Salinity stress effects on growth and nitrate assimilation in bean seedlings likely to be mediated via nitric oxide
Автор: Dhamgaye S., Gadre R.
Журнал: Журнал стресс-физиологии и биохимии @jspb
Статья в выпуске: 4 т.11, 2015 года.
Бесплатный доступ
Background : Salinity stress usually imposes adverse effects on plant systems, but the severity depends upon plant species, growth status and genotype, nutritional and environmental conditions etc. Present study analyses salinity effects on growth and in vivo nitrate reductase activity (NRA) in Phaseolus vulgaris seedlings to work out the mechanism. Results : Supply of 10-200 mM NaCl with 10 mM KNO 3 for 24 h in continuous light reduced the overall growth of the bean seedlings, with perfect -ve correlation for seedling weight, root length and shoot length. Salinity effect with 10 mM NH 4Cl was lesser and with 10 mM NH 4NO 3 was intermediary. NaCl treatment with 10 mM KNO 3 reduced the fresh wt of the root as well as shoot tissue but increased in vivo NRA exerting strong correlation and more prominent effect in the root tissue. Very high NaCl concentration prominently increased NRA at 1, 10 and 50 mM KNO 3 showing inverse gradation in effect. Salt treatment with NH 4NO 3, reduced the in vivo NRA of the root tissue, but the stress parameters, like proline and peroxidase were increased. Conclusions : The salinity stress effects on NRA are less severe with NH 4+, more prominent for root and more effective at low NO 3- concentration. Inverse correlation between decrease in fresh mass and increase in NRA with salinity suggests the involvement of NR in the synthesis of nitric oxide and the observed effect of stress is the balance of two factors reduced assimilatory effect and increased nitric oxide stress.
Nacl effects, phaseolus vulgaris, nitrate reductase, nitrate assimilation, nitric oxide stress
Короткий адрес: https://sciup.org/14323933
IDR: 14323933
Текст научной статьи Salinity stress effects on growth and nitrate assimilation in bean seedlings likely to be mediated via nitric oxide
Abiotic stresses, such as drought, salinity, extreme temperatures and chemical toxicity are serious threats to agriculture and the natural status of the environment. Salinity is a major agricultural constraint that leads to a number of physiological, biochemical and molecular changes which adversely affect plant growth and productivity (Wang et al., 2001). Soil salinity stresses plants in two ways: high concentrations of salts in the soil make it harder for roots to extract water and high concentrations of salts within the plant can be toxic. Processes, such as, seed germination, seedling growth and vigour, vegetative growth, flowering and fruit set are adversely affected by high salt concentration, ultimately causing diminished economic yield and also quality of produce (Sairam and Tyagi 2004). Phaseolus vulgaris (french bean or common bean) is an important and popular legume crop grown for green vegetable and dry seeds. It is valued for its high protein content (23%) and is also rich in calcium, phosphorus and iron. Nitrogen is one of the limiting nutrients for the plant growth. Higher plants acquire nitrogen from soil either as nitrate or ammonium ions, with nitrate being the important nitrogen source for crop plants. Nitrate is converted to ammonium by the sequential action of the cytosolic nitrate reductase (NR) and the chloroplastic nitrite reductase (NiR). Nitrate reductase (NR, EC 1.6.6.1) is a substrate inducible enzyme being subjected to regulation by a number of nutritional and environmental factors (Srivastava 1980). Nitrate reductase activity (NRA) is often correlated with the overall nitrogenous status of the system. In addition to nitrate assimilatory function,
NR has been reported to be involved in the synthesis of NO (Rockel et al ., 2002, Yamasaki and Sakihama 1999, Yamasaki and Sakihama 2000). Nitric oxide (NO) is a bioactive signaling molecule exerting both the beneficial and harmful effects in plant cells and is involved in different stresses (See Magdolena and Jolanta 2007). In the present study, short term salinity effects on overall growth and in vivo nitrate reductase activity were studied in bean seedlings with an insight to elucidate the mechanism.
