951). Protein Measurement byt the Folin Reagent. J. Biol. Chem. 193, 265-275. Available at: http://www.life.illinois.edU/biochem/355/articles/L owryJBC193_265.pdf.
- Maruri-Lopez, I., Aviles-Baltazar, N. Y., Buchala, A., and Serrano, M. (2019). Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 10, 1-11. doi:10.3389/fpls.2019.00423.
- Massacci, A., Nabiev, S. M., Pietrosanti, L., Nematov, S. K., and Chernikova, T. N. (2008). Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gasexchange analysis and chlorophyll fluorescence imaging. Plant Physiol Biochem, 46(2), 189-95 doi:10.1016/j.plaphy.2007.10.006.
- Matysik, J., Alia, A., Bhalu, B., and Mohanty, P. (2002). Molecular mechanism of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 82(5), 525-532.
- Moursy, M., and Hussein, M. (2008). Evaluating Water Stress Influence on Growth and Photosynthetic Pigments of Two Sugar Beet Varieties Evaluating Water Stress Influence on Growth and Photosynthetic Pigments of Two Sugar Beet Varieties. Research Journal of Agriculture and Biological Sciences, 4(6): 936941
- Muthayya, S., Sugimoto, J. D., Montgomery, S., and Maberly, G. F. (2014). An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 1324, 7-14. doi:10.1111/nyas.12540.
- Nakano, Y., and Asada, K. (1981). Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 22, 867-880. doi:10.1093/oxfordjournals.pcp.a076232.
- Noctor, G., Reichheld, J.-P., and Foyer, C. H. (2018). ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 80, 3-12. doi:https://doi.org/10.1016/j.semcdb.2017.07.013
- Palanog, A. D., Swamy, B. P. M., Shamsudin, N. A. A., Dixit, S., Hernandez, J. E., Boromeo, T. H., et al. (2014). Grain yield QTLs with consistent-effect under reproductive-stage drought stress in rice. F. Crop. Res. 161, 46-54. doi:10.1016/j.fcr.2014.01.004.
- Rahbarian, R., Ganjeali, A., and Najafi, F. (2011). Drought Stress Effects on Photosynthesis, Chlorophyll Fluorescence and Water Relations in Tolerant and Susceptible Chickpea (Cicer Arietinum L.) Genotypes. Acta biologica Cracoviensia. Series botanica, 53(1), 47-56 doi:10.2478/v10182-011-0007-2.
- Rani, P. L., Reddy, R., and Rani, L. (2018). Tillering behaviour and yield of different duration rice research article tillering behaviour and yield of different duration rice varieties under different dates of sowing under aerobic culture. International Journal of Current Research, 8(5), 30143-30146. Raskin, I. (1992). Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 439-463. doi:10.1146/annurev.pp.43.060192.002255.