MATERIALS AND METHODS
Plant growth conditions and treatment
Seeds of Phaseolus vulgaris cv. Rajmah, purchased from a local dealer were surface sterilized with 0.1 % HgCl 2 for 1-2 minutes followed by thorough washing with distilled water. The seedlings were raised in plastic pots containing acid washed sand for 4-5 days in continuous light of intensity 30 Wm-2 supplied by fluorescent tubes at 28 ± 3 °C. They were watered with ½ strength Hoagland’s solution (pH 6.0) containing no nitrogen. Uniformly grown seedlings were used for various salt treatments. For this the seedlings were placed on the test tubes containing ¼ strength Hoagland’s solution in the presence of desired concentration of NaCl for 24 h in continuous light supplied by fluorescent tubes. The root and shoot tissue of the treated seedlings were used for various analyses.
Analytical procedures
In vivo NRA of the treated material was assayed by colorimetric estimation of nitrite according to the method of Srivastava (1975). The proline content of the tissue was estimated by the method of Bates et al. (1973). The peroxidase activity of the phosphate buffer extract of the treated material was assayed using the substrate guaicol by monitoring ∆A436nm according to the method of Putter (1974). Results expressed in tables are the average values of at least four independent experiments with ± SE. Significance of difference obtained for various treatments was tested by student’s ‘t’ test.
RESULTS AND DISCUSSION
Growth effects
Salt stress reducing plant growth has been reported in several species, such as, sunflower cultivars (Noreen and Ashraf 2008), black seeds (Hussain et al., 2009), leaves and roots of tomato (Debouba et al., 2007), soybean (Tuncturk 2008, Amirjani 2010), rice (Lin and Kao 1995) and linseed genotypes (Khan et al., 2007). Further, growth reduction is observed in sensitive genotype of rice, whereas no significant effect is seen on tolerant genotype (Pattangul and Thitisaksakul 2008). However, under high saline conditions growth is affected even in tolerant genotypes of wheat (Hameed et al., 2008). In the present study, the growth parameters of the bean seedlings during salinity stress were evaluated under different nutritional conditions. Supply of 10-200 mM NaCl in the presence of 10 mM KNO3 for 24 h to bean seedling caused gradual reduction in fresh weight, root length and shoot length of the seedlings with the effect being most prominent for shoot length (Table 1). Correlation analyses using Microsoft XY scatter chart had yielded the correlation coefficient, R2, values between NaCl supply and these parameters. Strong correlation for the fr wt., root length and shoot length was observed with R2 values being 0.832, 0.826 and 0.772, respectively. NaCl treatment in the presence of 10 mM NH4Cl affected the fr wt, root length and shoot length of the seedlings to lesser extent depending upon the concentration (Table 1). Further, correlation coefficient, R2, values were also lower being respectively, 0.496, 0.436 and 0.600 for fr wt, root length and shoot length. Prolonged salt treatment of the seedlings for 48 h in the presence of KNO3 caused a significant reduction in fresh wt and shoot length, however, root length remained almost unaffected except at lower concentration of salt causing some reduction (Table 2). Very strong correlation for the fr wt. and NaCl treatment was observed with R2 value being 0.951, while for root length and shoot length the values were 0.392 and 0.699, respectively. Supply of NaCl in the presence of 10 mM NH4NO3 to bean seedlings affected the morphological growth parameters depending upon the concentration. Thus, the fresh wt, the root and shoot lengths of the seedlings was reduced gradually with increasing concentration of the salt, however, the dry wt remained almost unaffected (Table 3). Salt treatment varied fr wt and shoot length with no correlation, dry wt with lesser degree of correlation and root length with stronger correlation having R2 values of 0.330, 0.470, 0.531 and 0.695, respectively. However, prominent reduction in dry mass during NaCl treatment in mungbean seedlings have been reported (Saha et al., 2015). The results of the study related to growth of the seedlings during salinity stress indicates that the severity of effect seems to depend upon nitrogenous salt with more conspicuous effect in the presence of nitrate than ammonium and intermediary effects in the presence of both.
Effects on nitrate assimilation
Nitrate reductase catalyses the first and ratelimiting step of nitrate assimilation in fungi, algae and higher plants (Campbell, 2001) that often limits plant growth and productivity. Environmental stress caused due to salinity also affects the enzyme activity. Inhibition of NRA by salt treatment has been reported in tomato and maize leaves, barley roots and linseed genotypes (Debouba et al., 2006, Debouba et al., 2007, Baki et al., 2000, Omarov et al., 1998, Khan et al., 2007). In the present study, NRA in root and shoot tissue of the seedlings along with fresh mass of the tissues during salt treatment were analyzed. Incubation of bean seedlings with NaCl and KNO3 for 24 h caused gradual reduction in fresh weight of the root as well as shoot tissue of the seedlings with the effect being more prominent for the former (Table 4). On the other hand, NaCl supply increased the in vivo nitrate reductase activity of both the tissues with a significant increase in root tissue (Table 4). Strong – ve correlation for the fr wt of root and shoot tissue was observed with R2 values being 0.617 and 0.793, respectively, but for NRA very strong +ve correlation having R2 value of 0.896 resulted for the root tissue. Salt treatment with NH4Cl for 24 h caused reduction in fresh weight of the root tissue at 100 and 200 mM NaCl only, while the shoot tissue weight varied slightly at all the concentrations (Table 4). On the other hand, NaCl supply with NH4Cl increased the in vivo NRA of root system at higher concentration but decreasing the same at lower concentration. For shoot tissue the activity remained unaffected at all the concentrations except at 100 mM causing an increase (Table 4). Correlation between NaCl treatment and fr wt and NRA of the shoot was not apparent, but for the root tissue R2 values of 0.486 and 0.769 were observed for fr wt and NRA, respectively. NaCl treatment of the seedlings for 48 h resulted in pronounced reduction in fresh weight of the root as well as shoot tissue of the seedlings with the effect being more prominent for the later (Table 5). Supply of 200 mM NaCl increased the in vivo nitrate reductase activity of both the tissues with a more significant increase in root tissue. However, slight inhibition resulted at other concentrations in shoot tissue and in root tissue the effect varied being increased at 10 mM salt but decreased at 20 and 50 mM salt (Table 5). Correlation analyses yielded very strong correlation for the fr wt of root and shoot tissue with R2 values being 0.930 and 0.871, respectively, while for NRA the correlation was observed for the root tissue only with R2 value of 0.655. Thus apparent reduction in tissue mass but corresponding increase in NRA at high salt concentration suggests the involvement of NR in the synthesis of reactive nitrogen species. NR has been reported to be involved in the synthesis of NO (Rockel et al., 2002, Yamasaki and Sakihama 1999, Yamasaki and Sakihama 2000). NO serves as a signaling molecule in plants and involved in various plant stress responses (Magdolena and Jolanta 2007). Also in salinity stress, NO donor pretreatment caused an effect of better survival (Uhida et al.
2002). Hence, in bean seedlings NR seems to have a role in salinity induced oxidative stress. However, treatment of bean seedlings with 10 and 100 mM NaCl with NH4NO3 decreased the in vivo NRA in root tissue prominently, but increased in shoot tissue (Table 6). The proline content of the root as well as shoot was significantly increased at 100 mM NaCl. The peroxidase activity in the roots was prominently increased at both the concentrations of salt, but in the shoot, increase was observed at 100 mM salt only (Table 6).
Table 1 . Effect of supply of NaCl in the presence of two different nitrogenous salts on total weight, root length and shoot length of the seedlings. Five days old bean seedlings were treated with varying concentrations of NaCl for 24 h in the presence of KNO 3 , 10 mM or NH 4 Cl, 10 mM at continuous light intensity of 30 Wm-2 and temperature 26 ± 2 °C
|
Nitrogenous salt + NaCl, mM |
Growth Parameter |
||
|
Seedling wt, mg |
Root length, cm |
Shoot length, cm |
|
|
KNO 3 , 10 mM |
|||
|
+NaCl, 0 |
1184± 70 (100) |
8.0±0.4 (100) |
5.0±0.5 (100) |
|
10 |
1151± 76 ( 97) |
8.2±1.3 (103) |
4.1±0.2 ( 82) |
|
20 |
1057±122 ( 89) |
7.7±1.0 ( 96) |
3.9±0.4* ( 78) |
|
50 |
1112± 47 ( 97) |
7.6±1.2 ( 95) |
3.6±0.2** ( 72) |
|
100 |
1004± 79* ( 85) |
7.2±1.4 ( 90) |
3.1±0.1 ** ( 62) |
|
200 |
931±166 ( 79) |
7.0±0.8 ( 88) |
2.7±0.2*** ( 54) |
|
R2 values |
0.832 |
0.826 |
0.772 |
|
NH 4 Cl, 10 mM |
|||
|
+NaCl, 0 |
1271± 72 (100) |
7.8±0.6 (100) |
3.9±0.2 (100) |
|
10 |
1422± 62 (112) |
6.7±0.5 ( 86) |
4.5±0.4 (115) |
|
20 |
1106± 61 ( 87) |
6.9±0.3 ( 88) |
3.9±0.4 (100) |
|
50 |
1137±105 ( 89) |
6.9±0.4 ( 88) |
3.5±0.5 ( 90) |
|
100 |
1039± 91* ( 82) |
6.8±0.4 ( 87) |
3.2±0.4 ( 82) |
|
200 |
1023±130 ( 80) |
6.4±0.3* ( 82) |
3.2±0.4 ( 82) |
|
R2 values |
0.496 |
0.436 |
0.600 |
Values relative to control are given in parentheses. Level of significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
Table 2 . Effect of prolonged supply of NaCl in the presence of KNO 3 on Total weight, Root length and Shoot length of the seedlings. Five days old bean seedlings were treated with varying concentrations of NaCl for 48 h in the presence of 10 mM KNO 3 at continuous light intensity of 30 Wm-2 and temperature 26 ± 2 °C
|
Nitrogenous salt + NaCl, mM |
Growth Parameter |
||
|
Total wt, mg |
Root length, cm |
Shoot length, cm |
|
|
KNO 3 , 10 mM |
|||
|
+NaCl, 0 |
1333±176 (100) |
10.5±0.6 (100) |
6.1±1.0 (100) |
|
10 |
1280± 69 ( 96) |
9.8±0.6 ( 93) |
4.9±0.3 ( 80) |
|
20 |
1193± 42 ( 89) |
9.2±0.4 ( 88) |
4.7±0.6 ( 71) |
|
50 |
1103± 52 ( 83) |
10.2±0.8 ( 97) |
3.7±0.5* ( 61) |
|
100 |
996± 62* ( 75) |
10.5±0.8 (100) |
3.4±0.2* ( 56) |
|
200 |
806± 17** ( 60) |
10.8±0.7 (103) |
3.0±0.3* ( 49) |
|
R2 values |
0.951 |
0.392 |
0.699 |
Values relative to control are given in parentheses. Level of significance: *p ≤ 0.05, **p ≤ 0.01
Table 3 . Effect of supply of NaCl in the presence of 10 mM NH 4 NO 3 on Total weight, Root length and Shoot length of the seedlings. Five days old bean seedlings were treated with varying concentrations of NaCl for 24 h in the presence of 10 mM NH 4 NO 3 at continuous light intensity of 30 Wm-2 and temperature 26 ± 2 °C
|
Nitrogenous salt + NaCl, mM |
Growth Parameter |
|||
|
Seedling wt, mg |
Root length, cm |
Shoot length, cm |
||
|
Fresh |
Dry |
|||
|
NH 4 NO 3 , 10 mM |
||||
|
+NaCl, 0 |
933±41 (100) |
294±30 (100) |
10.5±0.4 (100) |
3.5±0.2 (100) |
|
10 |
876±64 (94) |
281±25 (96) |
10.3±0.7 (98) |
3.5±0.2 (100) |
|
20 |
829±83 (89) |
273±26 (95) |
10.3±0.5 (98) |
3.3±0.3 (94) |
|
50 |
831±64 (89) |
300±19 (102) |
10.2±0.7 (97) |
2.9±0.2* (83) |
|
100 |
829±68 (89) |
310±24 (105) |
9.8±0.6 (93) |
3.0±0.1* (86) |
|
200 |
827±72 (89) |
309±16 (105) |
9.9±0.7 (94) |
3.0±0.2* (86) |
|
R2 values |
0.330 |
0.531 |
0.470 |
0.695 |
Values relative to control are given in parentheses. Level of significance: *p ≤ 0.05
Table 4 . Effect of supply of NaCl in the presence of two different nitrogenous salt on fresh weight and in vivo nitrate reductase activity of root and shoot tissue of the seedlings. Five days old bean seedlings were treated with varying concentrations of NaCl for 24 h in the presence of KNO 3 , 10 mM or NH 4 Cl, 10 mM at continuous light intensity of 30 Wm-2 and temperature 26 ± 2 °C
|
Nitrogenous salt |
Fresh weight, mg |
In vivo NRA, nmoles NO 2 h-1 g-1 fr wt |
||
|
+ NaCl, mM |
Root |
Shoot |
Root |
Shoot |
|
KNO 3 , 10 mM |
||||
|
+NaCl, 0 |
153±12 (100) |
980±73 (100) |
198± 5 (100) |
42±3 (100) |
|
10 |
114±13* ( 75) |
983±31 (100) |
206± 35 (104) |
49±2* (117) |
|
20 |
130±32 ( 85) |
856±70 (87) |
283± 37* (143) |
55±3** (131) |
|
50 |
132±14 ( 86) |
913±52 (93) |
267± 67 (135) |
56±6* (133) |
|
100 |
118± 9* ( 77) |
807±77 (82) |
304± 55* (156) |
52±6 (124) |
|
200 |
95±12** ( 62) |
743±78* (76) |
664±123*** (335) |
56±6* (133) |
|
R2 values |
0.617 |
0.896 |
0.793 |
0.309 |
|
NH 4 Cl, 10 mM |
||||
|
+NaCl, 0 |
206±25 (100) |
995± 61 (100) |
37± 5 (100) |
20±2 (100) |
|
10 |
235±16 (114) |
1164± 47* (117) |
32± 3 ( 86) |
18±2 ( 90) |
|
20 |
196±14 ( 96) |
867± 54 ( 87) |
34± 5 ( 92) |
23±3 (115) |
|
50 |
218±37 (106) |
1050±112 (106) |
43±17 (116) |
23±2 (115) |
|
100 |
161±19 ( 78) |
910± 68 ( 91) |
66±12* (178) |
30±7 (150) |
|
200 |
174±25 ( 84) |
1033± 95 (104) |
64±19 (173) |
23±2 (115) |
|
R2 values |
0.486 |
0.769 |
0.002 |
0.212 |
Values relative to control are given in parentheses.
Level of significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
Table 5 . Effect of supply of NaCl in the presence of KNO 3 on fresh weight and in vivo nitrate reductase activity of root and shoot tissue of the seedlings. Five days old bean seedlings were treated with varying concentrations of NaCl for 48 h in the presence of 10 mM KNO 3 at continuous light intensity of 30 Wm-2 and temperature 26 ± 2 °C
|
Nitrogenous salt + NaCl, mM |
Fresh weight, mg |
In vivo NRA, nmoles NO 2 h-1 g-1 fr wt |
||
|
Root |
Shoot |
Root |
Shoot |
|
|
KNO 3 , 10 mM |
||||
|
+NaCl, 0 |
174±23 (100) |
1110±158 (100) |
240± 40 (100) |
58±11 (100) |
|
10 |
181±24 (104) |
1119± 55 (101) |
307± 92 (128) |
54±01 (93) |
|
20 |
182±16 (105) |
952± 35 (86) |
196± 32 ( 82) |
53±04 (91) |
|
50 |
155±13 (89) |
919± 62 (83) |
200± 60 ( 83) |
51±08 (88) |
|
100 |
154±17 (89) |
754± 54* ( 68) |
231± 60 ( 96) |
50±05 ( 86) |
|
200 |
107±7* (61) |
655± 19** ( 59) |
587±127* (245) |
70±16 (121) |
|
R2 values |
0.930 |
0.871 |
0.665 |
0.441 |
Values relative to control are given in parentheses. Level of significance: *p ≤ 0.05, **p ≤ 0.01
Table 6 . Effect of supply of NaCl in the presence of 10 mM NH 4 NO 3 on in vivo NRA, Proline content and Peroxidase activity in root and shoot tissue of the seedlings.Five days old bean seedlings were treated with varying concentrations of NaCl for 48 h in the presence of 10 mM KNO 3 at continuous light intensity of 30 Wm-2 and temperature 26 ± 2 °C
|
NaCl conc., mM |
In vivo NRA, nmoles NO 2 h-1 g-1 fr wt |
Peroxidase activity, ∆A min-1 g-1 fr wt |
Proline content, mg g-1 fr wt |
|||
|
Root |
Shoot |
Root |
Shoot |
Root |
Shoot |
|
|
0 |
319±41 (100) |
93±11 (100) |
0.063±0.010 (100) |
0.151±0.011 (100) |
19±1 (100) |
410±30 (100) |
|
10 |
119± 47** (37) |
131± 23 (141) |
0.080±0.007 (127) |
0.149±0.015 (98) |
20±2 (114) |
457±30 (105) |
|
100 |
97± 25*** (30) |
129± 30 (139) |
0.080±0.009 (127) |
0.185±0.025 (122) |
28±2* (172) |
704±40** (147) |
Values relative to control are given in parentheses. Level of significance: **p ≤ 0.01, ***p ≤ 0.001
Thus, at high nitrogen level with a combination of NO3- and NH4+, nitrate assimilation appears to be inhibited by salt, although the other stress responsive parameters are increased. Hence, during salt treatment NH4+ tend to suppress NO3- induced NO synthesis.
The NaCl stress has been reported to increase Na+, Cl- and also NH4+ ion concentrations, but to decrease the NO3 – ion concentration (Debouba et al., 2007). In barley seedlings, both Cl- and SO42- salts severely inhibited uptake of NO3-, whereas nitrate reductase activity was protected from salt injury in situ (Aslam et al., 1984). Hence, it is likely that salt stress effect on NRA may be mediated via uptake of NO3-. Here it is worth to mention that transport of nitrate across the plasma membranes is a complex process, involving various transport systems and mechanisms. The high-affinity transport system displays Michaelis– Menten kinetics saturating at 0.2-0.5 mM nitrate and the low-affinity transport system operates at concentrations above 0.5 mM and usually displays non-saturating uptake kinetics. At supersaturating
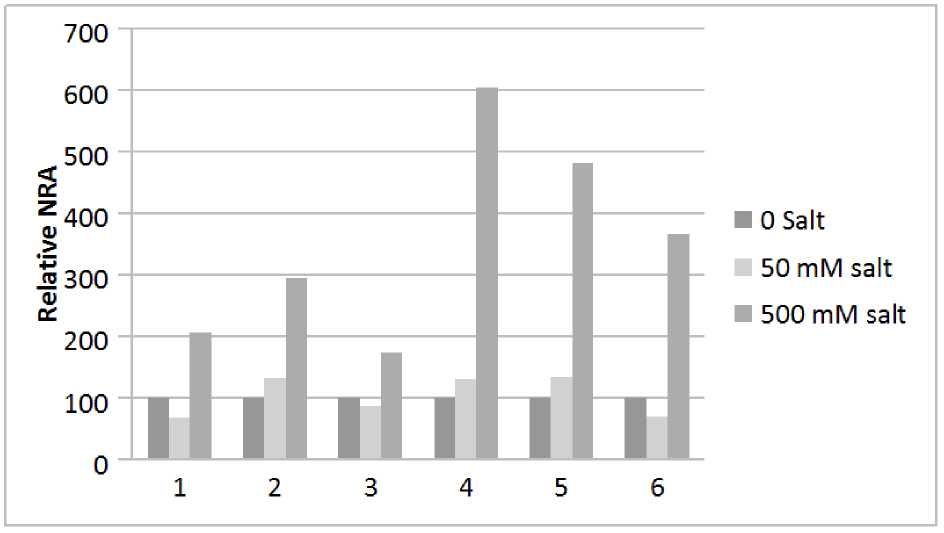
Figure 1. Effect of supply of NaCl in the presence of varying concentrations of KNO 3 on in vivo NRA of
root and shoot tissue of the seedlings. Five days old bean seedlings were treated with 50 and 500 mM NaCl in the presence of 1, 10 and 50 mM KNO 3 for 24 at continuous light intensity of 30 Wm-2 and temperature 26 ± 2 °C
1 - Shoot 1 mM KNO 3
4 - Root 1 mM KNO 3
2 – Shoot 10 mM KNO 3
5 – Root 10 mM KNO 3
3 – Shoot 50 mM KNO 3
6 – Root 50 mM KNO 3
nitrate concentrations there is passive diffusion through ion channels. In the present system when the effect of 50 and 500 mM NaCl in the presence of 1, 10 and 50 mM KNO3 on in vivo NRA in root and shoot tissue of the seedlings was analysed, the activity was slightly decreased in the root tissue at 50 mM salt with 1 mM KNO3 but in the shoot tissue it increased. High salt concentration of 500 mM increased the activity in both the tissues with a highly significant increase in root tissue (Fig. 1, Columns 1 and 4). At 10 mM KNO 3 the enzyme activity was increased slightly and prominently at 50 as well as 500 mM salt, respectively, in both the tissues (Fig. 1, Columns 2 and 5). Treatment in the presence of 50 mM KNO 3 caused a reduction in activity at 50 mM salt, but enhanced it at 500 mM salt in both the tissues (Fig. 1,
Columns 3 and 6). This suggests that high salinity increases high affinity as well as low affinity active transport for NO 3 - and also passive diffusion through ion channels with the last one being least effective. Further, in shoot tissue the effect is less prominent, as transport of nitrate from root to shoot is lesser.
CONCLUSION
Salinity stress with KNO3 reduces growth of the bean seedlings, but increases in vivo NRA. Increase in NRA by NaCl treatment is more prominent in the root tissue, at longer duration and low NO3 concentration. Inverse correlation observed for growth and NRA suggests involvement of NR in NO synthesis during saline stress. Salinity stress with NH4NO3 decreases NRA, but increases proline content and
peroxodase activity. Thus NR balances reduced assimilation and increased NO stress during salinity.
Список литературы Salinity stress effects on growth and nitrate assimilation in bean seedlings likely to be mediated via nitric oxide
- Amirjani, M.R. (2010) Effect of salinity stress on growth, mineral composition, proline content, antioxidant enzymes of soybean. Amer. J of Plant Physiol., 5(6), 350-360
- Aslam, M., Huffaker, R.C. and Rains, D.W. (1984) Early effects of salinity on nitrate assimilation in barley seedlings. Plant Physiol., 76, 321-325
- Baki, G.K., Siefritz, F., Man, H.M., Weiner, H., Kaldenhoff, R. and Kaiser, W.M. (2000) Nitrate redutase in Zea mays L. under salinity. Plant Cell Environ., 23, 515-521
- Bates, L.S., Waldren, R.P. and Teare, I.D. (1973) Rapid determination of free proline for water-stress studies. Plant Soil, 39, 205-207
- Campbell, W.H. (2001) Structure and function of eukaryotic NAD(P)H:nitrate reductase. Cell Mol. Life Sci., 53, 194-204
- Debouba, M., Gouia, H., Valadier, M.H., Ghorbel, M.H. and Suzuki, A. (2006) Salinity-induced tissue specific diurnal changes in nitrogen assimilatory enzymes in tomato seedlings grown under high and low nitrate medium. Plant Physiol. Biochem., 44(5-6), 409-419
- Debouba, M., Dghimi. H.M., Suzuki, A., Ghorbel, M.H. and Gouia, H. (2007) Changes in growth and activity of enzymes involved in nitrate reduction and ammonium assimilation in tomato seedlings in response to NaCl stress. Annals of Botany, 99(6), 1143-1151
- Hameed, A., Naseer, S., Iqbal, T., Syed, H. and Haq, A. (2008) Effects of NaCl salinity on seedling growth, senescence, catalase and protease activities in two wheat genotypes differing in salt tolerance. Pak J. Bot., 40(3), 1043-1051
- Hussain, K., Majeed, A., Nawaz, K., Khizar, H.B. and Nissar, M.F. (2009) Effect of different levels of salinity on growth and ion contents of black seeds. Curr. Res. J. Biol. Sci., 1(3), 135-138
- Khan, M.N., Siddiqui, M.H., Firoz, M., Mansoor, M., Khan, A. and Naeem, M. (2007) Salinity induced changes in growth, enzyme activities, photosynthesis, proline accumulation and yield in Linseed genotype. World J Agri. Sci., 3(5), 685-695
- Lin, C.C. and Kao, C.H. (1995) Disturbed ammonium assimilation is associated with inhibition of roots in rice seedlings caused by NaCl. Plant Growth Reg., 18, 233-238
- Magdalena, A. and Jolanta, F.W. (2007) Nitric oxide as a bioactive signaling molecule in plant stress responses. Plant Sci., 172, 876-887
- Noreen, S. and Ashraf, M. (2008) Alleviation of adverse effects of salt stress on sunflower by exogenous application of salicylic acid: growth and photosynthesis. Pak. J Bot., 40(4), 1657-1663
- Omarov, R.T., Sagi, M. and Lips, S.H. (1998) Regulation of aldehyde oxidase and nitrate reductase in roots of barley by nitrogen source and salinity. J. Exp. Bot., 49(322), 897-902
- Pattanagul, W. and Thitisakasakul, M. (2008) Effect of salinity stress on growth and Carbohydrate metabolism in three rice cultivars differing in salinity tolerance. Ind. J. Exp. Biol., 46, 736-742
- Putter, J. (1974) Peroxidases, In Bergmeyer, H.U. (ed) Methods of enzymatic analysis. Vol 2: Verlag Chemie-Academic Press, New York, pp 685-690
- Rockel, P., Strube, F., Rockel, A., Wildt, J.and Kaiser, W.M. (2002) Regulation of nitric oxide production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot., 53(366), 103-110
- Saha, P., Mukherjee, A. and Biswas, A.K. (2015) Modulation of NaCl induced DNA damage and oxidative stress in mungbean by treatment with sub lethal dose. Biol. Plantarum, 59(1), 139-146
- Sairam, R.K. and Tyagi, A. (2004) Physiology and molecular biology of salinity tolerance in plants. Curr. Sci., 86(3). 407-421
- Srivastava, H.S. (1975) Distribution of nitrate reductase in bean seedlings. Plant Cell Physiol., 16, 995-999
- Srivastava, H.S. (1980) Regulation of nitrate reductase in higher plants. Phytochem., 19, 725-733
- Tuncturk, M., Tunçturk, R. and Yasar, F. (2008) Changes in micronutrients, dry weight and plant growth of soybean (Glycine max L. Merrill) cultivars under salt stress. Afr. J Biotech., 7(11), 1650-1654
- Uhida, A., Jagandorf, A.T., Hibino, T., Takabe, T. and Takabe, T. (2002) Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci., 163, 515-523
- Wang, W.X., Vinocur, B., Shoseyov, O. and Altman, A. (2001) Biotechnology of plant osmotic stress tolerance: physiological and molecular considerations. Acta. Hort., 560, 285-292
- Yamasaki, H. and Sakihama, Y. (1999) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In vivo evidence for the NR-dependent formation of active nitrogen species. FEBS Lett., 468, 89-92
- Yamasaki, H. and Sakihama, Y. (2000) An alternate pathway for nitric oxide production in plants: New features of an old enzyme. Trends Plant Sci., 4, 128-129


